Preparation method of macromolecular low-migration phosphine oxide UV-LED photoinitiators
A UV-LED and photoinitiator technology, which is applied in chemical instruments and methods, photomechanical equipment, photoplate-making process of patterned surface, etc., can solve the problem of non-drying on the surface of light-curing products and high migration of low-molecular-weight initiators And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A kind of preparation method of macromolecule low migration phosphine oxide UV-LED photoinitiator, is characterized in that: comprise the following steps:
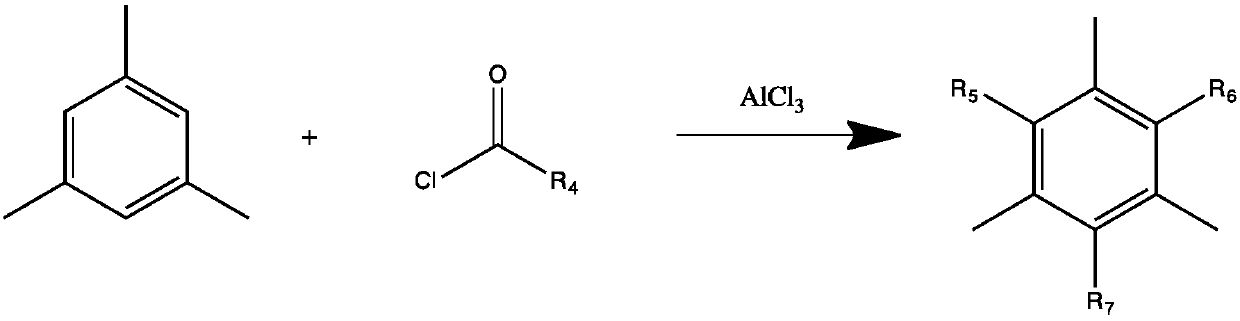
[0033] Step (1): Using 1,3,5-trimethylbenzene as a raw material, carry out Friedel-Crafts reaction with trichloroacetyl chloride or benzoyl chloride under the catalysis of Lewis acid or solid superacid to obtain intermediate a:
[0034]
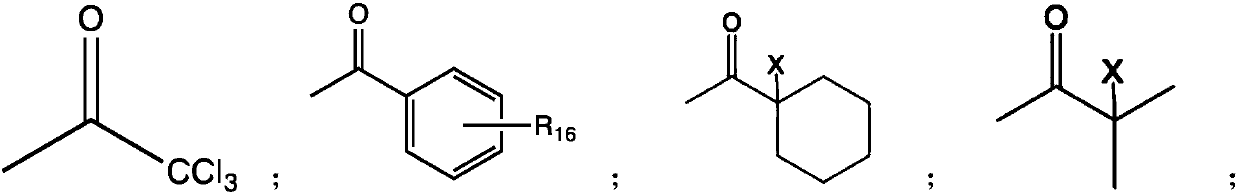
[0035] Wherein R4 is trichloromethyl, 2-chloroisopropyl, 2-bromoisopropyl, 1-chlorocyclohexyl, 1-bromocyclohexylbenzene ring or substituted benzene ring; R5, R6, R7 are the following Any one of the structures or hydrogen;
[0036]
[0037] Step (2): Intermediate a is hydrolyzed under acidic or alkaline conditions to obtain 2,4,6-trimethylbenzene substituted by mono-, di-, and tri-carboxylates:
[0038]
[0039] Wherein R8, R9, R10 are any one of the following structures or hydrogen respectively;
[0040]
[0041] Step (3): the product in the step (2) generates an acyl...
Embodiment 1
[0064] Example 1 Preparation of ethyl 3-benzoyl-2,4,6-trimethylbenzoylphosphonate
[0065]Put 500g of 1,3,5-trimethylbenzene and 150g of anhydrous aluminum trichloride into a 2000mL four-neck flask under nitrogen protection, start stirring and cool down to 0-5°C, slowly add 140g of benzoyl chloride dropwise, and react After 2 hours, add 100g of aluminum trichloride, then slowly add 180g of trichloroacetyl chloride dropwise and keep the temperature below 10°C. After the dropwise addition, continue to react for 2 hours, add the reaction solution to 2000ml of dilute hydrochloric acid in ice water for hydrolysis , stand to separate the organic layer, distill off the unreacted 1,3,5-trimethylbenzene for recycling to obtain 3-benzoyl-2,4,6-trimethylbenzoic acid, dissolve it In 500ml of dichloromethane, 150g of thionyl chloride was added dropwise, and after 1 hour of reaction, the unreacted raw materials and solvent were removed under reduced pressure to obtain 3-benzoyl-2,4,6-trimet...
Embodiment 2
[0066] Example 2 Preparation of 6-benzoyl-2,4-di(ethyl benzoylphosphonate)-1,3,5-trimethylbenzene
[0067] Put 500g of 1,3,5-trimethylbenzene and 150g of anhydrous aluminum trichloride into a 2000mL four-neck flask under nitrogen protection, start stirring and cool down to 0-5°C, slowly add 140g of benzoyl chloride dropwise, and react After 2 hours, add 250g of aluminum trichloride, then slowly add 360g of trichloroacetyl chloride dropwise and keep the temperature below 10°C. After the dropwise addition, continue to react for 2 hours, add the reaction solution to 2000ml of dilute hydrochloric acid in ice water for hydrolysis , let stand to separate the organic layer. Unreacted 1,3,5-trimethylbenzene was distilled off under reduced pressure for recycling to obtain 3-benzoyl-2,4,6-trimethyl-1,5-dibenzoic acid, which was dissolved in 500ml In dichloromethane, 300g of thionyl chloride was added dropwise, and after 1 hour of reaction, the unreacted raw materials and solvent were r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com