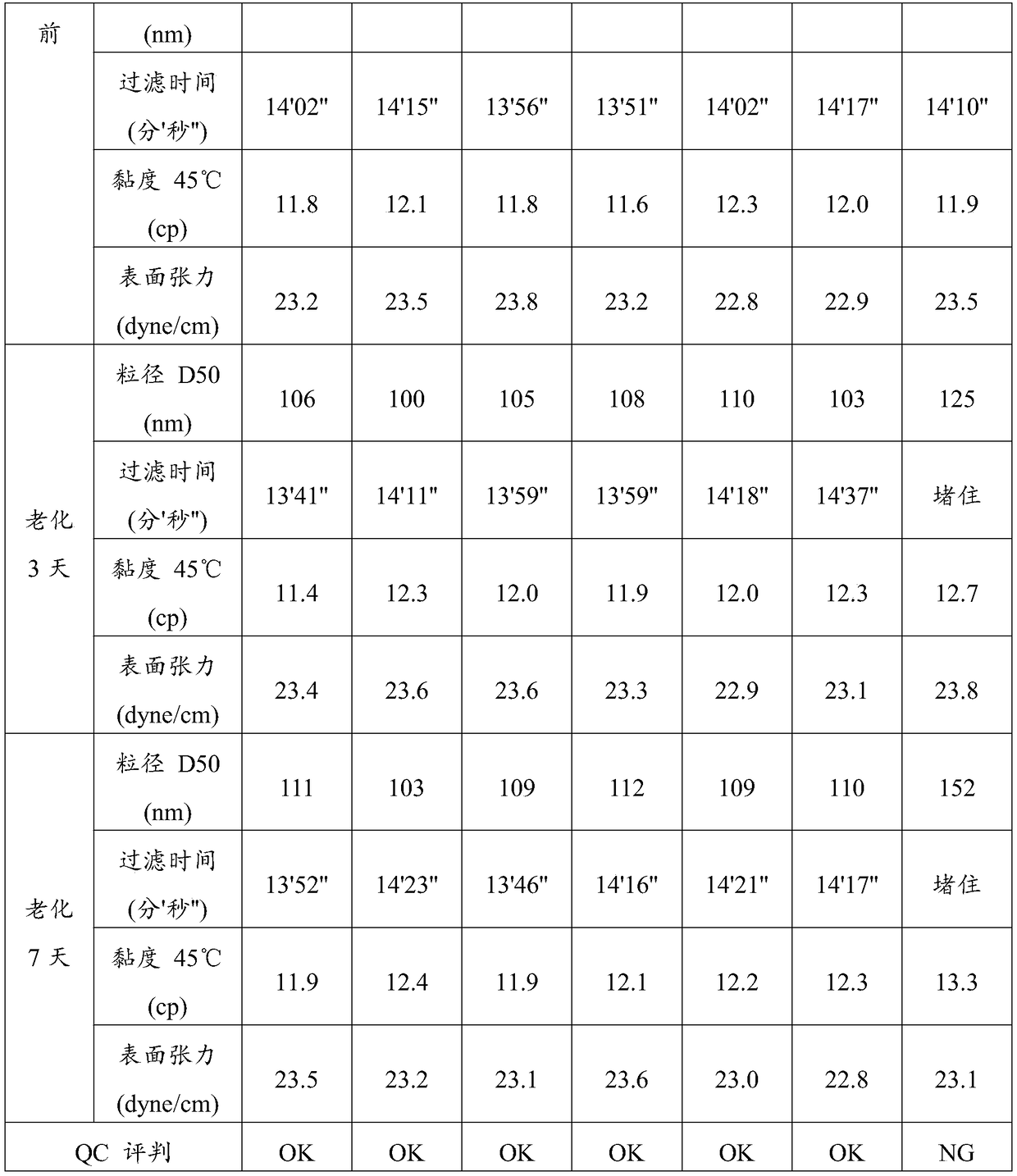
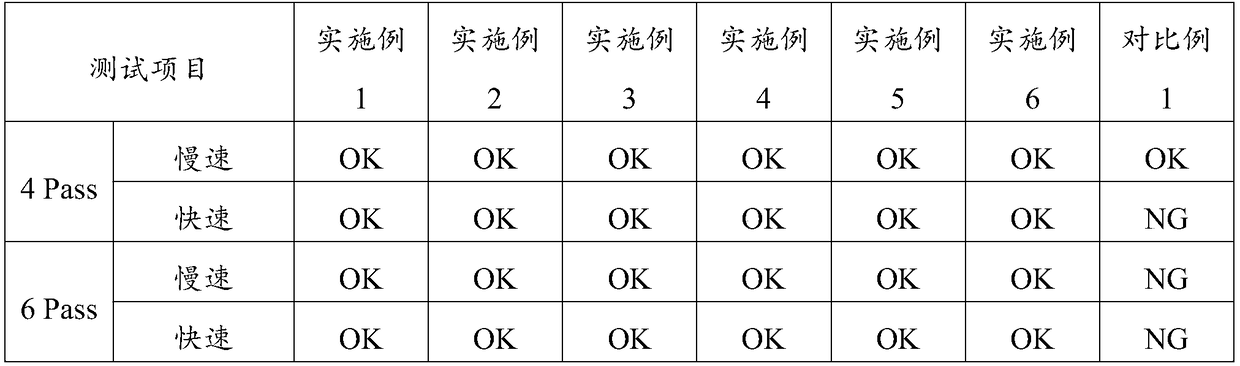
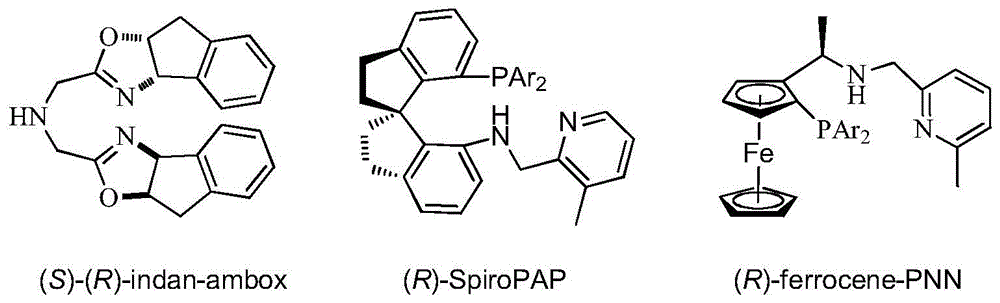
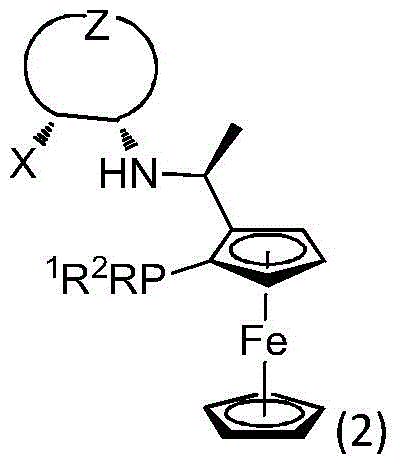
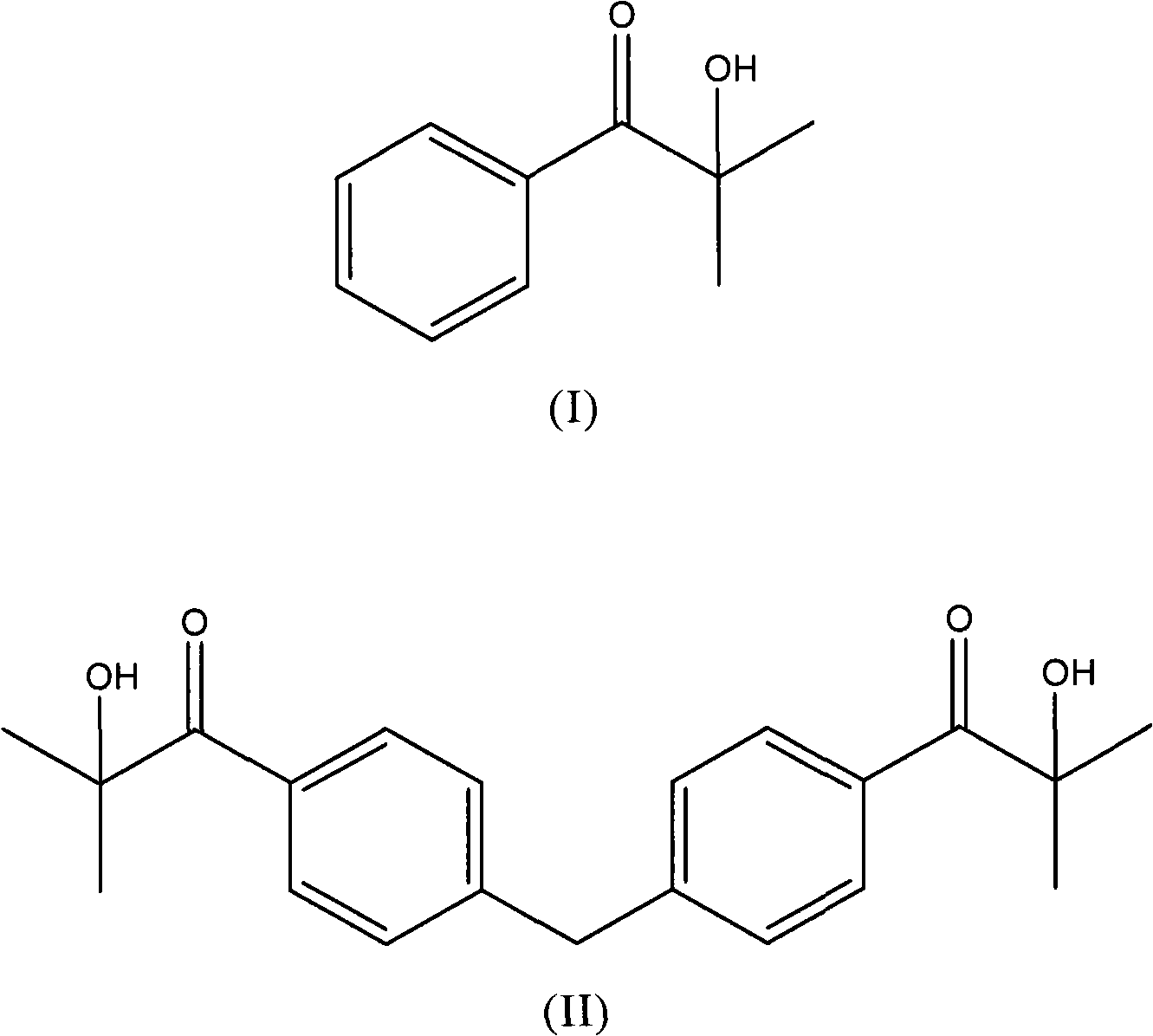
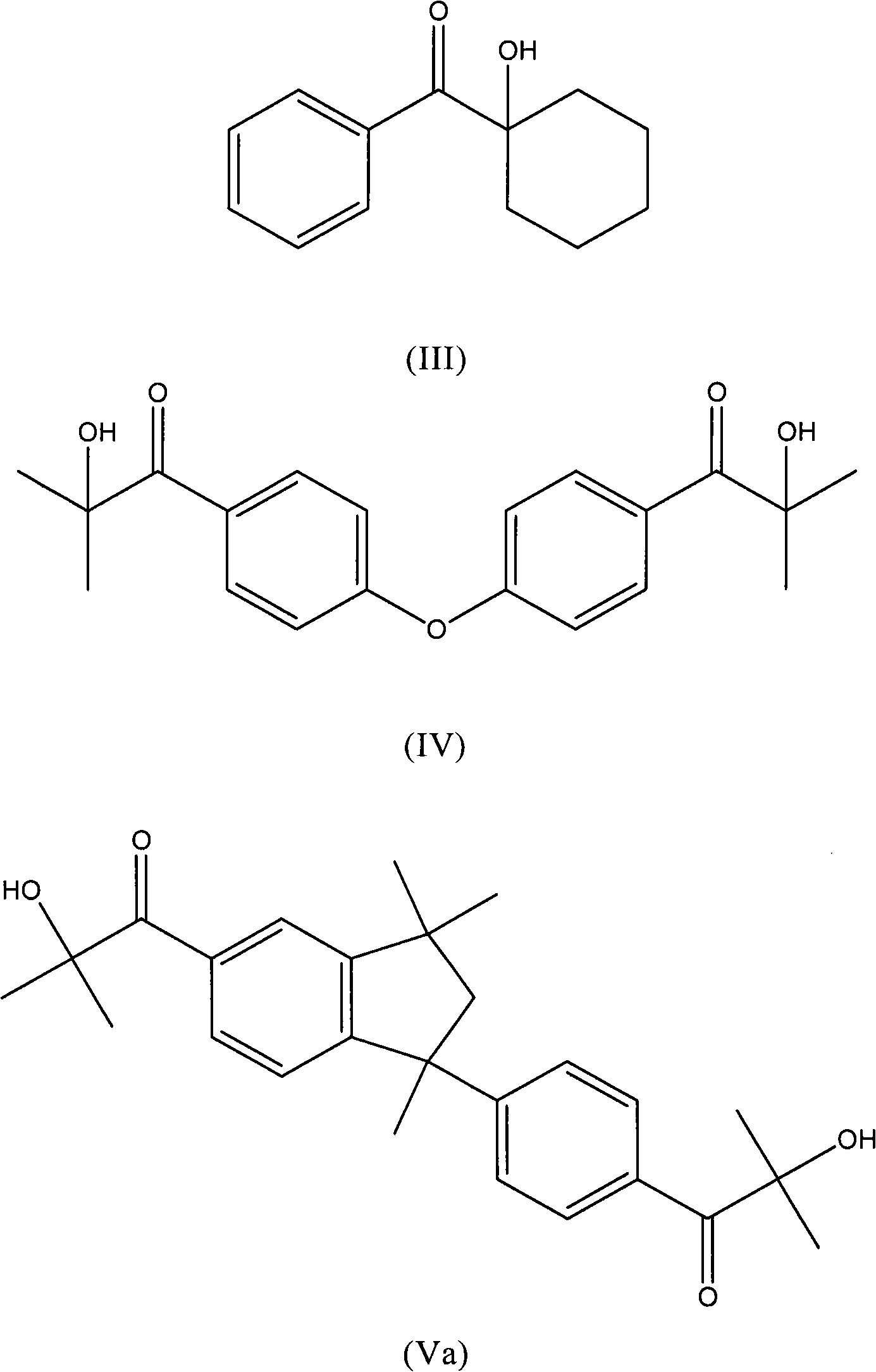
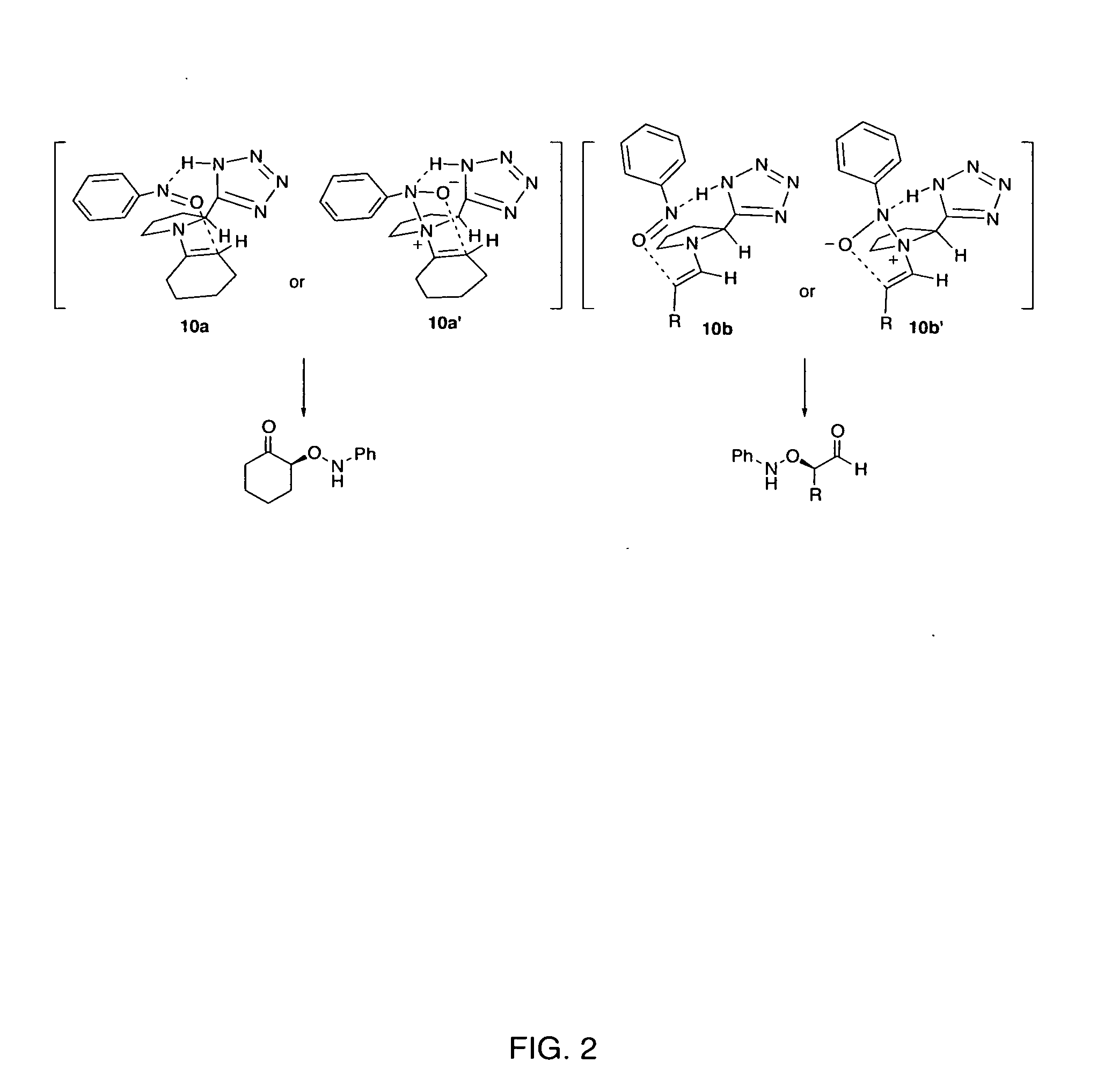
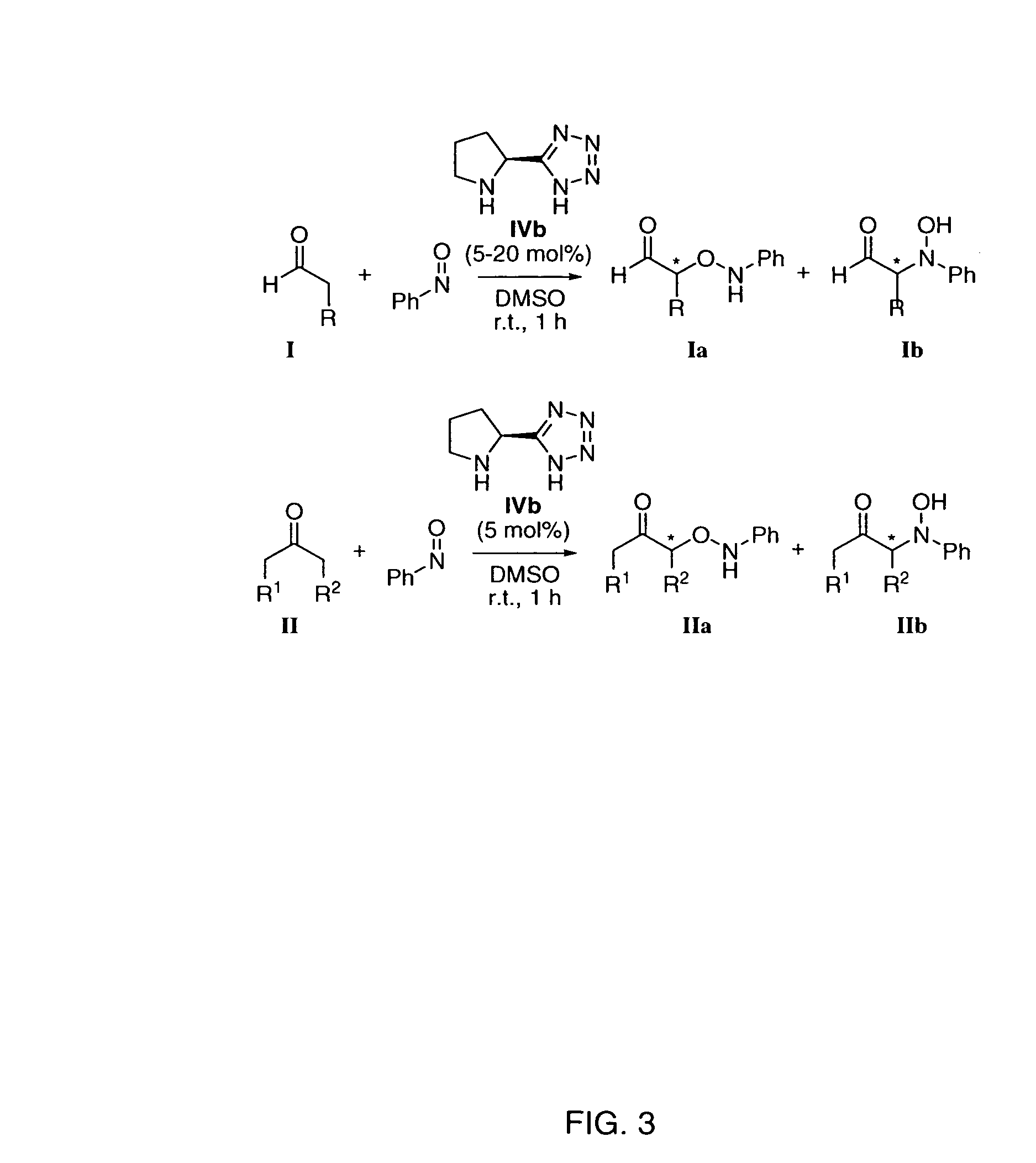
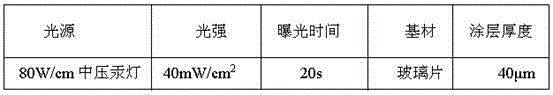
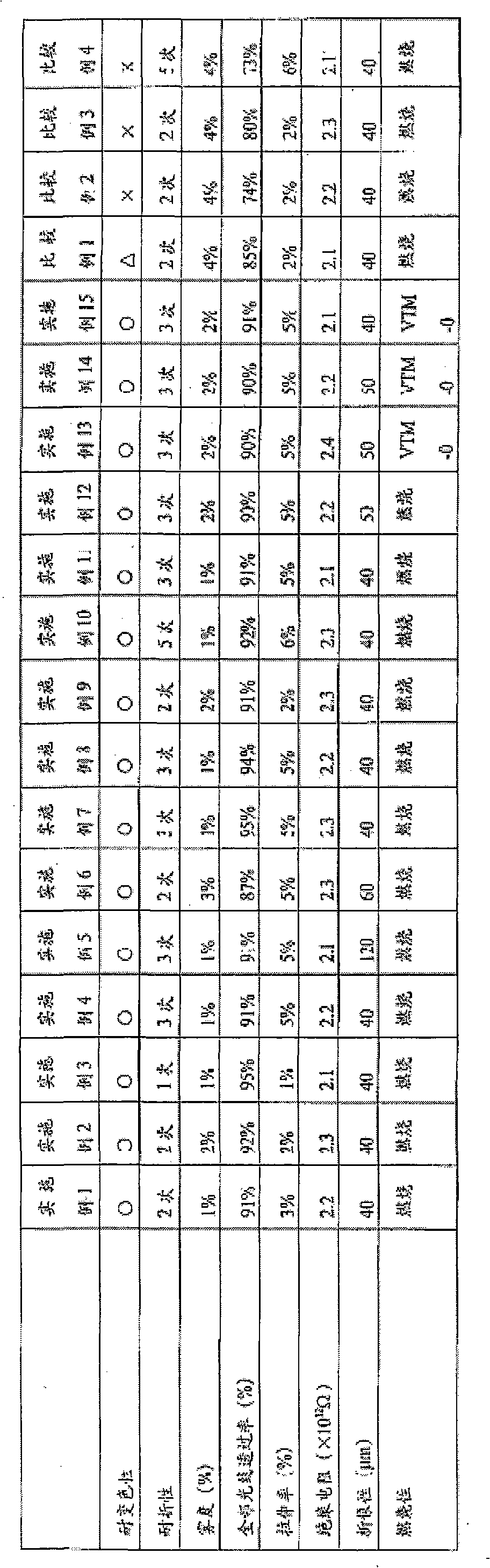
