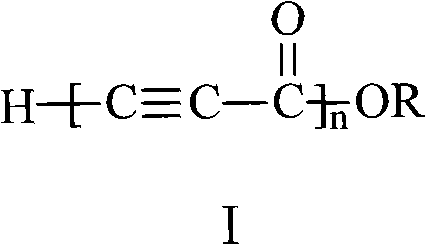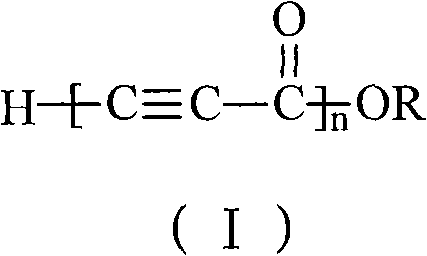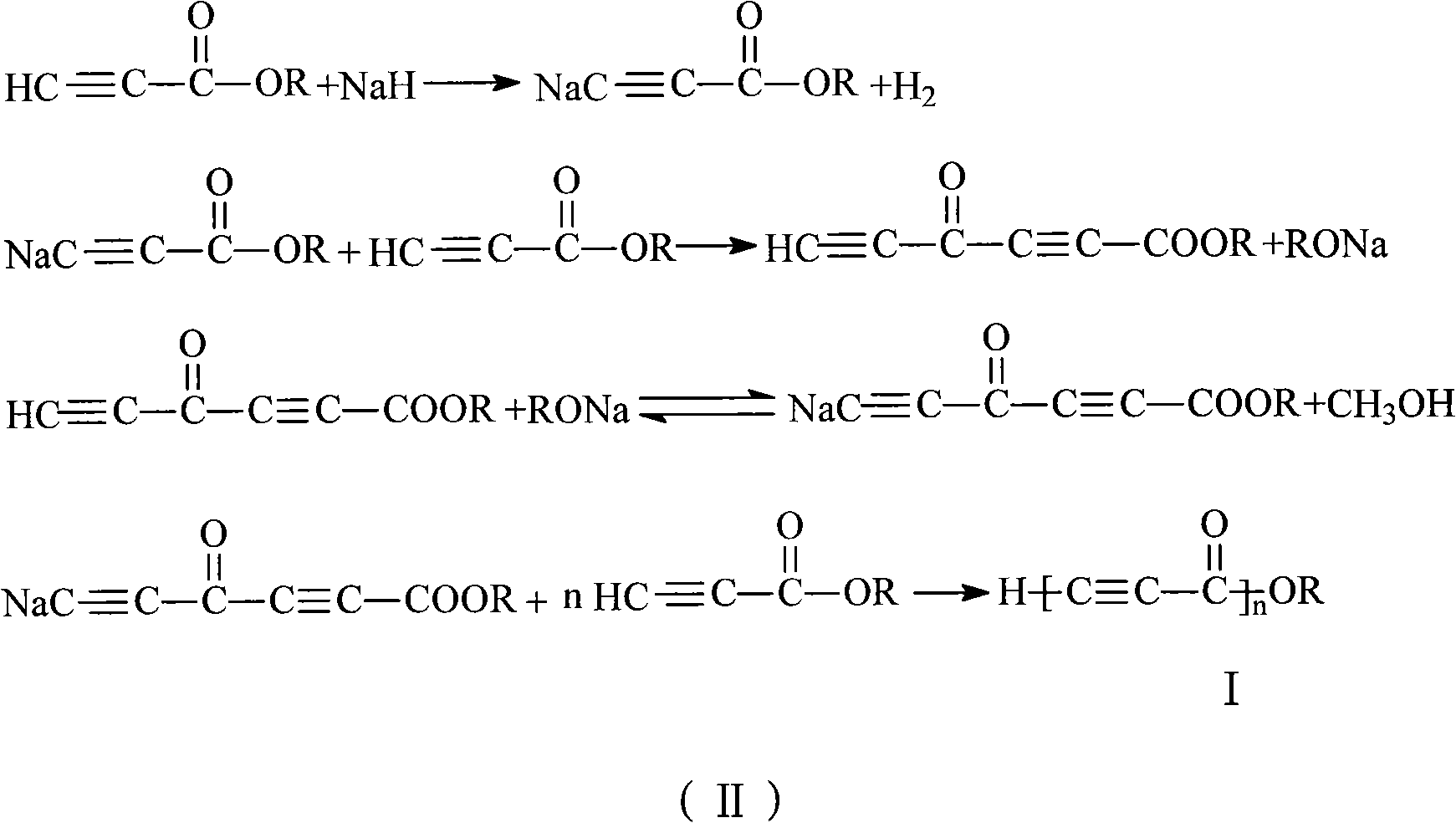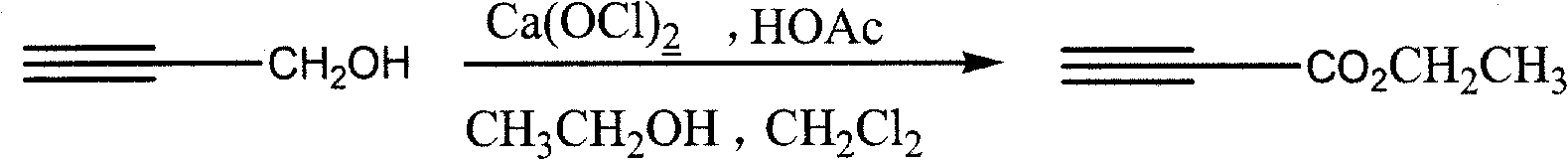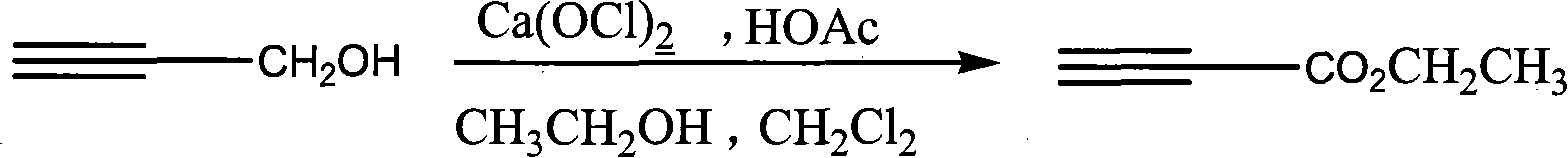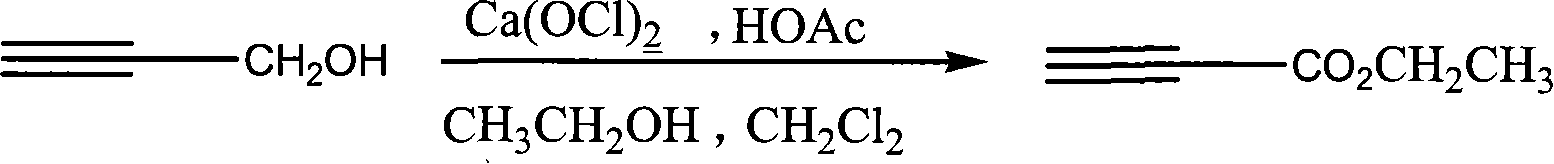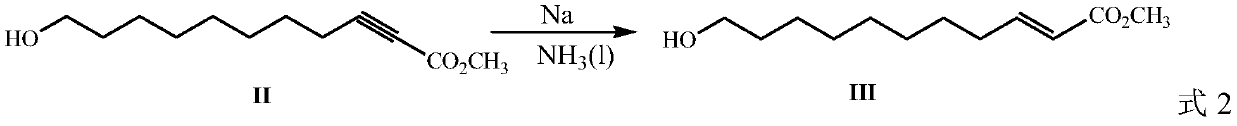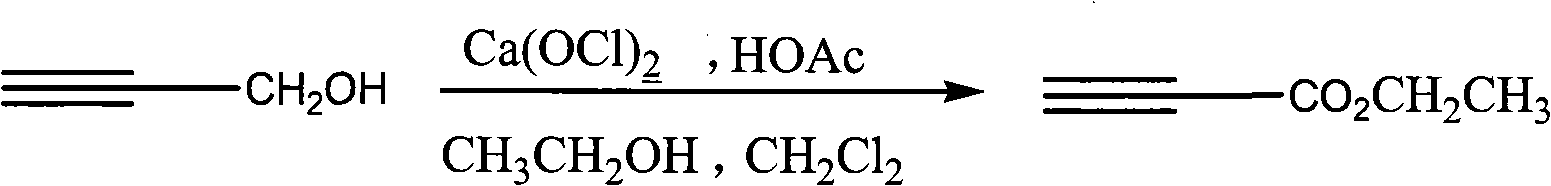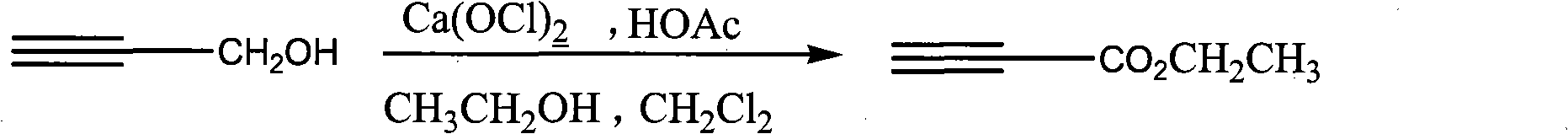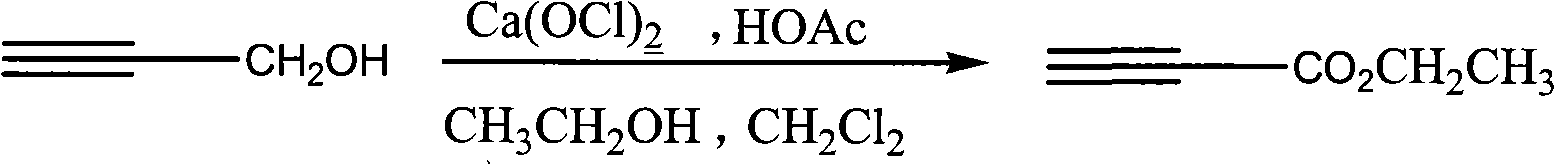Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Methyl propiolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methyl propiolate is an organic compound with the formula HC₂CO₂CH₃. It is the methyl ester of propiolic acid, the simplest acetylenic carboxylic acid. It is a colorless liquid that is miscible with organic solvents. The compound is a reagent and building block for the synthesis of other organic compounds, reactions that exploit the electrophilicity of the alkyne group.
