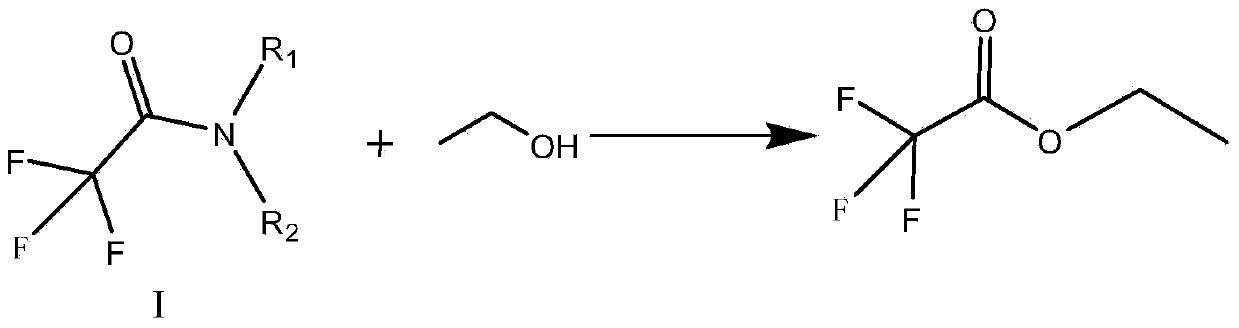
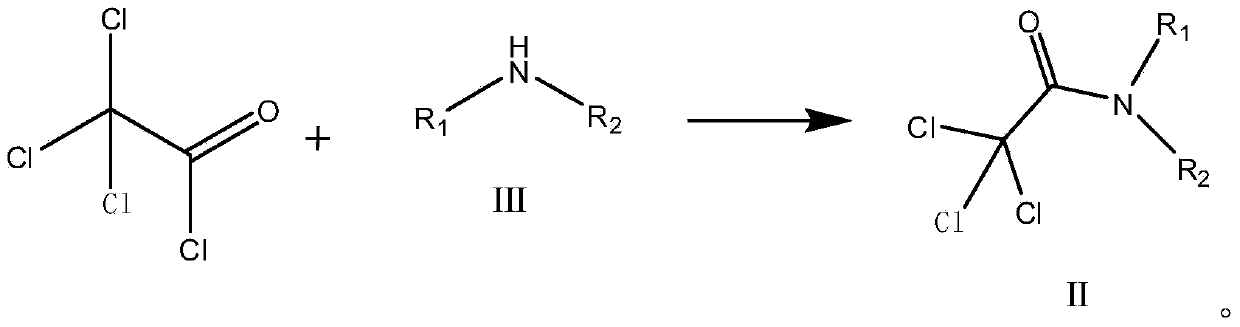
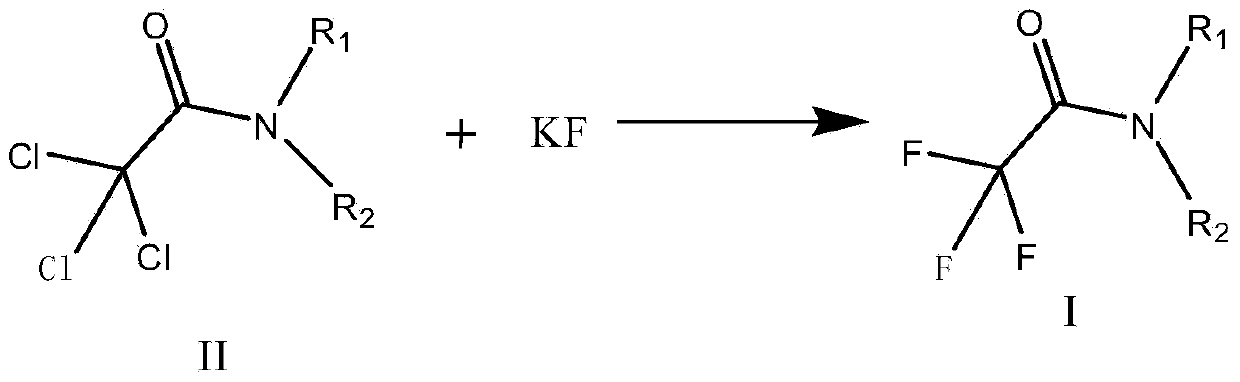Preparation method of trifluoroacetic acid ethyl ester and preparation method of intermediate of trifluoroacetic acid ethyl ester
A technology of ethyl trifluoroacetate and ethanol, applied in the field of preparation of ethyl trifluoroacetate and intermediates thereof, can solve the problems of large amount of three wastes, high equipment requirements, and high production cost, and achieves suppression of the production of ether and water, The effect of shortening reaction time and reducing product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Step 1. Add 73.2 g (1 mol) of methyl isopropylamine and 400 g of toluene into a 1000 ml dry flask, add 182 g (1 mol) of trichloroacetyl chloride dropwise at 20 to 30°C, and keep it for 0.5 to 1 h after the addition is completed. Add 182 g of water to wash the layers, and the organic layer is desolvated and concentrated under reduced pressure to obtain 214.1 g (0.98 mol) of N-methyl-N-isopropyl-2,2-trichloroacetamide. 98% yield, purity> 99.0%.