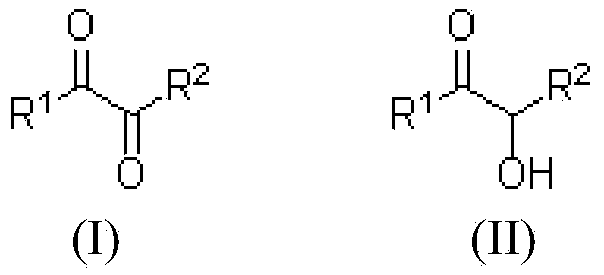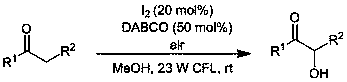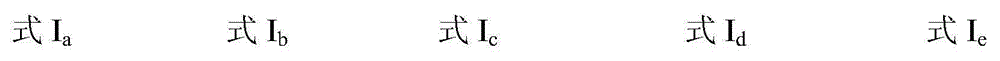Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Alpha hydroxyketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Macromolecule difunctional group alpha-hydroxy-ketone photoinitiator and preparation method thereof
InactiveCN102020726AIncrease the relative molecular massReduce volatilityOrganic compound preparationCarbonyl compound preparationLewis acid catalysisKetone
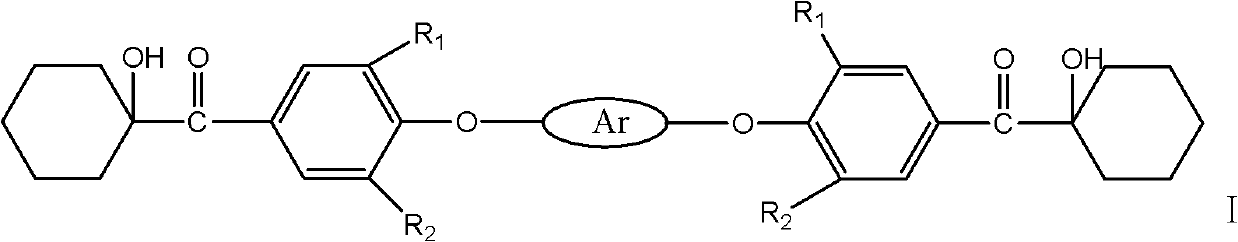
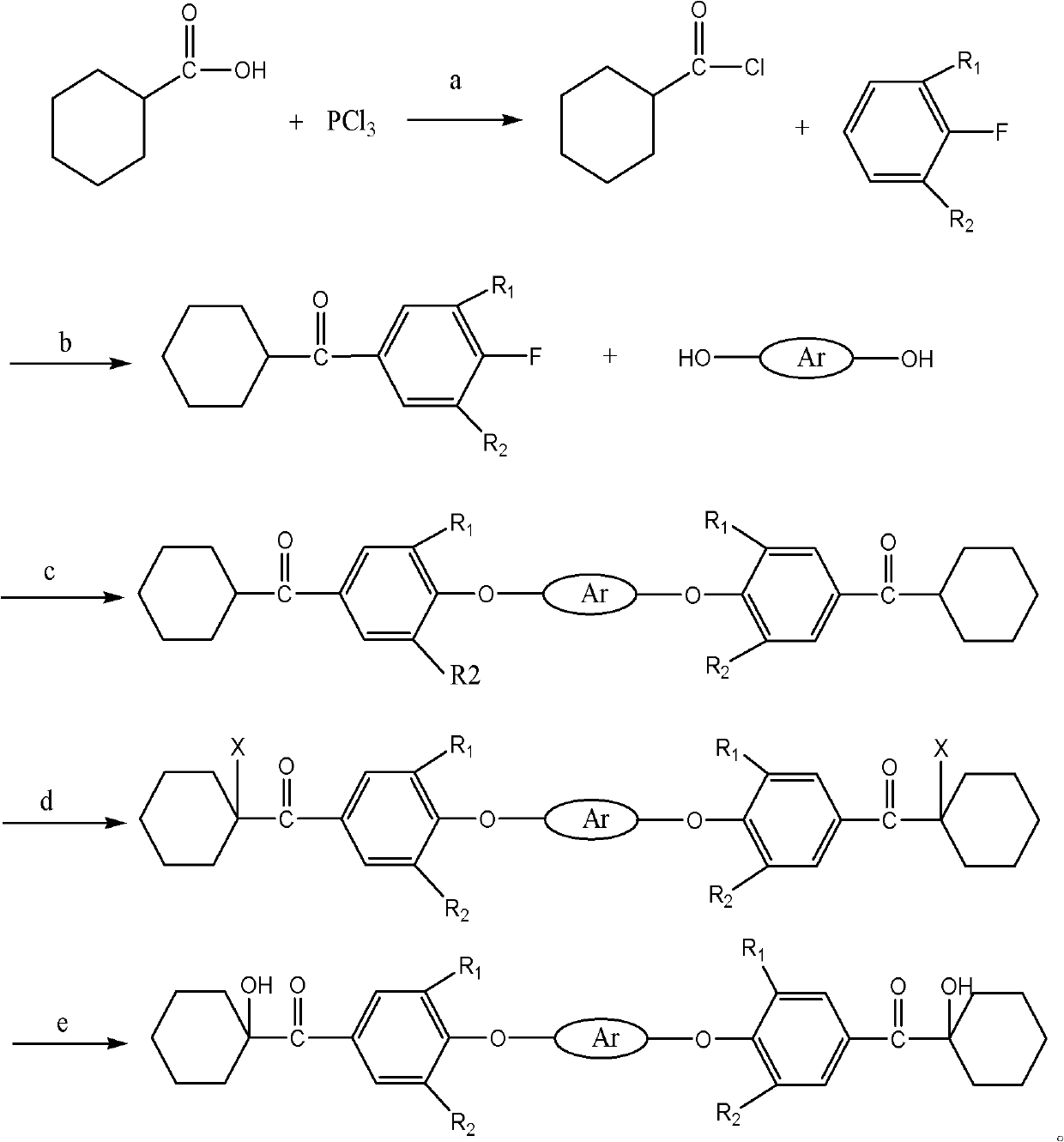
The invention discloses a macromolecule difunctional group alpha-hydroxy-ketone photoinitiator shown in the following formula and a preparation method thereof. The preparation method comprises: firstly, preparing acyl chloride by hexahydrobenzoic acid; carrying out Friedel-Crafts acylations reaction on the acyl chloride and halogeno benzene in the presence of catalysis by lewis acid; then, in the presence of the catalysis, synthetizing a diketone midbody by ketone and bisphenol; and carrying out alpha-site halogenating and hydrolyzing on the diketone midbody to obtain a target product by. The macromolecule difunctional group alpha-hydroxy-ketone photoinitiator has good compatibility with resin, the volatility of the difunctional group alpha-hydroxy-ketone photoinitiator is greatly improved if compared with corresponding micromolecule photoinitiato, but the photo-initiation activity is not obviously reduced. Most importantly, because of the influence by conjugation, the photoinitiator disclosed by the invention has obvious red shift of absorption wavelength, has higher light absorption efficiency and has good application prospect in the material industries of paint, ink and the like.