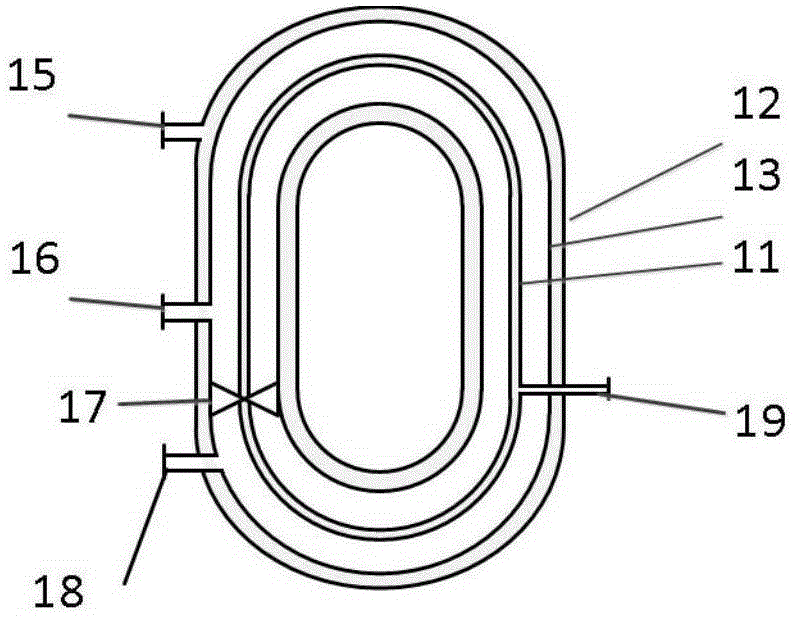
Medical anti-adhesion hydrogel dressing and preparation method thereof
ActiveCN105169465AImprove hydrophilic abilityPromote healingAbsorbent padsBandagesMicro structureMethacrylate
The present invention discloses a medical anti-adhesion hydrogel dressing, which comprises a hydrogel, wherein a methyl propiolate monomer, double or multi-functional group terminated-polyethylene glycol having unsaturated double bond, water and an initiator are further subjected to thermal initiation or photo initiation or radiation initiation polymerization so as to obtain the hydrogel, and the hydrogel has a micron porous structure. According to the present invention, the prepared hydrogel dressing has characteristics of no adhesion to wound, good water absorption and excellent mechanical property, and has a space micro-structure capable of loading drugs or growth factors so as to load various drugs or growth factors, achieve the functionalization treatment of the dressing, and achieve antibacterial, wound healing promoting and other functions.
Owner:朗肽生物制药股份有限公司
Synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives
InactiveCN102627522AOrganic compound preparationHydrocarbon from oxygen organic compoundsEthyl groupKetone
The invention discloses a synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives. The synthesis method of indenofluorene derivatives is characterized in that ester-group-containing compounds are formed through Diels-Alder reaction of methyl propiolate and indenocyclopentadienone, ethyl substituted indenofluorene derivatives are formed through hydrolysis, acid catalytic ring closing, carbonyl reduction and introduction of ethyl onto methylene, diketone compounds produced after ring closing react with lithium salt of 4, 4'-di-tert-butyl-2-brominated biphenyl, and then acidification and ring closing are conducted to obtain indenofluorene derivatives with spirofluorene. The synthesis method of isotruxene is characterized in that 1, 4-diphenyl-2, 3-di (carbalkoxy) fluorenone products are formed through the Diels-Alder reaction of indenocyclopentadienone and dimethyl acetylenedicarboxylate, isotruxene ketone is obtained through hydrolysis and acid catalytic ring closing and finally isotruxene is obtained; dibenzyl alcohol products are formed through the Diels-Alder reaction of 1, 4-butynediol and indenocyclopentadienone, and isotruxene is obtained through acetone protection, carbonyl reduction, acetone removal and polyphosphoric acid (PPA) ring closing; and oriented oxy substituted products, i.e. isotruxene ketone which is derived from isotruxene with methylene at a No.5 position being substituted, and corresponding diethyl substituted products are additionally obtained. The indenofluorene derivatives, the isotruxene and the mono-substituted isotruxene derivatives disclosed by the invention can be used in the field of organic electroluminescence and organic micro-molecule solar cells.
Owner:EAST CHINA NORMAL UNIV
Annular multilayer sleeve membrane reactor and method for producing methyl propiolate through same
ActiveCN105170033AImprove conversion rateHigh selectivityOrganic compound preparationCarboxylic acid esters preparationWater vaporEther
The invention discloses an annular multilayer sleeve membrane reactor. The reactor adopts an annular three-layer sleeve structure as the main body, and comprises an inner membrane sleeve, a middle membrane sleeve and an outer sleeve which are coaxially arranged from the inner part to the outer part, wherein exteriors of the inner membrane sleeve and the middle membrane sleeve are fixed and supported through supporting networks; a water vapor outlet is formed in the inner membrane sleeve; a material inlet and a material outlet are formed in the middle membrane sleeve; an air inlet is formed in the outer sleeve; a mixing component is arranged in the cavity of the middle membrane sleeve; a separating network is mounted in at the outlet of the middle membrane sleeve; a one-way valve is mounted between the material inlet and the material outlet. Methyl propiolate produced by the reactor can realize continuous removal of produced water during esterification reaction of the methyl propiolate, and improve the reaction conversion rate and selectivity, and meanwhile reduces the generationproduces of polymer and ether impurities.
Owner:WANHUA CHEM GRP CO LTD
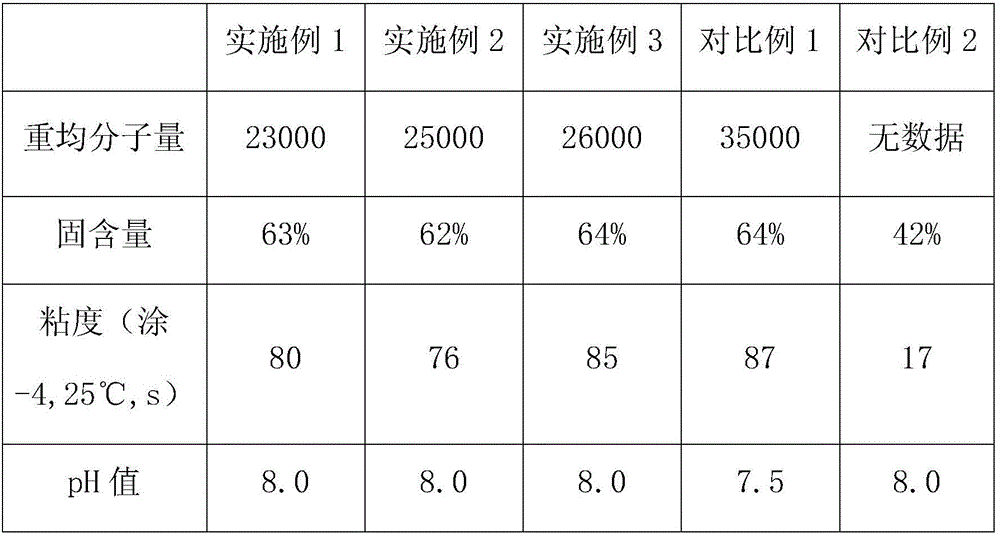
High-adhesion aqueous thermosetting acrylic resin, preparation method and applications thereof
The invention discloses a high-adhesion aqueous thermosetting acrylic resin, a preparation method and applications thereof. According to the present invention, a reaction monomer is modified with a silane coupling agent, a polymerization reaction is performed in an alcohol ether type solvent medium under the effect of an initiator, and neutralization salification is performed with an organic amine to prepare the acrylic resin, wherein the reaction monomer comprises, by mass, 0-30% of methyl propiolate, 0-30% of acrylic acid ester, 5-18% of (methyl) hydroxyalkyl acrylate, 1-10% of unsaturated carboxylic acid, and 0-20% of a vinyl aromatic compound. According to the present invention, the prepared acrylic resin coating material has excellent adhesion to the aluminum material, the paint film has characteristics of high luster, good fullness and high strength, and the excellent protection performance and the excellent decorative effect can be provided for the high-grade aluminum material.
