Environment-friendly preparation method of propiolic acid derivatives
A technology of propynoic acid and derivatives is applied in the field of compound preparation, which can solve the problems of high safety risk and large environmental pollution, and achieve the effects of high operational safety, little environmental pollution and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
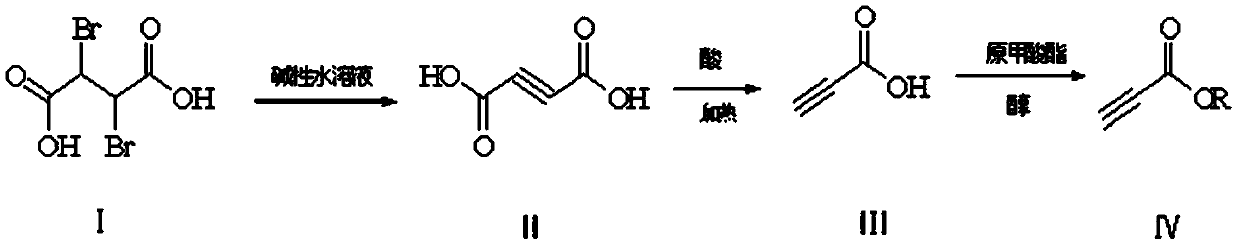
[0044] Embodiment one: the preparation of dipotassium salt of butynedioic acid
[0045] In a 2L three-neck flask, equipped with a thermometer and a condenser, put 417g of water and 60g of potassium hydroxide into it, and stir to dissolve it. At a temperature of 60°C, add 50g of 2,3-dibromosuccinic acid several times to control the temperature below 100°C. , The addition was completed in about 2 hours; heating and holding at 80°C, and reacting for 10 hours, the reaction of the raw materials was detected by HPLC, and it was set aside.
Embodiment 2
[0046] Example 2: Preparation of butynedioic acid disodium salt
[0047] In a 2L three-neck flask, equipped with a thermometer and a condenser, put 417g of water and 43g of sodium hydroxide into it, and stir to dissolve it. At a temperature of 60°C, add 50g of 2,3-dibromosuccinic acid several times to control the temperature below 100°C. The addition is completed in about 2 hours; heat and keep warm at 80°C, and react for 10 hours. The reaction of the raw materials is detected by HPLC, and it is set aside.
Embodiment 3
[0048] Embodiment three: the preparation of butynedioic acid dipotassium salt
[0049] In a 2L three-neck flask, equipped with a thermometer and a condenser, put 1000g of methanol and 60g of potassium hydroxide into it, and stir to dissolve it. At a temperature of 60°C, add 50g of 2,3-dibromosuccinic acid at one time, reflux, and react for 24 hours. The reaction of the raw materials was detected by HPLC, cooled to room temperature, and suction filtered to obtain 92 g of dipotassium butynedioic acid and potassium bromide mixture for subsequent use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com