Owner:CHANGSHANG NEWSUN CHEM IND
Thermosensitive recording medium
InactiveUS8461076B2Avoid yellowingReduce the amount requiredAblative recordingThermographyPolyesterMeth-
To provide a thermosensitive recording medium, containing: a support; a thermosensitive recording layer; and a surface layer, where the thermosensitive recording layer and the surface layer are provided over the support, wherein the thermosensitive recording layer contains a binding agent, a coloring agent, and a color developer, and wherein the surface layer contains polyester (meth)acrylate having at least three (meth)acryloyl groups, and an α-hydroxyketone-based polymerization initiator having a melting point of 80° C. or higher.
Owner:RICOH KK
Ultraviolet curing coating and preparation method thereof
InactiveCN105331255AImprove flatnessGood flexibilityPolyurea/polyurethane coatingsEpoxy resin coatingsPolyesterCross-link
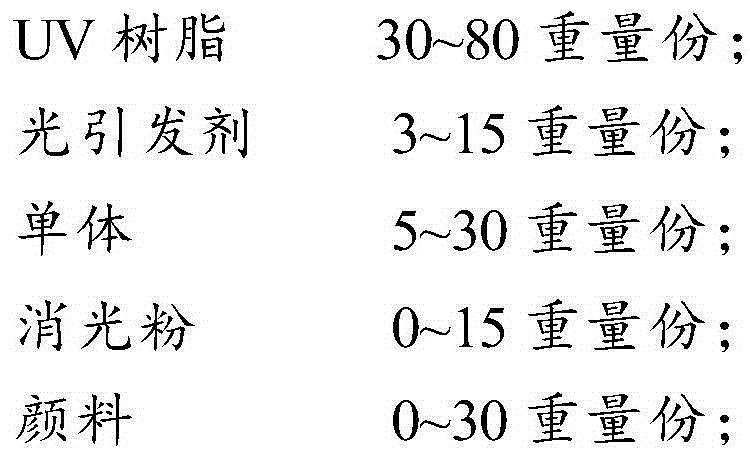
The invention provides an ultraviolet curing coating and a preparation method thereof. The ultraviolet curing coating is prepared from, by weight, 30-80 parts of UV resin, 3-15 parts of a photoinitiator and 5-30 parts of monomers, wherein the UV resin is selected from one or more of bisphenol A epoxy acrylic resin, polyester acrylic resin, polyurethane acrylic resin and organic silicon resin, the photoinitiator is selected from one or more of an aromatic ketone photoinitiator, an alpha-hydroxy ketone derivative, benzil, an acyl phosphorus oxide photoinitiator and an acyl phosphorus oxide derivative, and acrylic monomers and / or modified acrylic monomers are adopted as the monomers. Compared with the prior art, the ultraviolet curing coating has the advantages that the specific photoinitiator can be motivated to be decomposed into free radicals under LED irradiation, the free radicals interact with the UV resin and the monomers to be subjected to a cross-linking reaction to enable the ultraviolet curing coating to be cured, and meanwhile an obtained coating film is higher in flatness, capable of resisting scraping, better in flexibility and higher in hardness.
Owner:展辰新材料集团股份有限公司 +5
LED-UV jet ink and preparation method thereof
The invention provides LED-UV jet ink, which comprises, by weight, the following components: 10-85 part of a monomer, 5-20 parts of a photoinitiator and 10-40 parts of color pulp. The monomer comprises one or more of a ring structure, hydroxyl group, ethoxy group, propoxy group, amine group and amino group; the photoinitiator is an acyl phosphine oxide photoinitiator, an alpha-hydroxyketone photoinitiator, an alpha-amino ketone photoinitiator or a thianthracene photoinitiator. The LED-UV jet ink has good stability, and under the action of LED-UV light and the photoinitiator, the LED-UV jet ink, composed of the monomer, resin and color pulp, can be attached well to the surface of a substrate and improve the adhesion of the LED-UV jet ink. The test results show that the LED-UV jet ink has good stability and strong adhesion.
Owner:TRENDVISION TECH(ZHUHAI) CO LTD
Chiral tridentate nitrogen-phosphine-oxygen ligands and application of related ligands in asymmetric catalytic reactions
ActiveCN105732725AAir stabilizationRegulatory steric hindranceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitrogenAsymmetric hydrogenation
The invention relates to PNHO tridentate ligands and application of related ligands in asymmetric hydrogenation and similar reactions. The novel tridentate nitrogen-phosphine-oxygen ligands are first tridentate nitrogen-phosphine-oxygen ligands containing ferrocenyl planar chiral phosphine, and are successfully applied to high-efficiency high-selectivity asymmetric hydrogenation and other similar reactions of simple aromatic ketone, alpha-hydroxyketone and beta-ketone ester. Compared with other dominant tridentate ligands, the ligands provided by the invention have the advantages of simple synthesis route and low cost, can easily implement large-scale synthesis, are stable in air, and have high activity and high selectivity in asymmetric hydrogenation reactions of carbon-oxygen double bonds.
Owner:WUHAN CATALYS TECH CO LTD
Process for the preparation of aromatic alpha-hydroxy ketones
InactiveCN102015603AOrganic compound preparationCarbonyl compound preparation by condensationHydrogen halideBromine
Process for the preparation of aromatic alpha-hydroxyketones (aromatic a- hydroxyketones) that does not require the use of chlorine, sulfuryl chloride or bromine and comprises the halogenation of an intermediate aromatic ketone with a hydrogen halide in the presence of an oxidising compound.