Owner:FOSHAN KINNO METAL TECH
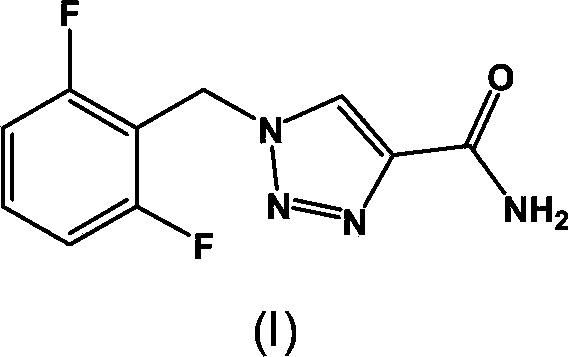
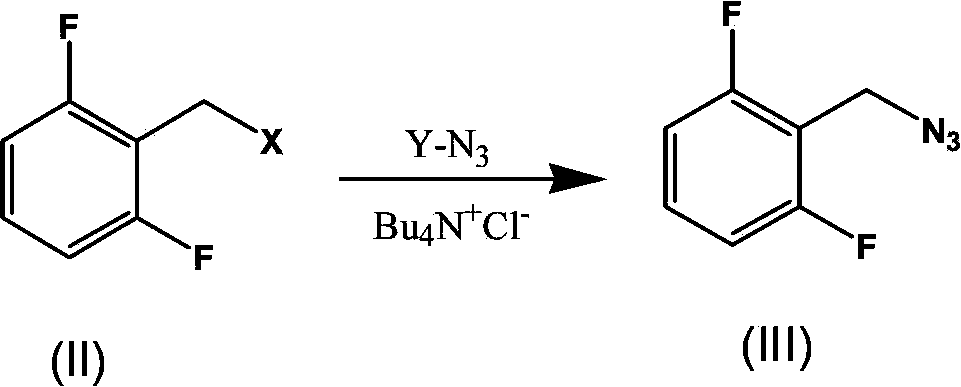
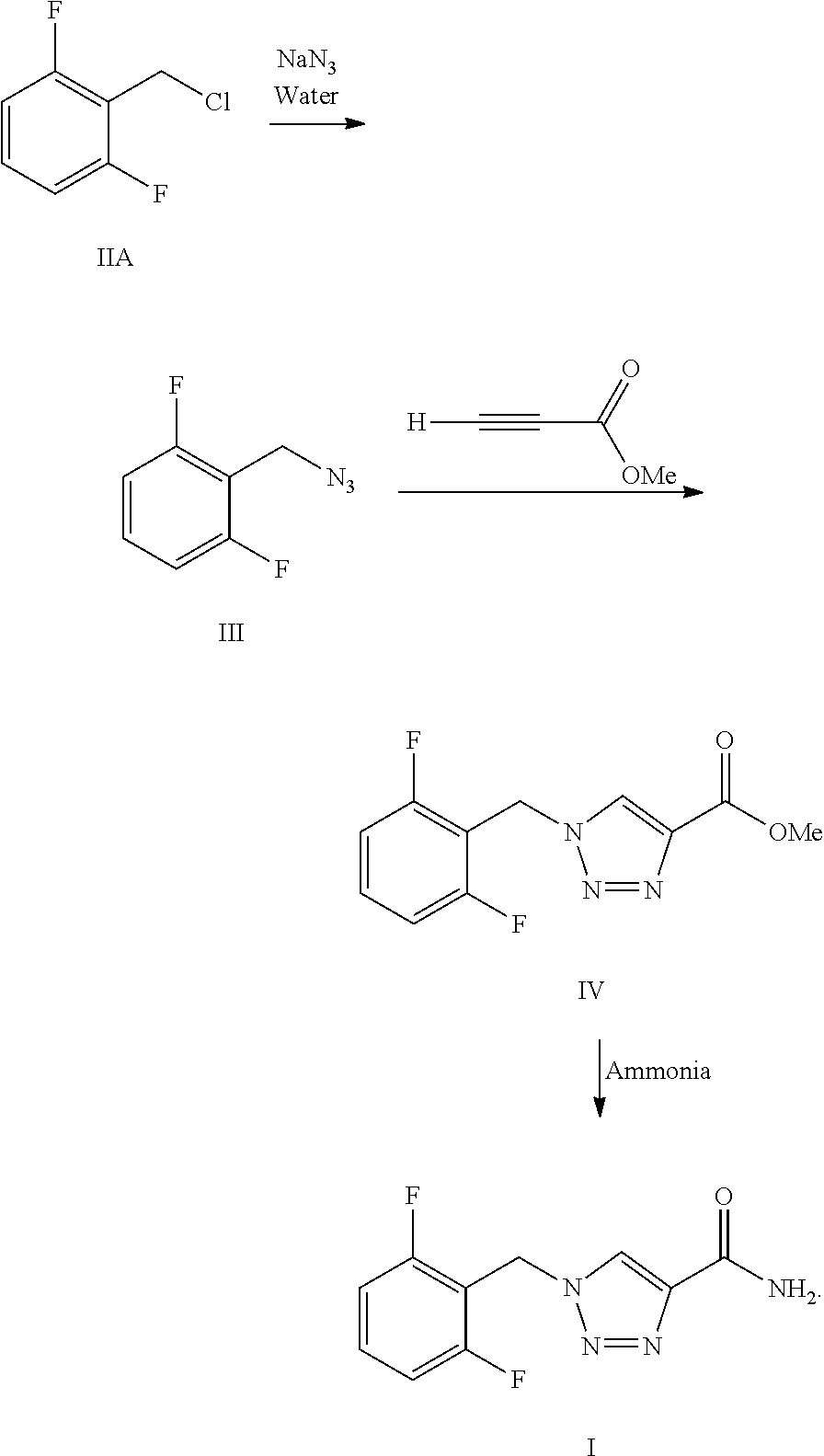
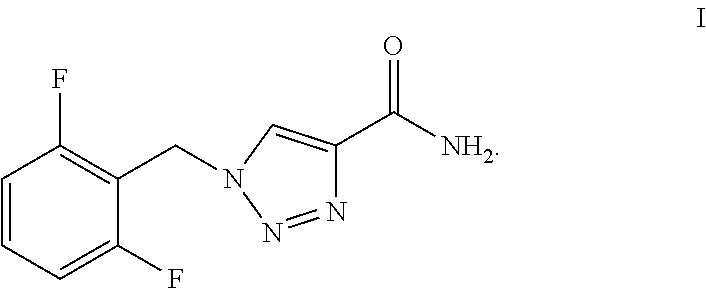
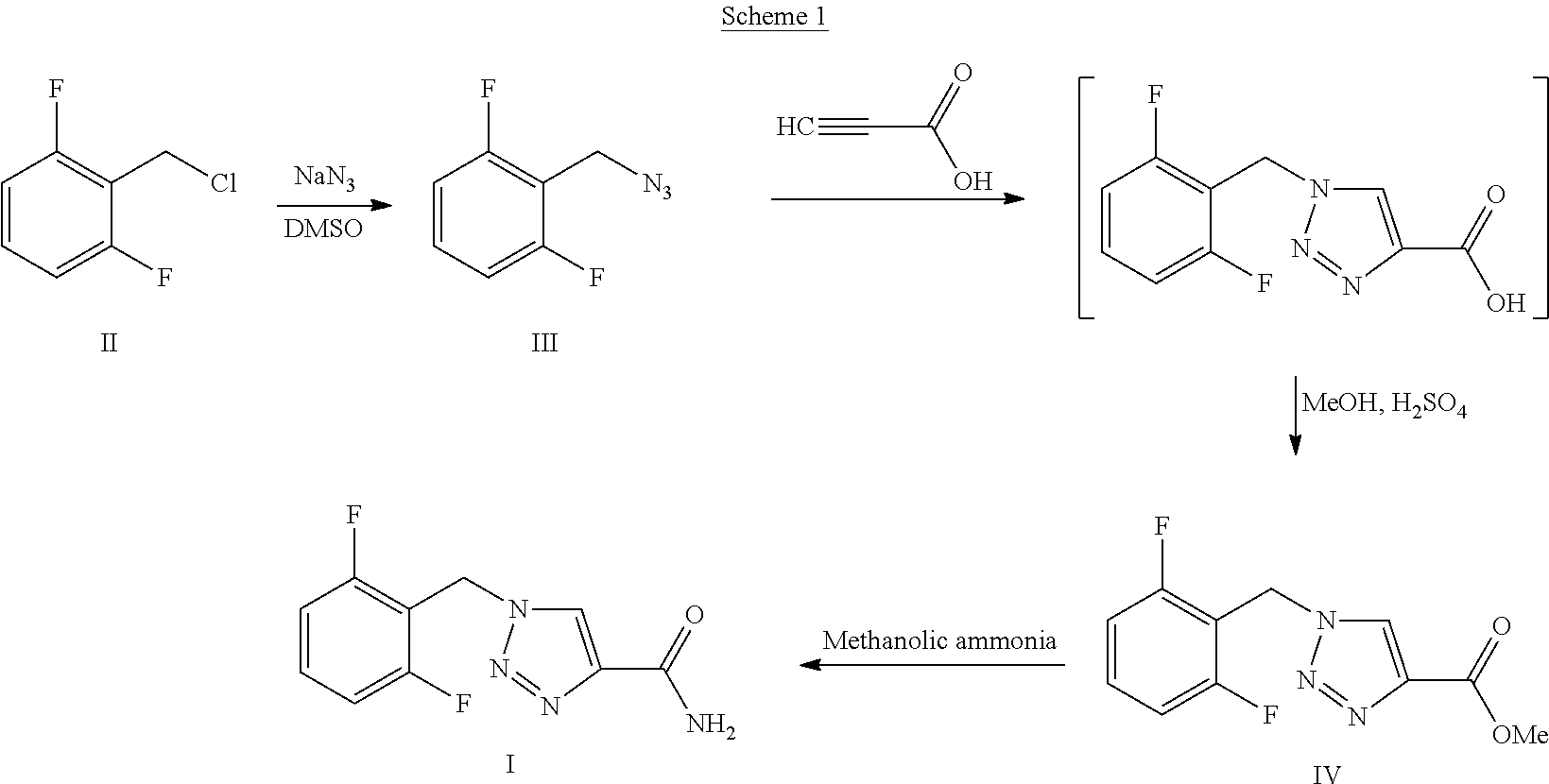
Synthesis process of rufinamide
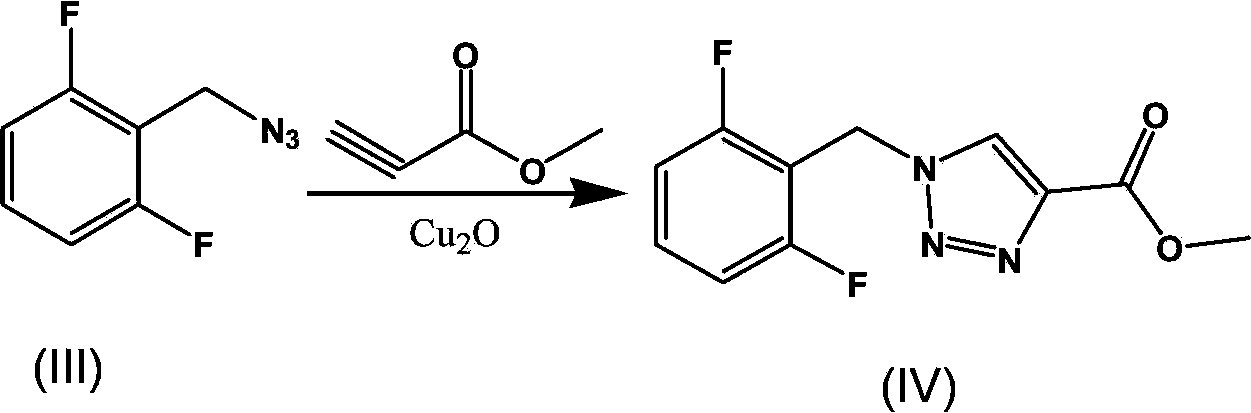
The invention provides an improved synthesis process for synthesizing rufinamide. The process comprises the following steps of a, reacting 2, 6-difluorobenzyl halide with azide and tetrabutylammonium chloride to obtain 2-(azide methyl)-1,3-difluorobenzene; b, reacting 2-(azide methyl)-1, 3-difluorobenzene with methyl propiolate under the catalyzing of nano-cuprous oxide (Cu2O) to obtain 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-methyl formate; and c, reacting 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-methyl formate with ammonium hydroxide to obtain rufinamide. The process has the advantages of short reaction time and high catalytic efficiency, and is simple and safe to operate.
