A fluorophenylindole compound, its application as a red organic light-emitting material and its preparation method
A technology of substituted phenylindole and luminescent materials, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems limited to the field of medicine, few researches on up-conversion luminescent materials, less preparation and research of luminescent materials, etc. problems, to achieve the effects of reducing interactions, enhancing upconversion luminescence performance, and enhancing chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: 3,5-bis(3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)allylidene)cyclohexanone (I) preparation:
[0042] In a 250 ml round bottom flask, dissolve cyclohexanone (1 mmol) and 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)acrolein (2 mmol) In 50 ml of ethanol or methanol, 10 ml of 15% potassium hydroxide solution was added dropwise to the solution under rapid stirring, and the reaction was stirred at room temperature for 10-15 hours. Afterwards, the reaction solution was poured into 150 ml of water, and the resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized from ethanol-acetone mixed solvent and dried in vacuum to obtain a red solid with a yield of 68%.
[0043] 1 H NMR (300MHz, CDCl 3 / TMS)δ:1.67-1.71(m,14H),2.30(t,J=5.4Hz,4H),4.86-4.96(m,2H),6.50(d,J=12.0,15.3Hz,1H),6.55 (d,J=15.3Hz,1H),7.01-7.25(m,10H),7.32-7.43(m,6H),7.49(d,J=7.8Hz,2H),7.53(d,J=8.4Hz, 2H); 13 C NMR (1...
Embodiment 2
[0044] Example 2: 3,5-bis(3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)allylidene)cyclohexanone (I) preparation:
[0045] In a 250 ml round bottom flask, cyclohexanone (1 mmol) and 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)acrolein (2.2 mmol) Dissolve in 50 ml of ethanol or methanol, add 20 ml of 20% sodium hydroxide solution dropwise to the solution under rapid stirring, and react with stirring at room temperature for 10-15 hours. Afterwards, the reaction solution was poured into 150 ml of water, and the resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized from ethanol-acetone mixed solvent and dried in vacuum to obtain a red solid with a yield of 72%. ESI-MS m / z:677.3(M+H) + .
Embodiment 3
[0046] Example 3: 3,5-bis(3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)allylidene)cyclohexanone (I) Fluorescence performance test
[0047] It is 2 * 10 that the compound of embodiment 1 is formulated into concentration -5 mol / L dichloromethane solution. Measure its ultraviolet absorption spectrum on a HORIBA Jobin Yvon Aqualog absorption and three-dimensional fluorescence scanning spectrometer with a 1 cm fluorescent cell, and measure its fluorescence spectrum on a Perkin Elmer LS-55 fluorescence spectrophotometer, the results are as follows figure 1 and figure 2 shown.
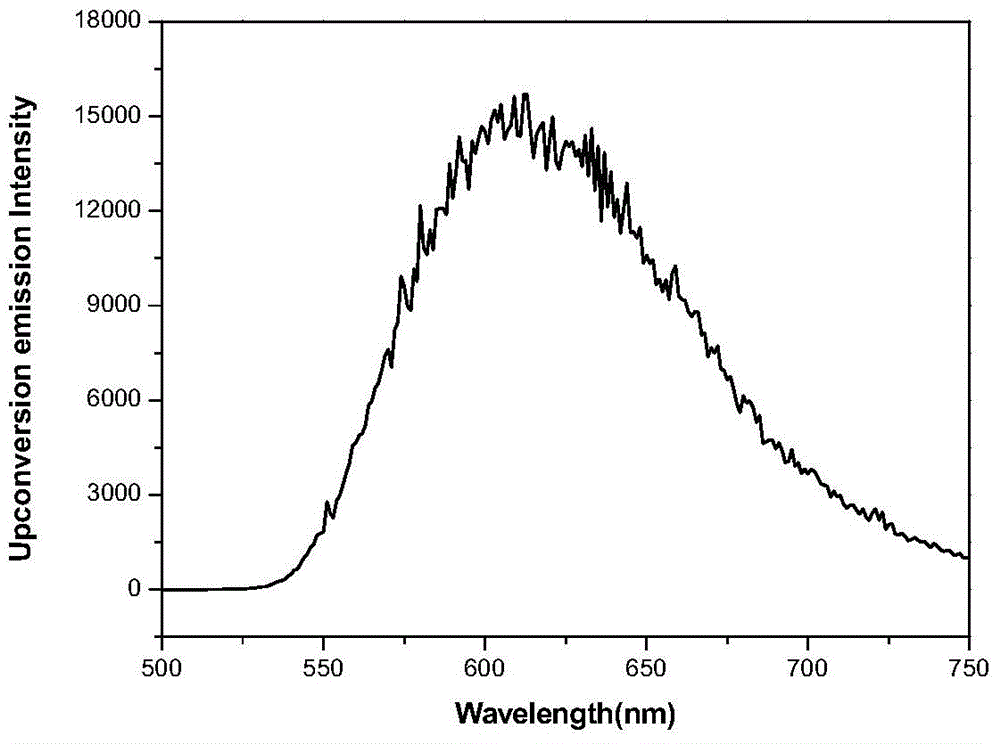
[0048] It is 2 * 10 that the compound of embodiment 1 is formulated into concentration -4 mol / L dichloromethane solution, with the femtosecond Ti of 800nm wavelength: sapphire laser has measured the up-conversion luminescence characteristic of embodiment 1 molecule, and its up-conversion fluorescence spectrum sees image 3 .
[0049] Depend on figure 1 It can be seen that the molecules of Example...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com