A quinuclidone compound, its application as a solid-state green light-emitting organic luminescent material and its preparation method
A technology of quinuclidinone and quinuclidinone hydrochloride is applied in the field of organic light-emitting materials to achieve the effects of improving absorption and transmission speed, improving processing performance and practicability, and reducing coplanarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
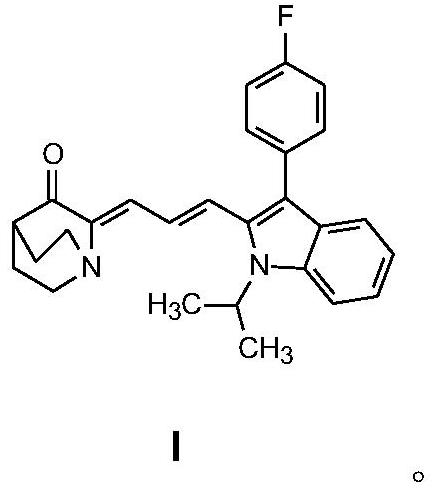
[0028] Example 1: 2-(3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)allylidene)-3-quinuclidinone (I) preparation of
[0029] In a 250 mL round bottom flask, mix 3-quinuclidone hydrochloride (1 mmol) and 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)propene Aldehyde (1 mmol) was dissolved in 100 mL of ethanol, 15 mL of potassium hydroxide solution with a mass fraction of 15% was added dropwise to the solution under rapid stirring, and the reaction was stirred at room temperature for 10 hours. Afterwards, the reaction solution was poured into 150 mL of water and left to stand, and the resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized from ethanol and dried in vacuo to obtain a yellow solid with a yield of 73%.
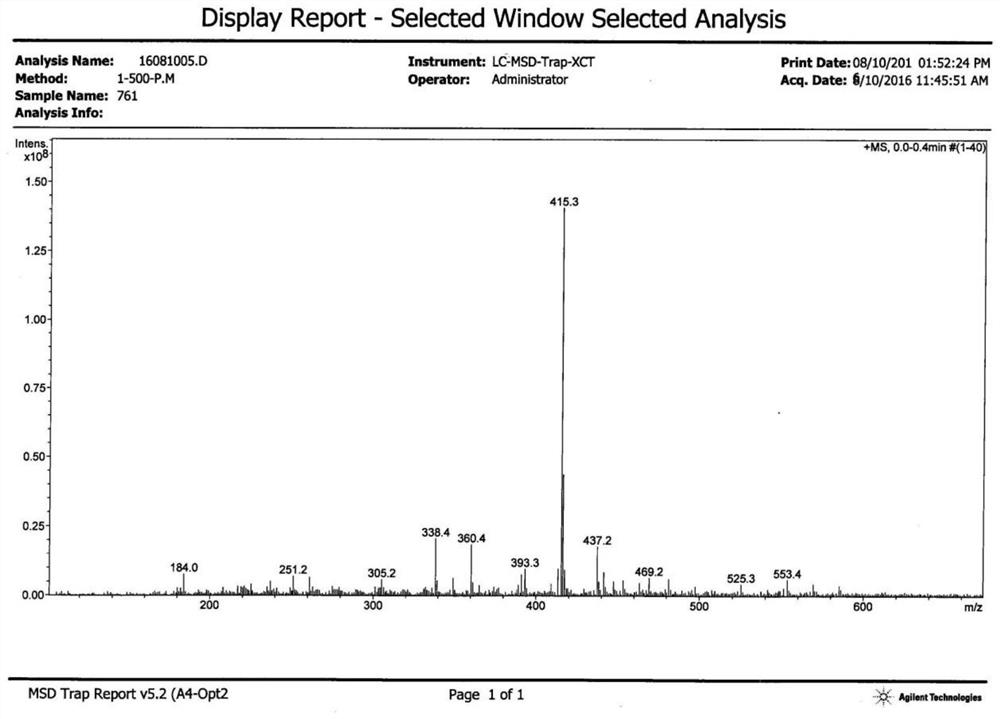
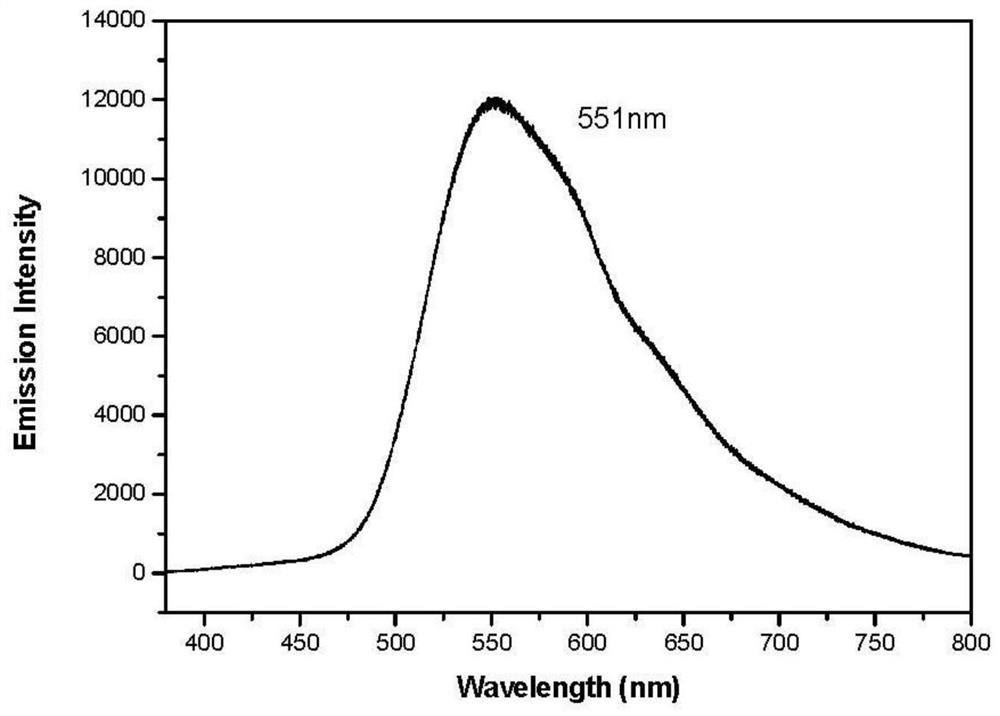
[0030] The obtained product was determined to be the target product by proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum, and mass spectrometry. T...
Embodiment 2
[0032] Example 2: 2-(3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)allylidene)-3-quinuclidinone (I) preparation of
[0033] In a 250 mL round bottom flask, mix 3-quinuclidone hydrochloride (1 mmol) and 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)propene Aldehyde (1.1 mmol) was dissolved in 100 mL of methanol, 20 mL of 25% sodium hydroxide solution was added dropwise to the solution under rapid stirring, and the reaction was stirred at room temperature for 9 hours. Afterwards, the reaction solution was poured into 150 mL of water and left to stand, and the resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized from ethanol and dried in vacuo to obtain a yellow solid with a yield of 77%.
Embodiment 3
[0034] Example 3: 2-(3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)allylidene)-3-quinuclidinone (I) preparation of
[0035]In a 250 mL round bottom flask, mix 3-quinuclidone hydrochloride (1 mmol) and 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)propene Aldehyde (1.1 mmol) was dissolved in 100 mL of methanol, 15 mL of 20% potassium hydroxide solution was added dropwise to the solution under rapid stirring, and the reaction was stirred at room temperature for 13 hours. Afterwards, the reaction solution was poured into 150 mL of water and left to stand, and the resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized from ethanol and dried in vacuo to obtain a yellow solid with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com