Owner:XUHE TIANJIN YIYAO KEJI YOUXIAN GONGSI +1
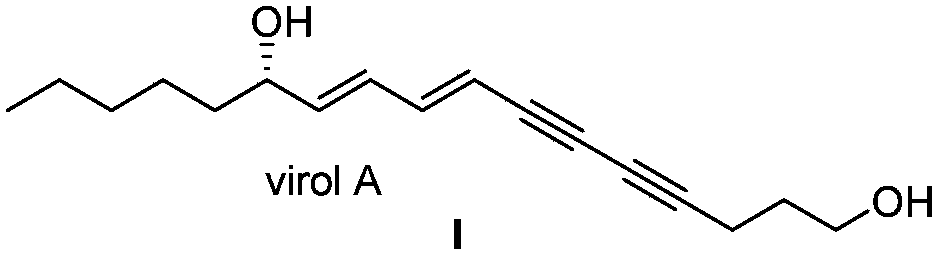
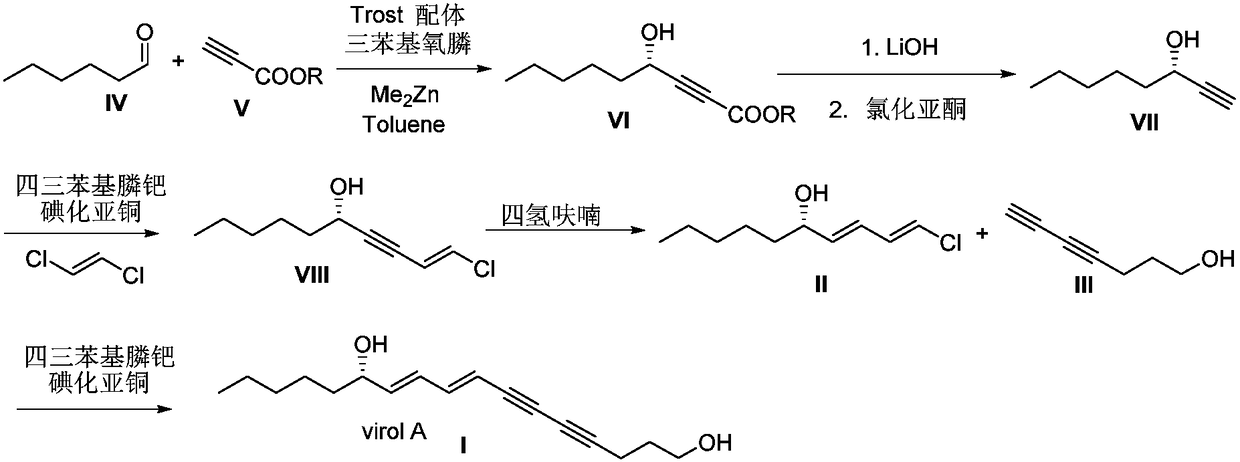
Synthetic method for (S)-Virol A from water hemlock extract
ActiveCN105348044AGreen preparationEfficient preparationOrganic compound preparationPreparation by hydrogenation1-HeptanolAlkyne
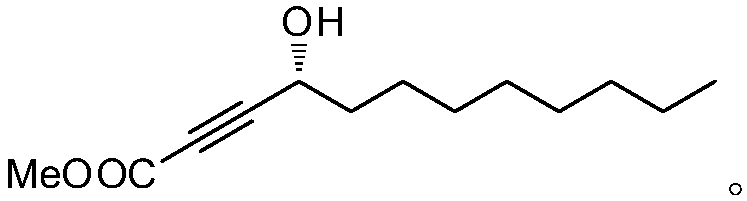
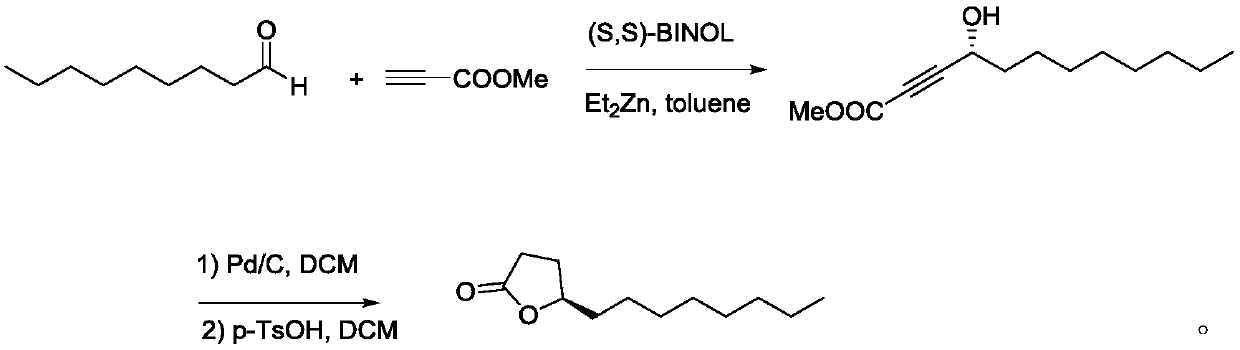
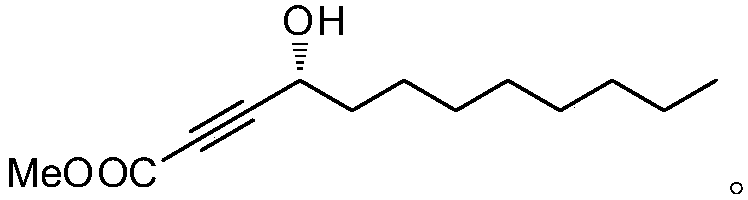
The invention relates to a synthetic method for (S)-Virol A from a polyacetylene water hemlock extract. The synthetic method for (S)-Virol A from the polyacetylene water hemlock extract is an asymmetric synthetic method, and comprises the following steps: firstly, under catalysis of a chiral ligand, methyl propiolate and dimethyl zinc are reacted to prepare an alkynyl zinc reagent, hexanal is subjected to asymmetric addition reaction to obtain a chiral intermediate (S)-4- hydroxy-2-alkyne methyl nonanoate, through ester group removal to obtain acetylene, the processed intermediate is coupled with vinylidene chloride, then selective reduction is carried out to obtain a product, and finally the product is coupled with 4,6-diyne-1-heptanol to obtain the (S)-Virol A. The flow of the method is simple and easy to realize, and the method is environmental-friendly.
Owner:HENAN AGRICULTURAL UNIVERSITY
Synthesis method of grain storage injurious insect trogoderma pheromone
ActiveCN108117531AHigh purityHigh yieldOrganic chemistryInsect catchers and killersAlcoholSynthesis methods
The invention discloses a synthesis method of grain storage injurious insect trogoderma pheromone. The structure of the grain storage injurious insect trogoderma pheromone is shown in the description;the synthesis method of the grain storage injurious insect trogoderma pheromone takes methyl propiolate and propionaldehyde as starting raw materials, and the raw materials react according to a reasonable ratio to synthesize an important intermediate compound chiral alcohol ester; then catalytic hydrogenation reaction is carried out to synthesize the grain storage injurious insect trogoderma pheromone. The synthesis method provided by the invention has the advantages of simple synthesis route, simple and easy-to-obtain raw materials, low synthesis cost, moderate reaction conditions and simplicity in separation, purification and operation; a target compound with high purity can be directly obtained, and the yield and the optical purity are high; meanwhile, a toxic solvent is not used so that an operation process is safer; a prepared product can be prepared into a lure for monitoring, preventing and controlling storage injurious insects and has commercial application value.
Owner:天驰药业有限公司
Flow battery positive electrode electrolyte based on tetrathiafulvalene dicarboxylic acid ethyl ester and preparation method of flow battery positive electrode electrolyte
ActiveCN112271314AEasy to synthesizeEase of mass productionRegenerative fuel cellsElectrolytic agentMethyl carbonate
The invention relates to a flow battery positive electrode electrolyte based on tetrathiafulvalene dicarboxylic acid ethyl ester and a preparation method of the flow battery positive electrode electrolyte. Firstly, carbon disulfide and methyl propiolate are used as raw materials, tetrathiafulvalene dicarboxylic acid methyl ester is synthesized under the catalysis of tributylphosphine, and then tetrathiafulvalene dicarboxylic acid ethyl ester is obtained through ester exchange. The tetrathiafulvalene dicarboxylic acid ethyl ester is dissolved into an ethylene carbonate and dimethyl carbonate mixed solution containing 1mol / L of LiPF6, so as to obtain the flow battery positive electrode electrolyte based on the tetrathiafulvalene dicarboxylic acid ethyl ester. The obtained positive electrodeelectrolyte has the advantages of high oxidation-reduction potential, high volume specific capacity, good cycling stability and the like.
