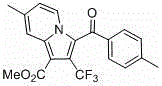
Perfluoroalkyl indolizine derivative and synthesis method thereof
A technology of perfluoroalkyl indolizine and perfluoroalkyl, which is applied in the field of perfluoroalkyl indolizine derivatives and synthesis thereof, can solve the problems of cumbersome reaction steps, complicated operation, difficult to obtain raw materials and the like, and achieves novel structure. , the effect of high regional selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
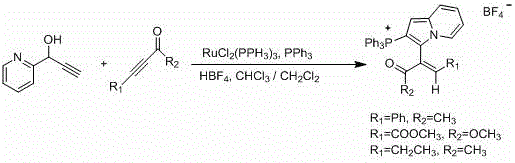
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Pyridine (158 mg, 2 mmol), ω bromoacetophenone (438 mg, 2.2 mmol), and acetonitrile (5 mL) were added to a round-bottomed flask, and reacted at 40 ° C for 4 hours to form a salt , then added methyl trifluoromethylpropiolate (152 mg, 1 mmol), N, N-diisopropylethylamine (258 mg, 1 mmol) and reacted for 7 hours, cooled to room temperature, spin-dried the solvent, and The pure product was isolated by chromatography. white solid. Yield 90%. Structural formula:
[0024]
[0025] Chinese name: methyl 3-benzoyl-2-(trifluoromethyl)indolizine-1-carboxylate
[0026] English name: Methyl 3-benzoyl-2-(trifluoromethyl)indolizine-1-carboxylate
[0027] Molecular weight: 347.08
[0028] Appearance: white solid
[0029] Melting point: 78.0-79.2 °C
[0030] H NMR spectrum (500MHz, CDCl 3, Internal standard: TMS): δ: 3.98 (s, 3H), 6.89-6.92 (m, 1H), 7.28-7.31 (m, 1H), 7.49-7.52 (m, 2H), 7.63-7.66 (m, 1H) , 7.85-7.87 (m, 2H),8.37-8.40 (m, 2H) ppm;
[0031] C NMR sp...
Embodiment 2
[0034] Add pyridine (158 mg, 2 mmol), 2-bromo-4'-methoxyacetophenone (502 mg, 2.2 mmol) and acetonitrile (5 mL) as solvent in a round-bottomed flask, and react 4 at 40° C. Salt was formed after 1 hour, then added methyl trifluoromethylpropiolate (152 mg, 1 mmol), N, N-diisopropylethylamine (258 mg, 1 mmol) and reacted for 7 hours, then cooled to room temperature, and the solvent was vortexed Drying, the pure product was separated by column chromatography. white solid. Yield 91%. Structural formula:
[0035]
[0036] Chinese name: methyl 3-benzoyl-(4-methoxy)-2-(trifluoromethyl)indolizine-1-carboxylate
[0037] English name: Methyl 3-(4-methoxybenzoyl)-2-(trifluoromethyl)indolizine-1-carboxylate
[0038] Molecular weight: 377.09
[0039] Appearance: white solid
[0040] Melting point: 129.4-130.7°C
[0041] H NMR spectrum (500MHz, CDCl 3, Internal standard: TMS): δ: 3.87 (s, 3H), 3.95 (s, 3H), 6.82-6.85 (m, 1H), 6.93-6.96 (m, 2H), 7.22-7.25 (m, 1H) , 7.81 -7.84 (m,...
Embodiment 3
[0045] Pyridine (158 mg, 2 mmol), 2-bromo-4'-nitroacetophenone (537 mg, 2.2 mmol) and acetonitrile (5 mL) were added to a round bottom flask, and reacted at 40 °C for 4 hours After forming a salt, add methyl trifluoromethylpropiolate (152 mg, 1 mmol), N, N-diisopropylethylamine (258 mg, 1 mmol) and react for 7 hours, cool to room temperature, and spin the solvent Drying, the pure product was separated by column chromatography. white solid. Yield 93%. Structural formula:
[0046]
[0047] Chinese name: methyl 3-benzoyl-(4-nitro)-2-(trifluoromethyl)indolizine-1-carboxylate
[0048] English name: methyl 3-(4-nitrobenzoyl)-2-(trifluoromethyl)indolizine-1-carboxylate
[0049] Molecular weight: 392.06
[0050] Appearance: white solid
[0051] Melting point: 176.0-177.9°C
[0052] H NMR spectrum (500MHz, CDCl 3, Internal standard: TMS): δ: δ 3.97 (s, 3H), 7.01-7.04 (m,1H), 7.37-7.41 (m, 1H), 7.97-8.00 (m, 2H), 8.32-8.34 (m, 1H), 8.40-8.41 (d,1H ), 8.73-8.75 (d, 1H) ppm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com