Trifluoromethyl pyrrolo isoquinoline derivative and synthesis method thereof
A kind of technology of trifluoromethylpyrrole and isoquinoline, applied in the direction of organic chemistry etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
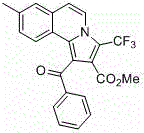
[0024] Add isoquinoline (65~84.5 mg, 0.5~0.65 mmol), phenylacetylene (51 mg, 0.5 mmol), methyl trifluoromethylpropiolate (114 mg, 0.75 mmol) into a round bottom flask, bromide Cuprous (7.2 mg, 10 mol%), toluene (5 mL) as solvent, nitrogen protection, reaction at room temperature for 16 to 24 hours; then add copper bromide (11.2 mg, 10 mol%) as a catalyst, base pyridine ( 40 mg, 0.5 mmol), heated to 100~120°C and reacted for 18~24 hours, cooled to room temperature, diluted with saturated brine, extracted three times with ethyl acetate, and the combined organic layer was washed with anhydrous Na 2 SO 4 After drying, the pure product was separated by column chromatography. Pale yellow solid.
[0025] Structural formula:
[0026]
[0027] Chinese name:
[0028] 1-benzoyl-3-(trifluoromethyl)pyrrolo[2,1- a ]Methyl isoquinoline-2-carboxylate
[0029] English name:
[0030] Methyl 1-benzoyl-3-(trifluoromethyl)pyrrolo[2,1- a ]isoquinoline-2-carboxylate
[0031] Molecular we...
Embodiment 2
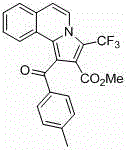
[0037] Add isoquinoline (65~84.5 mg, 0.5~0.65 mmol), p-methylphenylacetylene (58 mg, 0.5 mmol), methyl trifluoromethylpropiolate (114 mg, 0.75 mmol) into a round bottom flask , cuprous bromide (7.2 mg, 10 mol%), toluene (5 mL) as a solvent, nitrogen protection, reaction at room temperature for 16 to 24 hours; then add copper bromide (11.2 mg, 10 mol%) as a catalyst, Base pyridine (40 mg, 0.5 mmol), heated to 100~120°C and reacted for 18~24 hours, cooled to room temperature, diluted with saturated saline, extracted three times with ethyl acetate, and the combined organic layer was washed with anhydrous Na 2 SO 4 After drying, the pure product was separated by column chromatography. Pale yellow solid.
[0038] Structural formula:
[0039]
[0040] Chinese name:
[0041] 1-(4-methylbenzoyl)-3-(trifluoromethyl)pyrrolo[2,1- a ]Methyl isoquinoline-2-carboxylate
[0042] English name:
[0043] Methyl 1-(4-methylbenzoyl)-3-(trifluoromethyl)pyrrolo[2,1- a ]isoquinoline-2-car...
Embodiment 3
[0050] Add isoquinoline (65~84.5 mg, 0.5~0.65 mmol), m-methylphenylacetylene (58 mg, 0.5 mmol), methyl trifluoromethylpropiolate (114 mg, 0.75 mmol) into a round bottom flask , cuprous bromide (7.2 mg, 10 mol%), toluene (5 mL) as a solvent, nitrogen protection, reaction at room temperature for 16 to 24 hours; then add copper bromide (11.2 mg, 10 mol%) as a catalyst, Base pyridine (40 mg, 0.5 mmol), heated to 100~120°C and reacted for 18~24 hours, cooled to room temperature, diluted with saturated saline, extracted three times with ethyl acetate, and the combined organic layer was washed with anhydrous Na 2 SO 4 After drying, the pure product was separated by column chromatography. Pale yellow solid.
[0051] Structural formula:
[0052]
[0053] Chinese name:
[0054] 1-(3-methylbenzoyl)-3-(trifluoromethyl)pyrrolo[2,1- a ]Methyl isoquinoline-2-carboxylate
[0055] English name:
[0056] Methyl 1-(3-methylbenzoyl)-3-(trifluoromethyl)pyrrolo[2,1- a ]isoquinoline-2-car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com