Owner:FUZHOU UNIV
Process for the Preparation of Rufinamide
InactiveUS20110207938A1Economical and simpleDelayed reaction timeBiocideNervous disorderIodideCarboxylic acid
The present invention relates to a process for the preparation of rufinamide of formula I, which process comprises: (i) reacting a 2,6-difluorobenzylhalide of formula II, wherein X is chloride, bromide or iodide, with an azide to obtain 2-(azidomethyl)-1,3-difluorobenzene of formula III; (ii) reacting 2-(azidomethyl)-1,3-difluorobenzene of formula III with methyl propiolate to obtain methyl 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carboxylic acid of formula IV; and (iii) reacting methyl 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carboxylic acid of formula IV with ammonia to obtain rufinamide of formula I.
Owner:CIPLA LTD
Alkynyl-containing conjugated polymers and synthetic method thereof
The invention discloses alkynyl-containing conjugated polymers and a synthetic method thereof, and belongs to the field of macromolecular compound synthesis. The prior triple bond-containing conjugated polymers and synthesis have the problems of low conversion rate of a monomer, poor dissolution of products and the like. The polymers are shown as a general formula (I). The monomer and an initiator are added into a reactor in a molar ratio of 2-100:1, and reacts at a temperature of between 20 DEG C below zero and 70 DEG C for 1 to 24 hours; and the products are dissolved by hydrochloric acid, separated, and dried in vacuum to obtain the alkynyl-containing conjugated polymers, wherein the monomer is methyl acetylenecarboxylate, ethyl acetylenecarboxylate or butyl acetylenecarboxylate; and the initiator is solid sodium hydride, potassium hydride, sodium methoxide, sodium alcoholate or organolithium. The alkynyl-containing conjugated polymers and the method have the advantages of wide reaction temperature range, high conversion rate of the monomer and the like. In the formula, R is -CH3, -CH2CH3, or -CH2CH2CH2CH3, and n is between 10 and 500.
Owner:BEIJING UNIV OF CHEM TECH
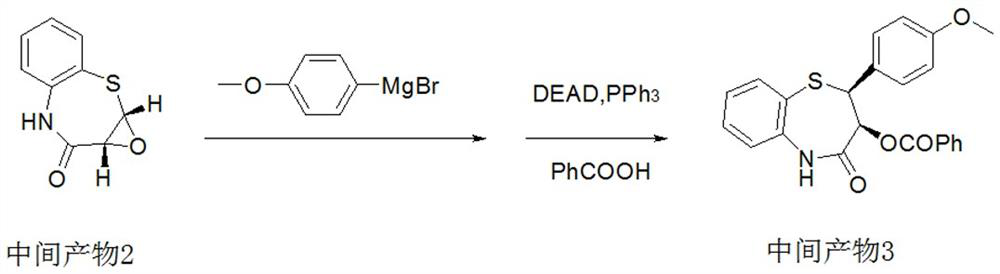
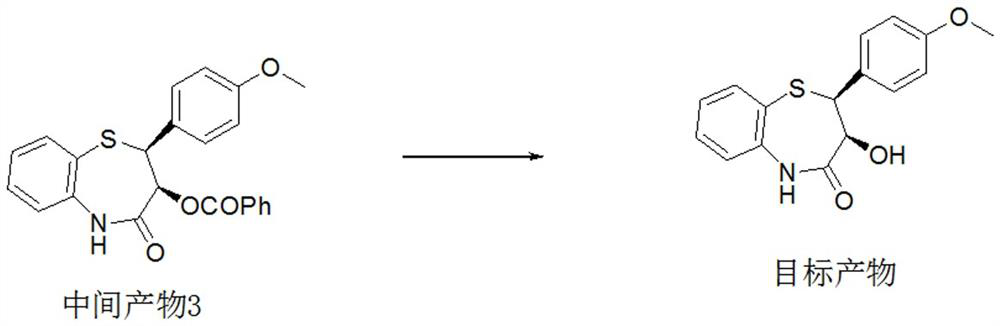
Preparation method of 2-(4-methoxyphenyl)-3-hydroxy-2,3-dihydro-1,5-benzothiazepine
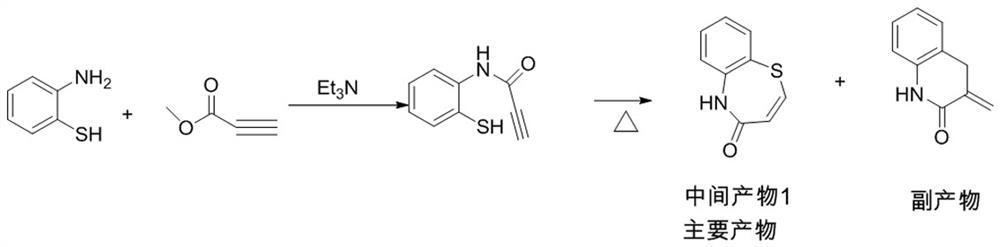
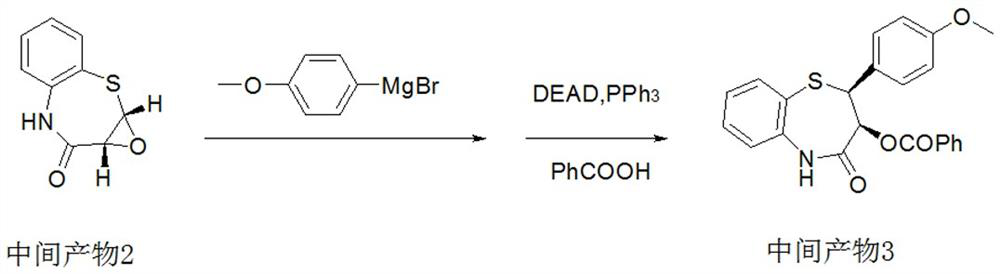
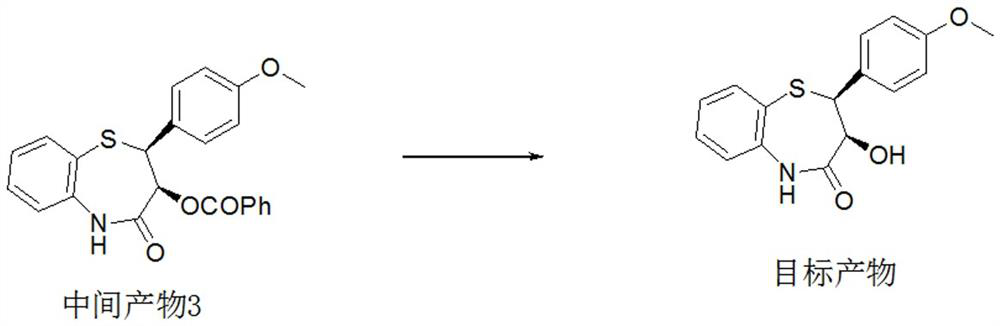
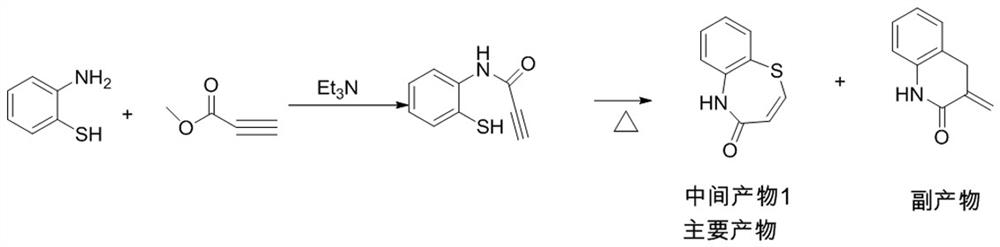
ActiveCN112279820BImprove efficiencyHigh yieldOrganic chemistryChemical recyclingOrtho positionHydrolysis
The invention provides a preparation method of 2-(4-methoxyphenyl)-3-hydroxyl-2,3-dihydro-1,5-benzothiazepine. The preparation method uses o-aminothiophenol and methyl propiolate as raw materials to synthesize an intermediate product 1, and then obtains an intermediate product 2 through selective epoxidation, and reacts the intermediate product 2 and 4-methoxyphenyl anion, and then Mitsunobu reaction afforded intermediate 3, which was then hydrolyzed to yield the product. During the ring formation process of the intermediate product 1, the tension of the Z-structure olefin is small, so the yield is high, and the subsequent epoxidation is an asymmetric epoxidation reaction, and the intermediate product 2 is obtained with high optical purity and high yield. The oxyphenyl anion is SN 2 Reaction, and then the ortho position undergoes configuration inversion to obtain the product. The whole process does not need to be split, and the total yield of the route is high and economical.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Process for synthesizing ethyl propiolate
InactiveCN101318900BNo pollution in the processRaw materials are easy to getOrganic compound preparationCarboxylic acid esters preparationReaction temperatureEthyl propiolate
The invention relates to a method for synthesizing ethyl propiolate, comprising the following steps of: using propargyl alcohol and ethanol as reaction raw materials; using dichloromethane as a solvent with a reaction temperature between 0 and 40 DEG C; and carrying out a one-step synthesis of the ethyl propiolate by using the reaction raw materials under actions of calcium hypochlorite and an acetic acid. The method has the advantages of easy acquisition of raw materials, convenient method, mild reaction conditions and no pollution to environment, and is capable of performing the reaction under a room temperature.
