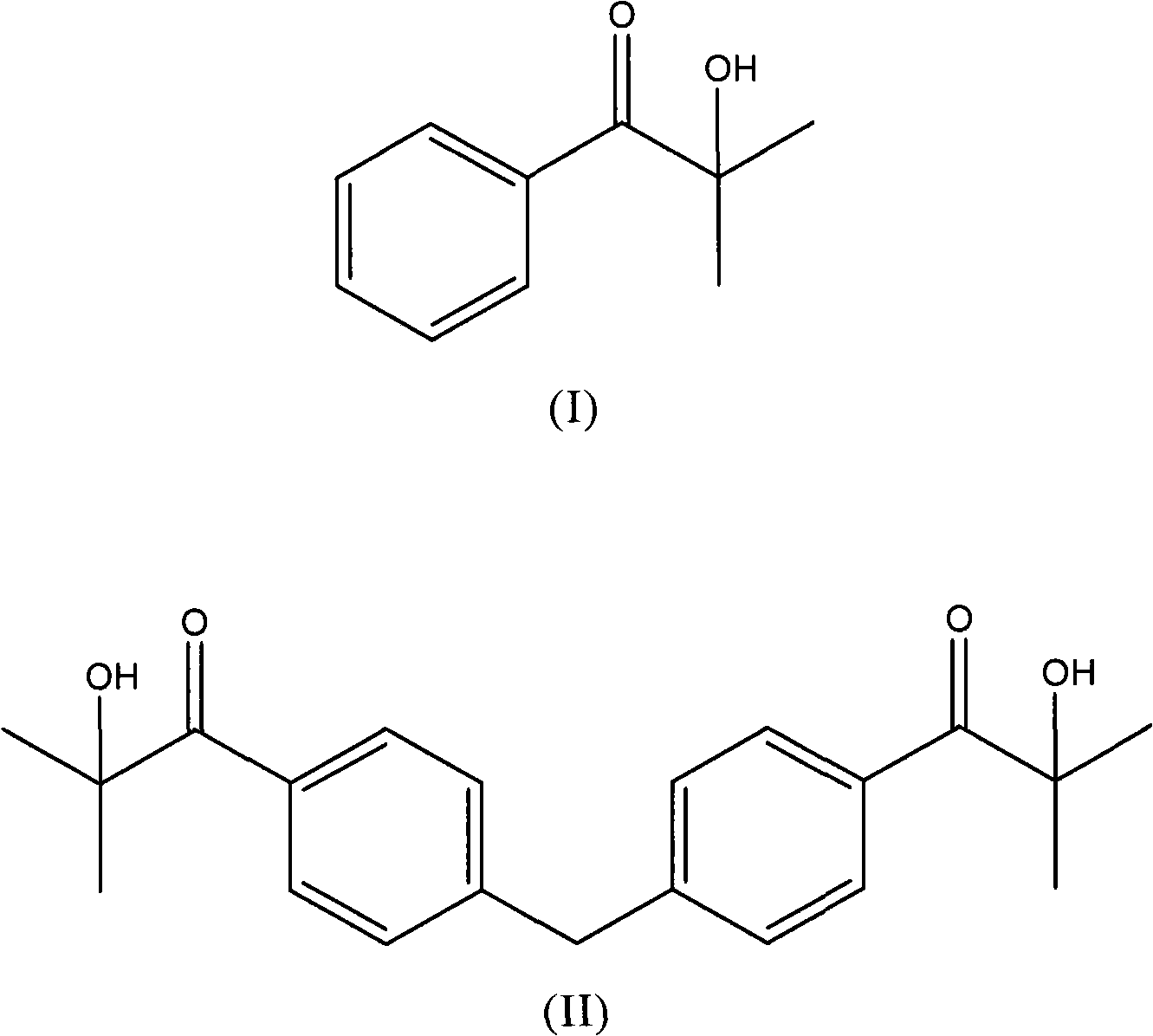
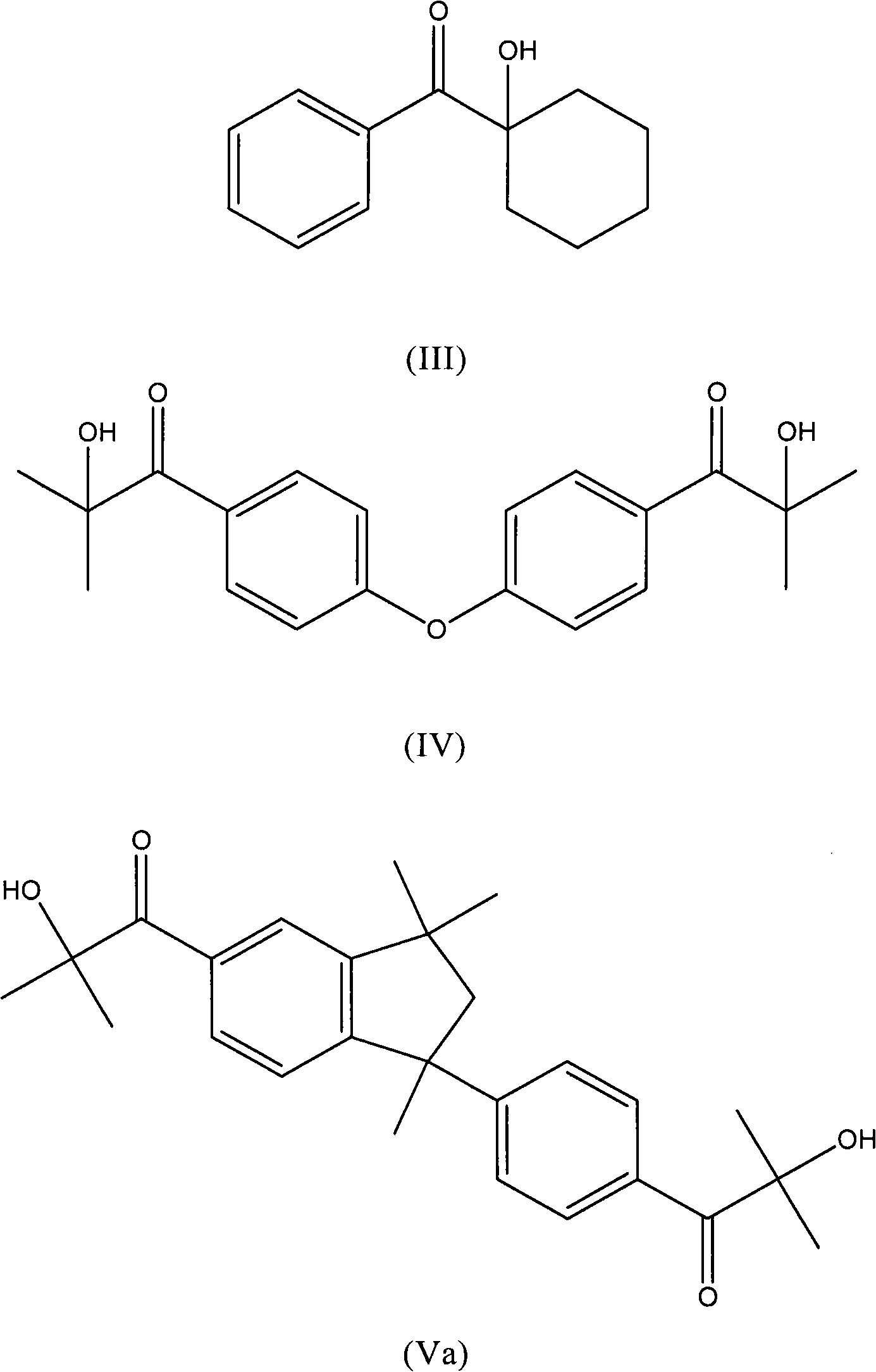
Process for the preparation of aromatic alpha-hydroxy ketones
A technology for hydroxy ketones and aromatic ketones, applied in the field of preparing aromatic α-hydroxy ketones, can solve the problems of not finding aromatic α-hydroxy ketones and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Preparation of 2-Hydroxy-2-methyl-propiophenone
[0068] a) Acylation
[0069] -Synthesis of 2-methyl-propiophenone (isobutyrophenone)
[0070] 123 g of aluminum chloride (1.02 mol) was added portionwise to a solution of 120 g of benzene (1.53 mol) and 108.2 g of isobutyryl chloride (1.02 mol) over 2 hours while stirring and maintaining the temperature at 5 °C . The mixture was kept under stirring for another 1 hour without cooling. By TLC (SiO 2 , toluene) detection reaction. The mixture was poured into ice with stirring. The organic layer was separated, the solvent was evaporated under vacuum, and the product was distilled at 163 °C and 160 mmHg to obtain 140 g of a colorless oil (95% yield), which was used in the next step.
