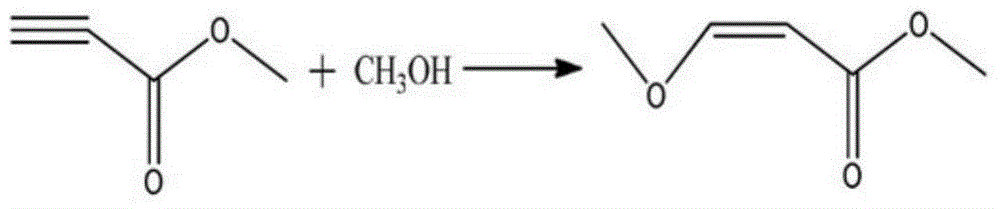
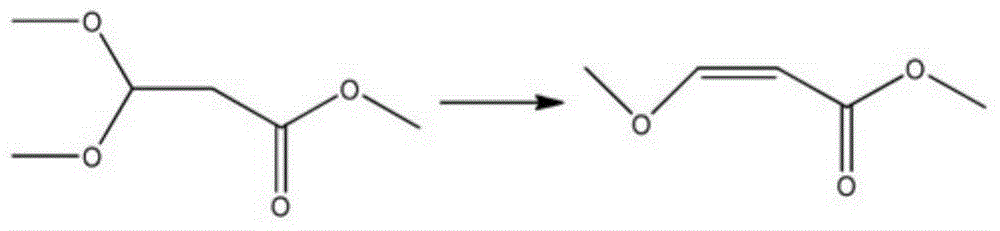
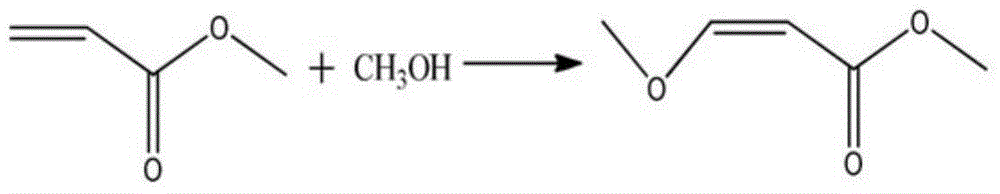
Preparation method of methyl 3-methoxyacrylate
A technology of methyl methoxyacrylate and methyl oxypropionate is applied in the field of preparation of methyl 3-methoxyacrylate, can solve problems such as low boiling point, strong volatility, etc. Ease of transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 38g (200mmol) methyl 3-methoxy-3-butoxypropionate, 190g methanol, and 1.9g potassium hydrogensulfate to a 500mL three-necked flask equipped with mechanical stirring, a thermometer and a reflux device, and heat to reflux after adding After 24 hours, the reflux device was changed to a vacuum distillation device, and the solvent was removed under reduced pressure. Under the protection of nitrogen, the residue was heated to 160 ° C for 5 hours for cracking reaction, and then distilled under reduced pressure to obtain 18.2 g of 3-methoxymethyl acrylate with a purity of 96%. The yield of methyl ester was 75.3%.
Embodiment 2
[0024] Add 38g (200mmol) methyl 3-methoxy-3-butoxypropionate, 190g methanol, and 0.38g potassium hydrogensulfate to a 500mL three-neck flask equipped with mechanical stirring, a thermometer and a reflux device, and react at 40°C after adding After 15 hours, the reflux device was changed to a vacuum distillation device, and the solvent was removed under reduced pressure. Under the protection of nitrogen, the residue was heated to 120 ° C for 5 hours for cracking reaction, and then distilled under reduced pressure to obtain 16.6 g of 3-methoxymethyl acrylate with a purity of 95%, which was converted to 3-methoxy-3-butoxypropionic acid The yield in terms of methyl ester was 68.0%.
Embodiment 3
[0026] Add 38g (200mmol) methyl 3-methoxy-3-butoxypropionate, 570g methanol, and 1.14g sodium bisulfate to a 1000mL three-neck flask equipped with mechanical stirring, a thermometer and a reflux device, and react at 55°C after adding After 10 hours, the reflux device was changed to a vacuum distillation device, and the solvent was removed under reduced pressure. Under the protection of nitrogen, the residue was heated to 180° C. for 5 hours, and then distilled under reduced pressure to obtain 18.2 g of methyl 3-methoxyacrylate with a purity of 97%. The yield of methyl ester was 76.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com