[0080] Step 2. Add 132.5g (2.28mol) KF, [bmin][PF6]692g, 214.1g (0.98mol) N-methyl-N-isopropyl-2,2-trichloroacetamide into a 1000ml flask. Incubate at 60-70°C for 4-5h, filter, and concentrate by distillation to obtain 157.3g (0.931mol) of N-methyl-N-isopropyl-2,2-trifluoroacetamide, with a yield of 94.3% and a purity of 99.4%.
[0081] Step 3. Add 184.2g (1.88mol) of concentrated sulfuric acid to a 500ml flask, and add 157.3g (0.931mol) of N-methyl-N-isopropyl-2,2-trifluoroacetamide dropwise at 95℃~100℃ And 45.2g (0.98mol) ethanol ...
Embodiment 2
[0083] Step 1. Add 146.4g (2mol) of methyl isopropylamine and 400g of toluene into a 1000ml dry flask, add 182g (1mol) of trichloroacetyl chloride dropwise at 20-30°C, and keep it for 0.5-1h after the addition. Add 182 g of water to wash the layers, and the organic layer is desolvated and concentrated under reduced pressure to obtain 215.9 g (0.988 mol) of N-methyl-N-isopropyl-2,2-trichloroacetamide. 99% yield, purity> 99.0%.
[0084] Step 2. Add 132.5 (2.28mol) KF, [bmin][PF6] 435g, 215.9g (0.988mol) N-methyl-N-isopropyl-2,2-trichloroacetamide into a 1000ml flask at 90 Incubate at -100°C for 4-5h, filter, distill and concentrate to obtain 158.5g (0.938mol) of N-methyl-N-isopropyl-2,2-trifluoroacetamide with a yield of 95% and a purity of 99.5%.
[0085] Step 3. Add 184.2g (1.88mol) of concentrated sulfuric acid to a 500ml flask, and add 158.5g (0.938mol) of N-methyl-N-isopropyl-2,2-trifluoroacetamide dropwise at 95℃~100℃ And 45.2g (0.98mol) ethanol mixed solution, after 2 hours ...
Embodiment 3
[0087] Step 1. Add 182.8g (2.5mol) of methyl isopropylamine and 400g of toluene into a 1000ml dry flask, add 182g (1mol) of trichloroacetyl chloride dropwise at 20-30°C, and keep the temperature for 0.5-1h after the addition. Add 182 g of water to wash the layers, and the organic layer is desolvated and concentrated under reduced pressure to obtain 215.9 g (0.988 mol) of N-methyl-N-isopropyl-2,2-trichloroacetamide. 99% yield, purity> 99.0%.
[0088] Step 2. Add 171.7g (2.96mol) KF, [bmin][PF6]863.6g, 215.9g (0.988mol) N-methyl-N-isopropyl-2,2-trichloroacetamide into a 1000ml flask Incubate at 70-80°C for 3-4 hours, filter, and concentrate by distillation to obtain 159.4g (0.943mol) of N-methyl-N-isopropyl-2,2-trifluoroacetamide with a yield of 96% and a purity of 99.5%.
[0089] Step 3. Add 184.2g (1.88mol) of concentrated sulfuric acid into a 500ml flask, and add 159.4g (0.943mol) of N-methyl-N-isopropyl-2,2-trifluoroacetamide dropwise at 95℃~100℃ And 45.2g (0.98mol) ethanol mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com