Owner:LAMBERTI SPA
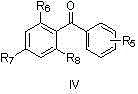
Process of making alpha-aminooxyketone/alpha-aminooxyaldehyde and alpha-hydroxyketone/alpha-hydroxyaldehyde compounds and a process making reaction products from cyclic alpha,beta-unsaturated ketone substrates and nitroso substrates
The present invention is directed to a process of making α-aminooxyketone and α-hydroxyketone compounds. The synthetic pathway generally involves reacting an aldehyde or ketone substrate and a nitroso substrate in the presence of a catalyst of the formula (IV): wherein Xa—Xc represent independently nitrogen, carbon, oxygen or sulfur and Z represents a 4 to 10-membered ring with or without a substituent and optionally a further step to convert the α-aminooxyketone compound formed to the α-hydroxyketone compound. The present invention results in α-aminooxyketone and α-hydroxyketone compounds with high enantioselectivity and high purity. The present invention is also directed to a catalytic asymmetric O-nitroso Aldol / Michael reaction. The substrates of this reaction are generally cyclic α,β-unsaturated ketone substrate and a nitroso substrate. This methodology generally involves reacting the cyclic α,β-unsaturated ketone substrate and the nitroso substrate in the presence of a proline-based catalyst, to provide a heterocyclic product.
Owner:JAPAN SCI & TECH CORP
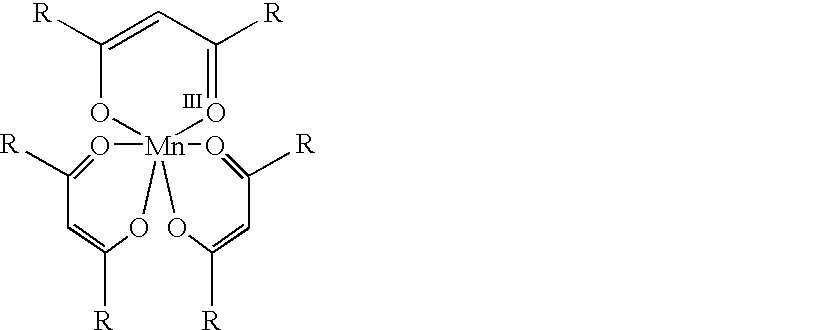
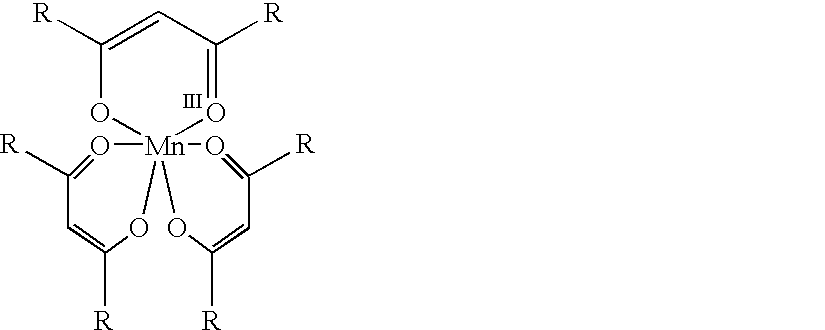
Conversion of alpha,beta-unsaturated ketones and alpha,beta-unsaturated esters into alpha-hydroxy ketones and alpha-hydroxy esters using Mn(III) catalyst, phenylsilane and dioxygen
InactiveUS20020120170A1Group 4/14 element organic compoundsOrganic compound preparationOxygenSide reaction
The present invention provides a novel process for the conversion of alpha,beta-unsaturated ketones. This invention is an improvement over existing processes in that it operates at neutral reaction conditions that prevent the formation of side reactions and that it is a single step, which proceeds with complete selectivity and gives a yield that is approximately 30% higher than the currently used processes. An example of this process is the conversion of 16-dehydroprogesterone into 17 alpha-hydroxyprogesterone.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
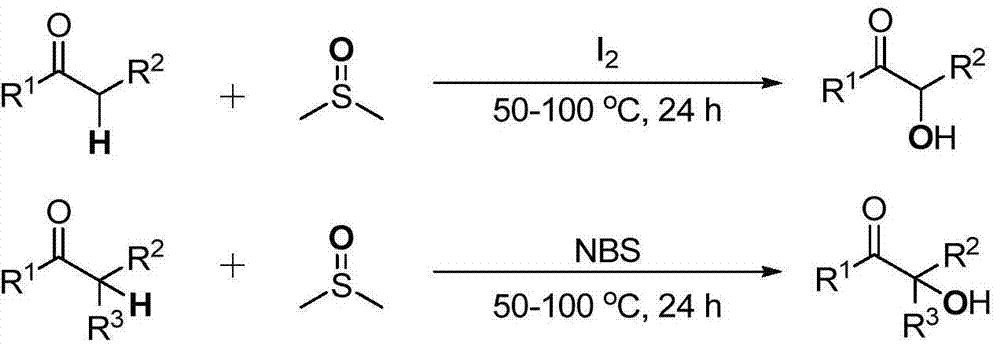
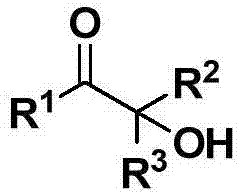
Cheap and efficient synthesis method of alpha-hydroxyketone compound
ActiveCN104710256AEmission reductionHigh yieldOrganic compound preparationHydroxy group formation/introductionSynthesis methodsEthyl acetate
The invention discloses a cheap and efficient synthesis method of an alpha-hydroxyketone compound. The synthesis method is characterized in that a carbonyl compound undergoes an oxidation hydroxylation reaction at 10-120DEG C under normal pressure with iodine simple substance, N-bromosuccimide, copper bromide, bromine simple substance, hydrogen bromide, N-iodosuccimide or hydrogen iodide as a catalyst, sulfoxide as an oxidant, water or sulfoxide as a hydroxy source and sulfoxide, ethyl acetate, N,N-dimethyl formamide, acetonitrile, toluene, 1,4-dioxane, 1,2-dichloroethane, tetrahydrofuran or H2O as a solvent, and converts into the alpha-hydroxyketone compound in a high selectivity manner. Compared with traditional synthesis methods, the method disclosed in the invention has the advantages of simple operation, high yield, simple conditions, easy purification, small waste discharge amount, simple reaction apparatus, and easy industrial production. The method has wide applicability and can be used for synthesizing various alpha-hydroxyketone compounds.
