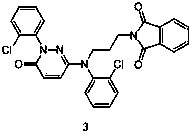
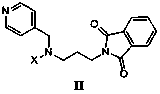
N, N-disubstituted-N'-phthaloyl group-1, 3-diamine derivative and preparation method thereof
A technology of phthaloyl and diamine derivatives, applied in organic chemistry, antiviral agents, etc., to achieve the effect of increasing diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 : N, N-dibenzyl-N'-phthaloyl-1, 3-diamine I-a preparation of
[0060]
[0061] Steps:
[0062] In a 250ml three-neck reaction flask, sodium (0.18g, 7.8mmol) was dissolved in absolute ethanol (100ml), cooled to 0°C with an ice bath, and the phthalimide 1 (11 g, 74.8 mmol) was added, followed by dropwise addition of acrolein (5.3 g, 93.6 mmol), and the reaction was stirred at room temperature for 2 hours after the drop was complete. The reaction solution was quenched with glacial acetic acid, extracted three times with ethyl acetate, and the organic phase was dried and concentrated to dryness to obtain a crude product. The crude product was purified by column chromatography to obtain 5.9 g of N-phthaloyl-3-aminopropanal 2 , yield 39%. HNMR (CDCl 3 ) d: 9.58 (s, 1H), 7.23-7.26 (m, 4H), 4.60-4.62 (m, 2H), 2.98-3.18 (m, 2H).
[0063] N-phthaloyl-3-aminopropanal 2 (2 g, 10 mmol) and triethylamine (2.1 ml) were dissolved in 1,2-dichloroethane (150 ml) so...
Embodiment 2
[0065] Example 2 : N-benzenesulfonyl-N-benzyl-N'-phthaloyl-1, 3-diamine I-b preparation of
[0066]
[0067] Steps:
[0068] N-benzyl-N'-phthaloyl-1,3-diamine 3 (2 g, 6.8 mmol) and triethylamine (2.1 ml) were dissolved in dichloromethane (150 ml) solution, cooled to 0 ° C with an ice bath, and then benzenesulfonyl chloride (952 mg, 6.8 mmol) was added dropwise , After the dropwise reaction was stirred at room temperature for 2 hours. The reaction solution was quenched with saturated aqueous sodium bicarbonate, extracted three times with dichloromethane, and the organic phase was dried and concentrated to dryness to obtain a crude product. The crude product was purified by column chromatography to obtain 2.9 g of N-benzenesulfonyl-N-benzyl-N'-phthaloyl-1,3-diamine I-b , yield 65%. HNMR (CD 3 OD) d: 7.73-7.77 (m, 4H), 7.43-7.65 (m, 5H), 7.07-7.15 (m, 5H), 4.25 (s, 2H), 3.42-3.45 (m, 2H), 3.01-3.10 (m, 2H), 1.62-1.69 (m, 2H).
Embodiment 3
[0069] Example 3 : N-3-pyridinesulfonyl-N-4-benzyl-N'-phthaloyl-1, 3-diamine I-c preparation of
[0070]
[0071] Steps:
[0072] N-benzyl-N'-phthaloyl-1,3-diamine 3 (2 g, 6.8 mmol) and triethylamine (2.1 ml) were dissolved in dichloromethane (150 ml) solution, cooled to 0 ° C with an ice bath, and then 3-pyridinesulfonyl chloride (1.2 g, 6.8 ml) was added dropwise mol), and the reaction was stirred at room temperature for 2 hours after dropping. The reaction solution was quenched with saturated aqueous sodium bicarbonate, extracted three times with dichloromethane, and the organic phase was dried and concentrated to dryness to obtain a crude product. The crude product was purified by column chromatography to obtain 1.9 g of N-3-pyridinesulfonyl-N-4-benzyl-N'-phthaloyl-1,3-diamine I-c , yield 65%. HNMR (CD 3 OD) d: 8.97-9.02 (m, 1H), 8.82-8.86 (m, 1H), 8.34-8.38 (m, 1H) 7.81-7.85 (m, 4H), 7.68-7.72 (m, 1H), 7.19- 7.22 (m, 2H), 7.12 -7.16 (m, 3H), 4.34-4.38 (m, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com