Owner:SUZHOU YUANFANG CHEM
Preparation method for zinc gel of alkaline zinc-manganese dioxide battery
InactiveCN103695714AImprove discharge performanceExtended service lifePrimary cell electrodesMethacrylatePowder mixture
The invention discloses a preparation method for zinc gel of an alkaline zinc-manganese dioxide battery. The preparation method comprises the following steps: (1) putting aluminum, bismuth and rare earth metals into a pressure fuse, heating to be molten, pressing galvanizing zinc to produce a zinc alloy, and crushing to produce zinc alloy particles; (2) mixing the zinc alloy particles with polyacrylic acid, or methacrylic acid, polyacrylic ester or methyl propiolate, polyacrylate or methyacrylate, and indium oxide powder, and grinding into a zinc powder mixture with particle size of 50-100 micrometers; (3) putting the zinc powder mixture and electrolyte prepared in advance into a hybrid reactor according to the volume ratio of 1:1, heating to 50-70 DEG C, agitating and mixing for 30-60 minutes at the agitation speed of 1000 revolutions / minute-2000 revolutions / minute, thus preparing zinc gel. According to the zinc gel of the alkaline zinc-manganese dioxide battery, prepared by adopting the preparation method, the discharge capability is greatly improved, the service life is effectively prolonged, and the zinc gel of the alkaline zinc-manganese dioxide battery is lead-free and mercury-free, thus being more environment-friendly.
Owner:高建军
Method for preparing royal jelly acid
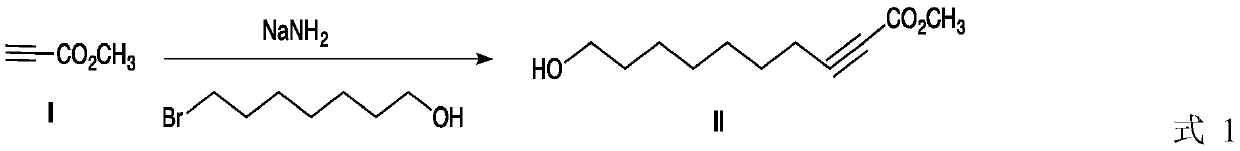
ActiveCN109942397AHigh purityHigh yieldPreparation from carboxylic acid saltsChemical synthesisBromine
The invention discloses a method for preparing royal jelly acid, and belongs to the field of chemical synthesis. The method comprises the steps that methyl propiolate and 7-bromine heptanol are adopted as initial raw materials, and in a solvent, 10-hydroxyl-2-methyl decylenate is obtained under the action of strong base sodamide; in a liquid ammonia solvent, metal sodium is utilized for carrying out trans-reduction, and (E)-10-hydroxyl-2-methyl decylenate is obtained; hydrolysis is carried out under the action of alkali, then acidizing is carried out, and the target product jelly acid is obtained. According to the method for preparing the jelly acid, the technical route has the advantages of being short, easy and convenient to operate and easy for industrial production, and the method forsynthesizing the royal jelly acid is economical, simple and convenient.
Owner:JIAXING UNIV
A kind of liquid flow battery cathode electrolyte based on ethyl tetrathiofulvalene dicarboxylate and preparation method thereof
ActiveCN112271314BEasy to synthesizeEase of mass productionRegenerative fuel cellsElectrolytic agentMethyl carbonate
The invention relates to a flow battery cathode electrolyte based on ethyl tetrathiofulvalene dicarboxylate and a preparation method thereof. The present invention first uses carbon disulfide and methyl propiolate as raw materials, synthesizes methyl tetrathiofulvalene dicarboxylate under the catalysis of tributylphosphine, and then obtains ethyl tetrathiofulvalene dicarboxylate through transesterification . Dissolve ethyl tetrathiofulvalene dicarboxylate to contain 1mol / L LiPF 6 In the mixed solution of ethylene carbonate and dimethyl carbonate, the anode electrolyte of flow battery based on ethyl tetrathiofulvalene dicarboxylate is obtained. The obtained cathode electrolyte has the advantages of high redox potential, high volume specific capacity, good cycle stability and the like.
Owner:FUZHOU UNIV
Method for synthesizing pesticide Silthiopham
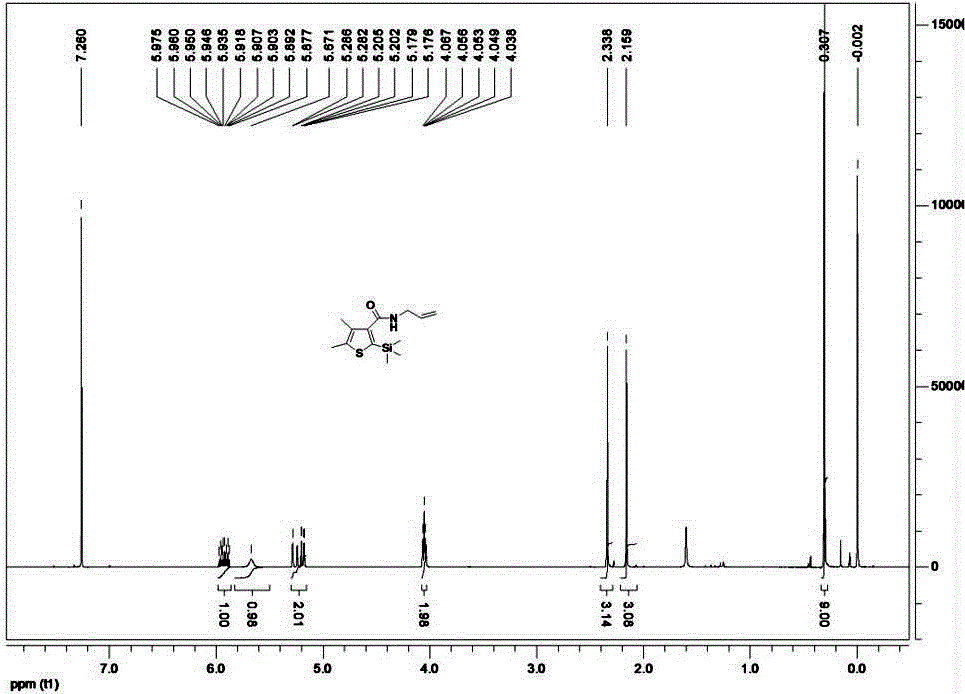
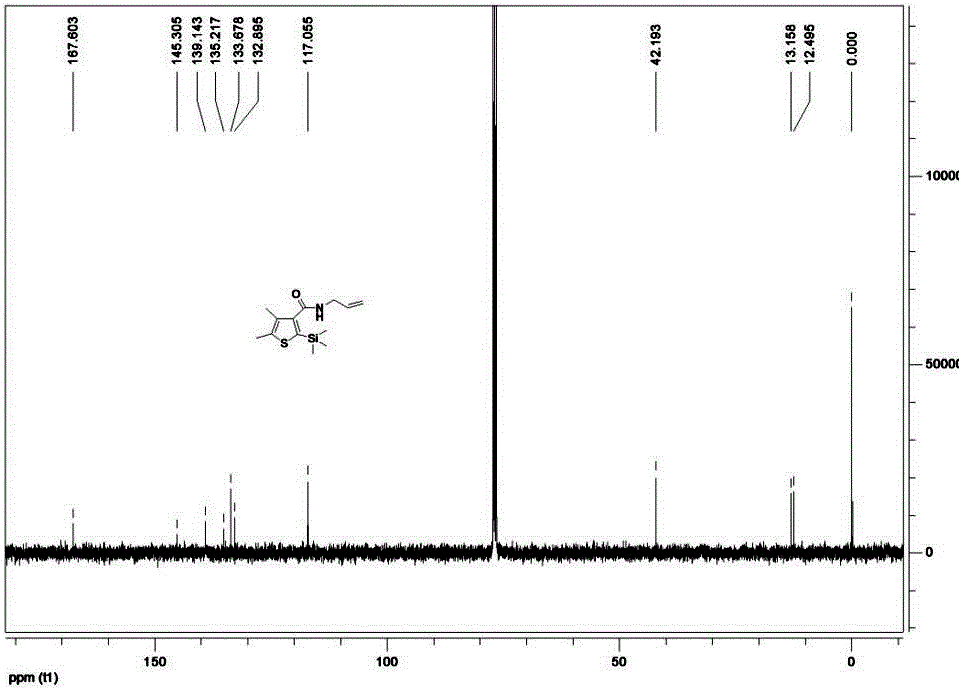
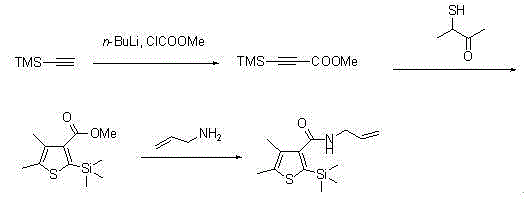
ActiveCN105085564AAvoid pollutionRaw materials are cheap and easy to getGroup 4/14 element organic compoundsOrganic baseOrganic synthesis
The invention belongs to the technical field of organic synthesis and provides a method for synthesizing a pesticide Silthiopham. The method is used for solving the problem of the pesticide Silthiopham synthesis process at present that the yield of exchange reaction is low. The method comprises the steps of (1) enabling trimethylsilyl acetylene, which serves as a raw material, to react with methyl chloroformate in the presence of an organic base under the protection of inert gas so as to obtain trimethylsilyl methyl propiolate; (2) enabling trimethylsilyl methyl propiolate to react with 3-mercapto-2-butanone in the presence of a basic catalyst so as to obtain 4,5-dimethyl-2-(trimethylsilyl)thiophen-3-methyl formate; and (3) enabling 4,5-dimethyl-2-(trimethylsilyl)thiophen-3-methyl formate to react with allyl amine in the presence of a catalyst, thereby obtaining the end product pesticide Silthiopham. According to the method, the used raw materials are cheap and readily-available, the route is simple, the overall yield is relatively high, and the consumption of reagents causing serious environmental pollution such as tert-butyl nitrous acid (t-BuONO) and thionyl chloride is avoided.
