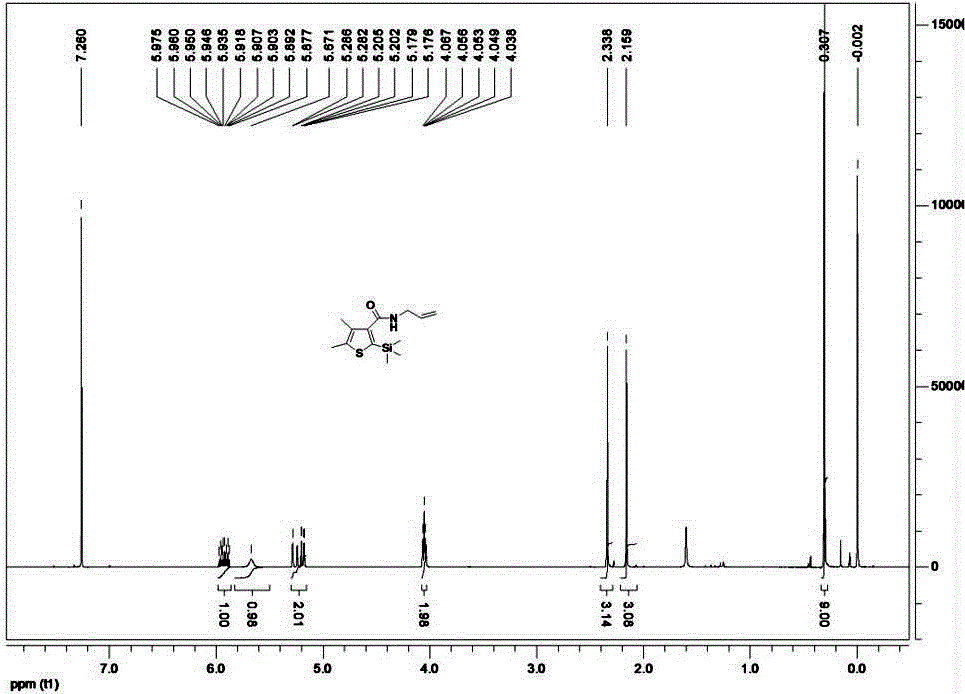
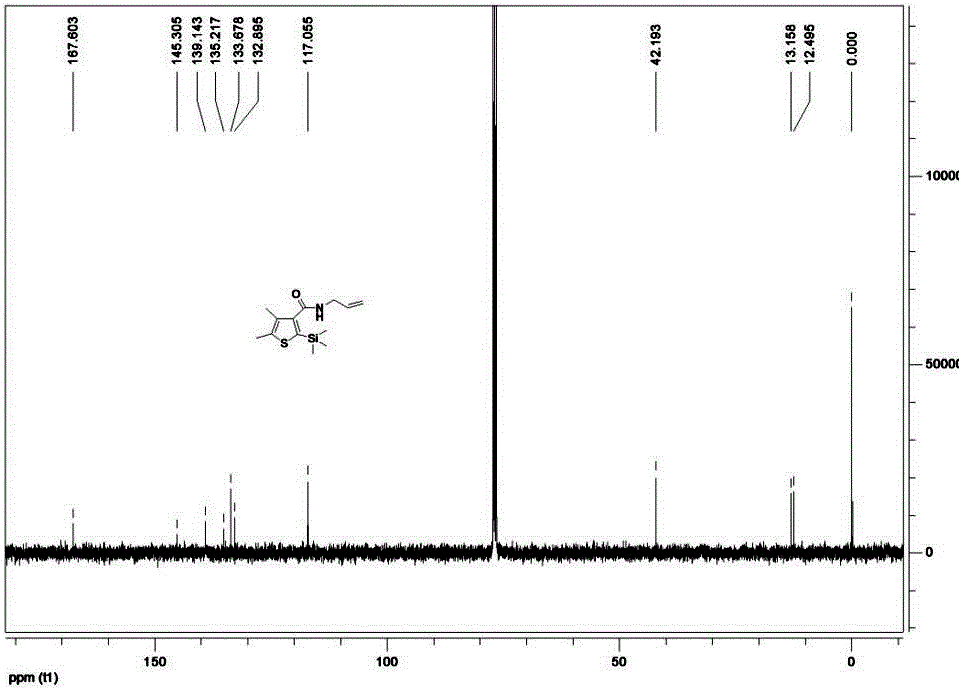
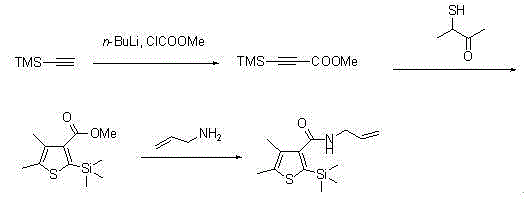
Method for synthesizing pesticide Silthiopham
A technology for the synthesis of silthiocarb and its synthesis method, which is applied in the field of high-efficiency synthesis of the pesticide silthiocarb, can solve the problems of low exchange reaction yields, and achieve the effects of cheap and easy-to-obtain raw materials, simple routes, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of methyl trimethylsilyl propiolate:
[0028] Under nitrogen protection, trimethylsilylacetylene (5.6 mL, 40.0 mmol) was added to dry THF, cooled to -78 °C, n-butyllithium (27.6 mL, 44.0 mmol) was slowly added to the system, and the Stir at temperature for 30 minutes, then warm to room temperature, and add methyl chloroformate (3.4 mL, 44.0 mmol), then stir the reaction for 2.5 hours. The reaction mixture was poured into ammonium chloride solution to quench the reaction, and ether was added for extraction. The organic phase was washed with water and brine and dried over anhydrous sodium sulfate. The solvent was removed in vacuo, and the obtained crude product was purified by column chromatography (5% EtOAc / PE) to obtain methyl trimethylsilylpropiolate 5.9 g (yield: 95.0%) as a yellow oily product.
[0029] (2) Preparation of methyl 4,5-dimethyl-2-(trimethylsilyl)thiophene-3-carboxylate:
[0030] Under nitrogen protection, methyl trimethylsilylpropiolat...
Embodiment 2
[0036] (1) Preparation of methyl trimethylsilyl propiolate:
