Preparation method of catalyst for preparing propylene by virtue of propane dehydrogenation
A propane dehydrogenation and catalyst technology, which is applied in the direction of chemical instruments and methods, physical/chemical process catalysts, hydrocarbons, etc., can solve the problems of pollution and high product cost, reduce energy consumption, reduce preparation costs, and reduce calcination temperature Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
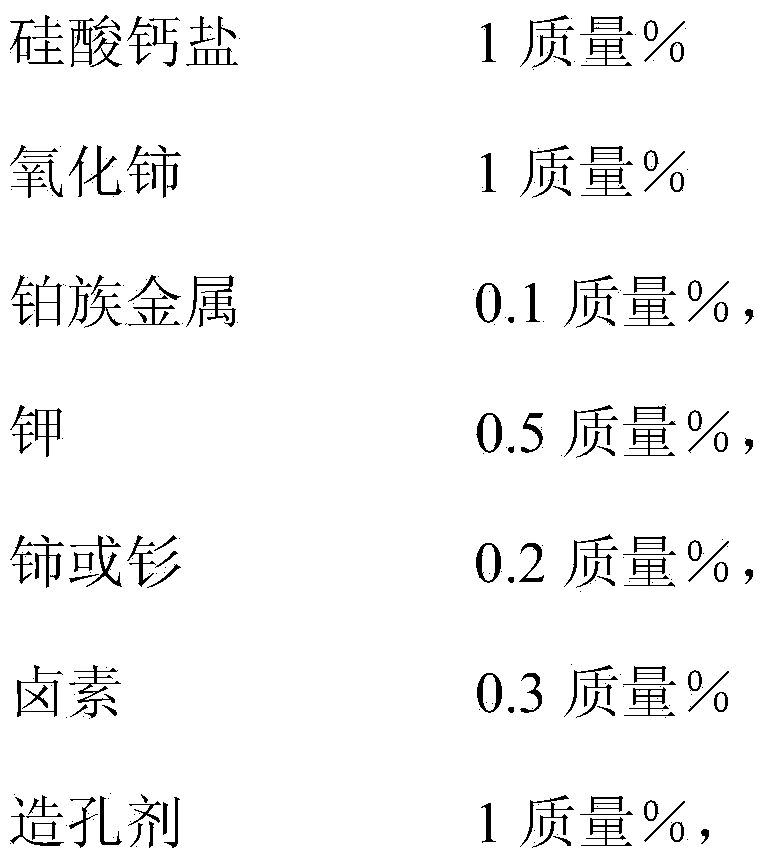
[0021] A method for preparing a catalyst for the dehydrogenation of propane to propylene, comprising an alumina carrier and active components with the following contents based on the carrier as a calculation basis:
[0022]
[0023] The balance is impurities;
[0024] The molar ratio of cerium or samarium to platinum group metal is 7:8; the platinum group metal is platinum; the halogen is chlorine;
[0025] The alumina carrier is impregnated with a soluble cerium or samarium compound solution, a calcium silicate salt solution, dried and calcined, and then impregnated with a platinum group metal compound and a hydrogen halide solution, dried, calcined, and then impregnated with a potassium salt solution. Drying and calcining; then mixing the above-mentioned corresponding amounts of cerium oxide and pore-forming agent, and calcining at 600° C. for 3 hours to obtain the catalyst for the dehydrogenation of propane to propylene.
Embodiment 2
[0027] A method for preparing a catalyst for the dehydrogenation of propane to propylene, comprising an alumina carrier and active components with the following contents based on the carrier as a calculation basis:
[0028]
[0029] The balance is impurities;
[0030] The molar ratio of cerium or samarium to platinum group metal is 7:8; the platinum group metal is platinum; the halogen is chlorine;
[0031] The alumina carrier is impregnated with a soluble cerium or samarium compound solution, a calcium silicate salt solution, dried and calcined, and then impregnated with a platinum group metal compound and a hydrogen halide solution, dried, calcined, and then impregnated with a potassium salt solution. Drying and calcining; then mixing the above-mentioned corresponding amounts of cerium oxide and pore-forming agent, and calcining at 600° C. for 3 hours to obtain the catalyst for the dehydrogenation of propane to propylene.
Embodiment 3
[0033] A method for preparing a catalyst for the dehydrogenation of propane to propylene, comprising an alumina carrier and active components with the following contents based on the carrier as a calculation basis:
[0034]
[0035] The balance is impurities;
[0036] The molar ratio of cerium or samarium to platinum group metal is 7:8; the platinum group metal is platinum; the halogen is chlorine;
[0037] The alumina carrier is impregnated with a soluble cerium or samarium compound solution, a calcium silicate salt solution, dried and calcined, and then impregnated with a platinum group metal compound and a hydrogen halide solution, dried, calcined, and then impregnated with a potassium salt solution. Drying and calcining; then mixing the above-mentioned corresponding amounts of cerium oxide and pore-forming agent, and calcining at 600° C. for 3 hours to obtain the catalyst for the dehydrogenation of propane to propylene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com