Owner:HANGZHOU NORMAL UNIVERSITY
Environment-friendly preparation method of propiolic acid derivatives
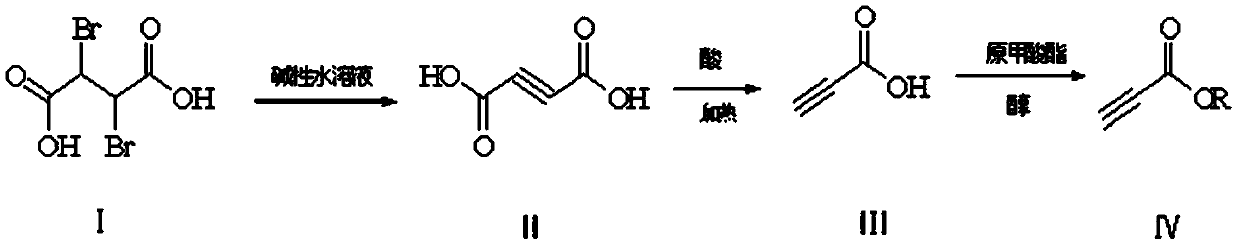
InactiveCN110698341AReduce pollutionImprove operational safetyOrganic compound preparationCarboxylic acid esters preparationButanedioic acidEngineering
The invention discloses an environment-friendly preparation method of propiolic acid derivatives. The preparation method comprises the following steps: (1) with 2,3-dibromosuccinic acid as a raw material, generating a butynedioic acid salt under alkaline conditions; (2) under an acidic condition, carrying out high-temperature decarboxylation to obtain propiolic acid; and (3) adding corresponding methanol or ethanol into propiolic acid in an extraction solvent, and preparing high-yield propiolate under acidic catalytic conditions under the condition that trimethyl orthoformate or triethyl orthoformate participates in dehydration. In the invention, the propiolic acid preparation method is friendly to environment and high in safety coefficient; and the method provided by the invention can beused for preparing methyl propiolate and ethyl propiolate, and has the advantages of small alcohol consumption, thorough reaction, high yield and easiness in separation.
Owner:安庆博曼生物技术有限公司
A lithium-ion battery negative electrode based on lithium tetrathiofulvalene dicarboxylate and preparation method thereof
ActiveCN112242517BEasy to makeEasy to recycleCell electrodesSecondary cellsCopper foilMethyl palmoxirate
The invention belongs to the technical field of lithium ion battery materials, and in particular relates to a lithium ion battery negative electrode based on lithium tetrathiofulvalene dicarboxylate and a preparation method thereof. The present invention first uses carbon disulfide and methyl propiolate as raw materials, synthesizes methyl tetrathiofulvalene dicarboxylate under the catalysis of tributylphosphine, and then obtains tetrathiofulvalene dicarboxylate through hydrolysis, acidification and lithiation. lithium carboxylate. The prepared lithium tetrathiofulvalene dicarboxylate, conductive agent and binder are mixed and dispersed in N-methylpyrrolidone, then coated on copper foil, dried and sliced to obtain tetrathiofulvalene dicarboxylate Lithium carboxylate negative electrode. The obtained negative electrode has the advantages of high discharge specific capacity, good cycle stability and the like.
Owner:FUZHOU UNIV
Process for synthesizing ethyl propiolate
InactiveCN101318900ANo pollution in the processRaw materials are easy to getOrganic compound preparationCarboxylic acid esters preparationReaction temperatureEthyl propiolate
The invention relates to a method for synthesizing ethyl propiolate, comprising the following steps of: using propargyl alcohol and ethanol as reaction raw materials; using dichloromethane as a solvent with a reaction temperature between 0 and 40 DEG C; and carrying out a one-step synthesis of the ethyl propiolate by using the reaction raw materials under actions of calcium hypochlorite and an acetic acid. The method has the advantages of easy acquisition of raw materials, convenient method, mild reaction conditions and no pollution to environment, and is capable of performing the reaction under a room temperature.
Owner:SUZHOU YUANFANG CHEM
A kind of medical anti-adhesive hydrogel dressing and preparation method thereof
ActiveCN105169465BImprove hydrophilic abilityPromote healingAbsorbent padsBandagesMicro structureMethacrylate
The present invention discloses a medical anti-adhesion hydrogel dressing, which comprises a hydrogel, wherein a methyl propiolate monomer, double or multi-functional group terminated-polyethylene glycol having unsaturated double bond, water and an initiator are further subjected to thermal initiation or photo initiation or radiation initiation polymerization so as to obtain the hydrogel, and the hydrogel has a micron porous structure. According to the present invention, the prepared hydrogel dressing has characteristics of no adhesion to wound, good water absorption and excellent mechanical property, and has a space micro-structure capable of loading drugs or growth factors so as to load various drugs or growth factors, achieve the functionalization treatment of the dressing, and achieve antibacterial, wound healing promoting and other functions.
Owner:朗肽生物制药股份有限公司
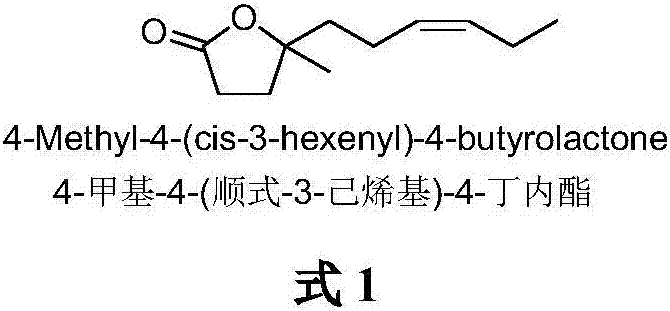
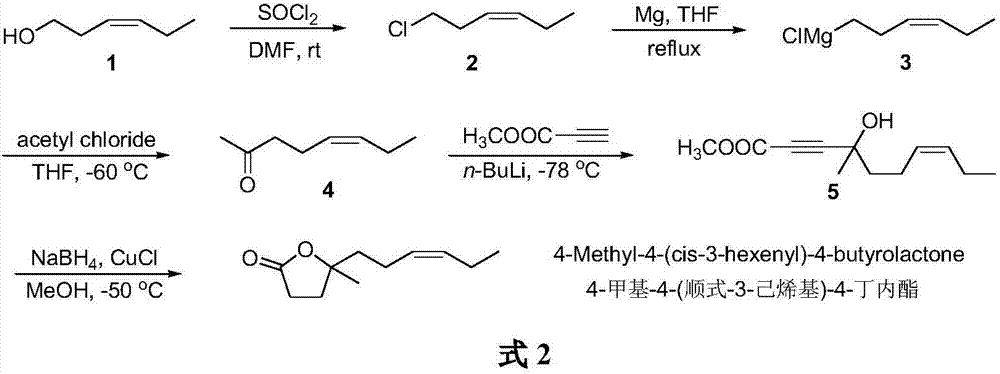
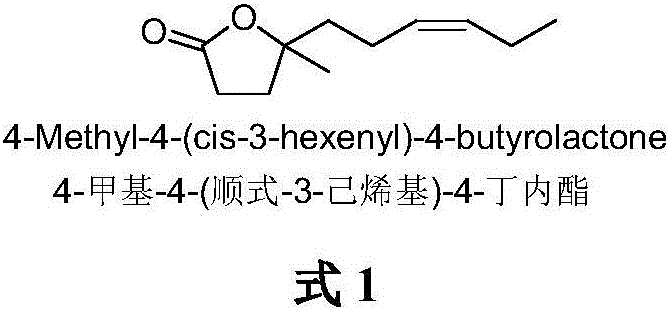
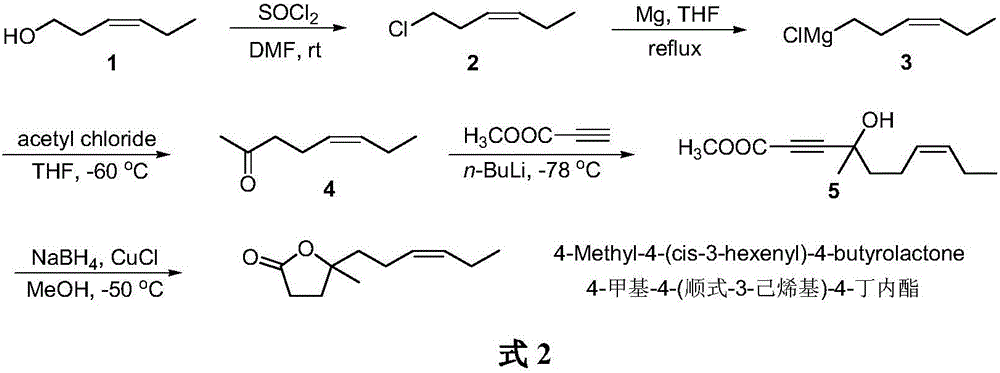
The synthetic method of 4-methyl-4-(cis-3-hexenyl)-4-butyrolactone
The invention belongs to the field of fine chemistry, and particularly relates to a synthetic method of 4-methyl-4-(cis-3-hexenyl)-4-butyrolactone. The method comprises the following steps: taking leaf alcohol as a starting material, enabling the leaf alcohol to react with thionyl chloride to generate cis-1-chloro-3-hexene, first reacting with metal magnesium to obtain a cis-3-hexenyl Grignard reagent, then reacting with acetyl chloride to generate cis-5-hexene-2-ketone, and then reacting with methyl propiolate in the presence of n-butyllithium to obtain cis-4-methy-4-hydroxyl-7-decene-2-acetylenic acid methyl ester, and finally directly performing the reaction cyclization with NaBH4 under the catalysis of CuCl to obtain the 4-methyl-4-(cis-3-hexenyl)-4-butyrolactone. The method is environment-friendly, low in cost and suitable for the industrialization production.