[0071] b) Halogenation
[0072] - Synthesis of 2-chloro-2-methyl-propiophenone (method A)
[0073] At 40°C, 31 g of a 12% aqueous solution of NaClO (0.05 mol) was added dropwise in 90' to 7.4 g of 2-methyl-propiophenone (0.05 mol) obtain...
Embodiment 2
[0081] Preparation of 2-Hydroxy-1-{3-[4-(2-Hydroxy-2-methyl-propionyl)-phenyl]-1,1,3-trimethyl-indan-5-yl}-2 -Methyl-propan-1-one and 2-hydroxy-1-{1-[4-(2-hydroxy-2-methyl-propionyl)-phenyl]-1,3,3-trimethyl- Mixture of indan-5-yl}-2-methyl-propan-1-one
[0082] a) Acylation
[0083] - Synthesis of 1-[4-(5-isobutyryl-1,3,3-trimethyl-indan-1-yl)-phenyl]-2-methyl-propan-1-one and 1-[ Mixture of 4-(6-isobutyryl-1,3,3-trimethyl-indan-1-yl)-phenyl]-2-methyl-propan-1-one
[0084] With stirring and keeping the temperature at 25°C, 14.66 g of aluminum chloride (110 mol) was added portionwise to 11.82 g of 1,3,3-trimethyl-1-phenyl-indane ( 50 mmol) and 13.38 g of isobutyryl chloride (123 mmol). The mixture was heated with stirring and maintained at 60° C. for a further hour; the viscosity of the mixture increased. By TLC (SiO 2 , toluene) detection reaction. The mixture was treated with 112 g of hydrochloric acid (4%) without exceeding 80°C. At 60°C, the organic layer was separa...
Embodiment 3
[0095] Preparation of 2-Hydroxy-1-{4-[4-(2-Hydroxy-2-methyl-propionyl)-phenoxy}-2-methyl-1-propan-1-one.
[0096] a) Acylation
[0097] - Synthesis of 1-[4-(4-isobutyryl-phenoxy)-phenyl]-2-methyl-propan-1-one
[0098] While stirring and keeping the temperature between 5°-15°C, 15.33 g of aluminum chloride (115 mmol) was added in portions to 8.6 g of diphenyl ether (50 mmol) and 11.97 g of in a solution of isobutyryl chloride (110 mmol). The mixture was kept under stirring at 15°C for a further 1 hour, then heated at 50°C for 1 hour. The mixture was treated with 100 ml of water with stirring. The organic layer was separated to give 11 g of a yellow oil which was used in the next step without purification. The sample was crystallized from petroleum ether at 40°-65°C to obtain a slightly white solid with a melting point of 54°C.
[0099] H1NMR (300MHz, CDCl 3 ): δ: 7.98 (d, 4H); 7.04 (d, 4H); 3.45-3.55 (m, 2H); 1.21 (d, 12H).
[0100] b) Halogenation
[0101] - Synthesis ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com