[0037] Under nitrogen protection, trimethylsilylacetylene (5.6 mL, 40.0 mmol) was added to dry tetrahydrofuran, cooled to -50°C, and methyl lithium (37.6 mL, 60.0 mmol) was slowly added to the system, and the temperature was kept at this temperature. was stirred for 30 minutes at low temperature, then warmed to room temperature, and methyl chloroformate (4.0 mL, 52.0 mmol) was added, and the reaction was stirred for 2.5 hours. The reaction mixture was poured into ammonium chloride solution to quench the reaction, and ether was added for extraction. The organic phase was washed with water and brine and dried over anhydrous sodium sulfate. The solvent was removed in vacuo, and the obtained crude product was purified by column chromatography (5% EtOAc / PE) to obtain methyl trimethylsilylpropiolate 5.8 g (yield: 93.0%) as a yellow oily product.
[0038] (2) Preparation of methyl 4,5-dimethyl-2-(trimethylsilyl)thiophene-...
Embodiment 3
[0044] (1) Preparation of methyl trimethylsilyl propiolate:
[0045] Under nitrogen protection, trimethylsilylacetylene (5.6 mL, 40.0 mmol) was added to dry THF, cooled to -60°C, phenyllithium (26.7 mL, 40.0 mmol) was slowly added to the system, and the temperature was kept at this temperature. It was stirred for 30 minutes at low temperature, then warmed to room temperature, and methyl chloroformate (4.7 mL, 60.0 mmol) was added, and the reaction was stirred for 2.5 hours. The reaction mixture was poured into ammonium chloride solution to quench the reaction, and ether was added for extraction. The organic phase was washed with water and brine and dried over anhydrous sodium sulfate. The solvent was removed in vacuo, and the obtained crude product was purified by column chromatography (5% EtOAc / PE) to obtain methyl trimethylsilylpropiolate 5.7 g (yield: 92.0%) as a yellow oily product.
[0046] (2) Preparation of methyl 4,5-dimethyl-2-(trimethylsilyl)thiophene-3-carboxylate:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com