Owner:新乡市博源生物科技有限公司
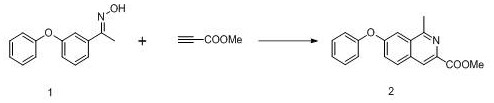
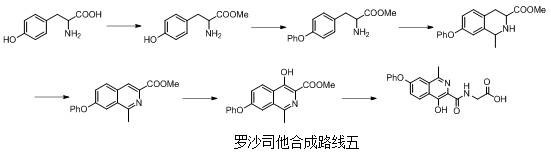
Preparation method of roxadustat key intermediate
The invention relates to a preparation method of a roxadustat key intermediate. The method is characterized in that an m-phenoxyacetophenone derivative and methyl propiolate are used as starting materials and are subjected to cyclization reaction under the catalysis of a catalyst, so that a roxadustat intermediate, namely, 1-methyl-7-phenoxyisoquinoline-3-methyl formate is synthesized. The synthesis route simplifies the introduction mode of isoquinoline C-1 methylation, and has the characteristics of simple process, convenience in operation, no need of column chromatography purification, high yield and the like.
Owner:TIANJIN LISHENG PHARM CO LTD
A method for synthesizing the pheromone of the grain storage pest trochaderma
ActiveCN108117531BHigh purityHigh yieldOrganic chemistryInsect catchers and killersSynthesis methodsSolvent
The invention discloses a synthesis method of grain storage injurious insect trogoderma pheromone. The structure of the grain storage injurious insect trogoderma pheromone is shown in the description;the synthesis method of the grain storage injurious insect trogoderma pheromone takes methyl propiolate and propionaldehyde as starting raw materials, and the raw materials react according to a reasonable ratio to synthesize an important intermediate compound chiral alcohol ester; then catalytic hydrogenation reaction is carried out to synthesize the grain storage injurious insect trogoderma pheromone. The synthesis method provided by the invention has the advantages of simple synthesis route, simple and easy-to-obtain raw materials, low synthesis cost, moderate reaction conditions and simplicity in separation, purification and operation; a target compound with high purity can be directly obtained, and the yield and the optical purity are high; meanwhile, a toxic solvent is not used so that an operation process is safer; a prepared product can be prepared into a lure for monitoring, preventing and controlling storage injurious insects and has commercial application value.
Owner:天驰药业有限公司
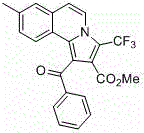
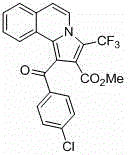
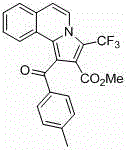
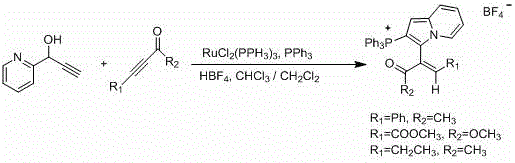
Trifluoromethyl pyrrolo isoquinoline derivative and synthesis method thereof
InactiveCN105884771AChange physiological activitySimple and fast operationOrganic chemistryArylIsoquinoline
The invention relates to a trifluoromethyl pyrrolo isoquinoline derivative and a synthesis method thereof. The structure of the compound is as shown in the description, wherein R1 is H or methyl or methoxyl or nitryl, and R2 is aryl or heterocyclic aryl. According to the method, polysubstituted isoquinoline, aromatic terminal alkyne and trifluoromethyl methyl propiolate prepared with a mature synthesis technique are adopted as raw materials, and the polysubstituted pyrrolo isoquinoline derivative containing trifluoromethyl is synthesized with a three-component step-by-step one-pot method. In the reactive oxidation process, a traditional oxidizing agent is omitted in the method, and oxygen in the air is adopted as an oxidizing agent, which embodies the concept of environment-friendly and sustainable chemistry. Regioselectivity is high, and the yield is high. Thus, the method is a new effective method for synthesizing polysubstituted pyrrolo isoquinoline derivative containing trifluoromethyl.
Owner:SHANGHAI UNIV
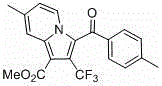
Perfluoroalkyl indolizine derivative and synthesis method thereof
InactiveCN106045994ANovel structureHigh regional selectivityOrganic chemistrySynthesis methodsStructural formula
The invention relates to a perfluoroalkyl indolizine derivative and a synthesis method thereof. A structural formula of the perfluoroalkyl indolizine derivative is as shown in the specification, wherein R1 refers to H, CH3 and CO2Me; R2 refers to hydrogen, chlorine, methoxyl, nitryl or methyl; RF refers to C1-C3 perfluoroalkyl. Easily acquirable pyridine derivatives, w-bromoacetophenone derivatives and perfluoroalkyl methyl propiolate mature in synthesis technique are adopted as raw materials for one-pot synthesis of the perfluoroalkyl indolizine derivative step by step. The perfluoroalkyl methyl propiolate used as the raw materials is subjected to Michael addition under certain conditions to obtain the perfluoroalkyl indolizine derivative, operations are greatly simplified, and high atom economy and environment friendliness are realized; due to adoption of acetonitrile as a solvent, low environment pollution is realized; high regioselectivity and high yield are achieved. Therefore, the synthesis method is a novel effective method for synthesis of the perfluoroalkyl indolizine derivative.
Owner:SHANGHAI UNIV
Synthesis method of paederus fuscipes sex pheromone
ActiveCN108623542AHigh optical activityWide variety of sourcesOrganic chemistryPest attractantsChemical synthesisAlcohol
The invention belongs to the technical field of chemical synthesis and in particular relates to a synthesis method of paederus fuscipes sex pheromone. An R configuration optical isomer of the paederusfuscipes sex pheromone has the following structure: the formula is shown in the description; the synthesis method of the R configuration optical isomer of the paederus fuscipes sex pheromone comprises the following steps: enabling nonanal and methyl propiolate to react, so as to obtain chiral alcohol ester; enabling the chiral alcohol ester to react, so as to obtain the paederus fuscipes sex pheromone. The paederus fuscipes sex pheromone prepared by the synthesis method has high optical activity; a synthesis route is simple and the cost is low; a prepared paederus fuscipes sex pheromone lureproduct can have a protection effect on field workers and resident in regions at which paederus fuscipes usually occurs, and has good commercial application value.
Owner:濮阳天健生物科技有限公司
Preparation method of 2-(4-methoxyphenyl)-3-hydroxy-2, 3-dihydro-1, 5-benzothiazepine ketone
The invention provides a preparation method of 2-(4-methoxyphenyl)-3-hydroxy-2, 3-dihydro-1, 5-benzothiazepine ketone. The preparation method comprises the following steps: taking o-aminothiophenol and methyl propiolate as raw materials to synthesize an intermediate product 1, carrying out selective epoxidation to obtain an intermediate product 2, reacting the intermediate product 2 with 4-methoxyphenyl anions, carrying out Mitsunobu reaction to obtain an intermediate product 3, and hydrolyzing to obtain the product. In the cyclization process of the intermediate product 1, the tension of Z-structure olefin is small so that the yield is high, subsequent epoxidation is an asymmetric epoxidation reaction, the obtained intermediate product 2 is high in optical purity and high in yield, the intermediate product 2 and 4-methoxyphenyl anions are subjected to SN2 reaction, configuration inversion is carried out in an ortho-position, and the product is obtained. The whole reaction process doesnot need to be split, the total yield of the route is high, and the method is economical.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
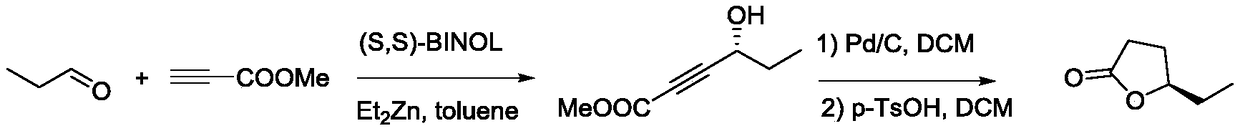
A kind of synthetic method of hemlock extract (s)-virol A
ActiveCN105348044BGreen preparationEfficient preparationOrganic compound preparationPreparation by hydrogenation1-HeptanolAlkyne
The invention relates to a method for synthesizing polyacetylenic hemlock extract (S)-Virol A. The asymmetric synthesis method of hemlock extract (S)-Virol A provided by the present invention, its preparation method is as follows: first under the catalysis of chiral ligand, propiolate methyl ester and dimethyl zinc reaction make alkynyl Zinc reagent, asymmetric addition reaction to hexanal to obtain chiral intermediate (S)-4-hydroxy-2-yne nonanoic acid methyl ester, after deesterification to alkyne, coupling with dichloroethylene, selective reduction , and finally coupled with 4,6-diyne-1-heptanol to obtain (S)-Virol A. The route of the invention is simple and easy to implement, and is friendly to the environment.
Owner:HENAN AGRICULTURAL UNIVERSITY
Synthetic method of 4-methyl-4-(cis-3-hexenyl)-4-butyrolactone
The invention belongs to the field of fine chemistry, and particularly relates to a synthetic method of 4-methyl-4-(cis-3-hexenyl)-4-butyrolactone. The method comprises the following steps: taking leaf alcohol as a starting material, enabling the leaf alcohol to react with thionyl chloride to generate cis-1-chloro-3-hexene, first reacting with metal magnesium to obtain a cis-3-hexenyl Grignard reagent, then reacting with acetyl chloride to generate cis-5-hexene-2-ketone, and then reacting with methyl propiolate in the presence of n-butyllithium to obtain cis-4-methy-4-hydroxyl-7-decene-2-acetylenic acid methyl ester, and finally directly performing the reaction cyclization with NaBH4 under the catalysis of CuCl to obtain the 4-methyl-4-(cis-3-hexenyl)-4-butyrolactone. The method is environment-friendly, low in cost and suitable for the industrialization production.
Owner:新乡市博源生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com