Owner:QINGDAO RUIJI MEDICAL TECH CO LTD
Electrochemical catalytic synthesis method of alpha-carbonyl ketone compounds
InactiveCN104313635ALow costReduce energy consumptionElectrolysis componentsElectrolytic organic productionSupporting electrolyteSynthesis methods
The invention discloses an electrochemical catalytic synthesis method of alpha-carbonyl ketone compounds, and belongs to the technical field of preparation of alpha-carbonyl ketone compounds. The electrochemical catalytic synthesis method comprises the following steps: in a single-chamber electrolytic cell, in a certain amount of electrolyte solution, with alpha-carbonyl ketone as a raw material and halide as a catalyst, performing constant-current electrolysis at a certain temperature and a certain current density to obtain the alpha-carbonyl ketone compounds. Compared with the existing other electrochemical methods for synthesizing the alpha-carbonyl ketone compounds, the electrochemical catalytic synthesis method provided by the invention has the advantages as follows: the single-chamber electrolytic cell and an inexpensive electrode material are used, and an oxidant is regenerated by an electrochemical method; supporting electrolyte is not added, and only a catalytic amount of halide is taken as the catalyst, so that the cost is greatly reduced and the posttreatment is simple; therefore, the electrochemical catalytic synthesis method is more convenient for industrial production.
Owner:BEIJING UNIV OF TECH +1
Process for preparing chiral aromatic alpha-hydroxy ketones using 2-hydroxy-3-oxoacid synthase
InactiveUS20060148042A1Improve efficiencyOrganic compound preparationRecombinant DNA-technologySynthonEnantiomer
A biotransformation process for preparing chiral aromatic-hydroxy ketones in high yields is described, using 2-hydroxy-3-oxoacid synthase, such as AHAS or TSAS. Optionally substituted arylaldehydes and -oxoacids react in this process to provide pure enantiomers, useful as synthons in the production of various drugs, an example being (R)-phenylacetyl carbinol.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Polymeric Alpha-Hydroxy Aldehyde and Ketone Reagents and Conjugation Method
ActiveUS20110230618A1Reduce chance of degradationPeptide/protein ingredientsPeptide preparation methodsHydrogen atomPolyethylene glycol
Provided herein are polymeric α-hydroxy aldehyde or α-hydroxy ketone reagents which can be conjugated to amine-containing compounds to form stable conjugates in a single-step reaction. In selected embodiments, the polymeric reagent itself incorporates an internal proton-abstracting (basic) functional group, to promote more efficient reaction. The substituent is appropriately situated, via a linker if necessary, to position the group for proton abstraction, preferably providing a 4- or 5-bond spacing between the abstracting atom and the hydrogen atom on the α-carbon. Also provided are methods of using the reagents and stable, solubilized conjugates of the reagents with biologically active compounds. In preferred embodiments, the polymeric component of the reagent or conjugate is a polyethylene glycol.
Owner:NEKTAR THERAPEUTICS INC
Green preparation method of alpha-hydroxyketone
ActiveCN108083999ASelectivityAvoid expensiveOrganic compound preparationCarbonyl compound preparationChromatographic separationAir atmosphere
The invention relates to a green preparation method of alpha-hydroxyketone. The method comprises the following steps: adding ketone, iodine, 1,4-diazabicyclo[2.2.2]octane and methanol into a glass reaction bottle in sequence; then stirring and reacting for 14 to 30h at room temperature in an air atmosphere under the irradiation of a 23W compact type fluorescent lamp, so as to obtain a reaction mixture; carrying out silica gel column chromatographic separation to obtain the pure alpha-hydroxyketone. The green preparation method provided by the invention has the characteristics of greenness, high efficiency, simplicity in operation, moderate conditions, wide applicability and easiness for industrialization.
Owner:NORTHWEST NORMAL UNIVERSITY
Polymeric alpha-hydroxy aldehyde and ketone reagents and conjugation method
ActiveUS8492503B2Reduce chance of degradationPeptide/protein ingredientsPeptide preparation methodsHydrogen atomPolyethylene glycol
Provided herein are polymeric α-hydroxy aldehyde or α-hydroxy ketone reagents which can be conjugated to amine-containing compounds to form stable conjugates in a single-step reaction. In selected embodiments, the polymeric reagent itself incorporates an internal proton-abstracting (basic) functional group, to promote more efficient reaction. The substituent is appropriately situated, via a linker if necessary, to position the group for proton abstraction, preferably providing a 4- or 5-bond spacing between the abstracting atom and the hydrogen atom on the α-carbon. Also provided are methods of using the reagents and stable, solubilized conjugates of the reagents with biologically active compounds. In preferred embodiments, the polymeric component of the reagent or conjugate is a polyethylene glycol.
Owner:NEKTAR THERAPEUTICS INC
Compound photoinitiator with low yellowing performance and high activity
The invention discloses a compound photoinitiator with low yellowing performance and high activity. A benzoyl ester hotoinitiator, an alpha-hydroxy-ketone hotoinitiator, a diphenyl ketone hotoinitiator and an acyl phosphine oxide photoinitiator are compouded according to a certain type and proportion. The compound photoinitiator with low yellowing performance and high activity, disclosed by the invention, can satisfy requirements of multiple fields on low yellowing performance and has the advantages of convenient use, low price and high photoinitiating activity. The compound photoinitiator with low yellowing performance and high activity can meet the use requirements of most fields with higher requirements on deep curing, low yellowing performance, high activity, good surface drying effect and so on by changing the mixing ratio of the compound photoinitiator.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
Punching-resistant LED-UV metal printing offset ink
The invention discloses punching-resistant LED-UV metal printing offset ink and relates to the technical field of metal printing offset ink. The punching-resistant LED-UV metal printing offset ink is prepared from ingredients in percentage by mass: 45 to 60% of UV resin, 5 to 20% of reactive diluents, 3 to 5% of dibenzoyl and derivative of the dibenzoyl, 4 to 4.5% of BAPO, 3 to 3.5% of alpha-hydroxyketone derivative, 2 to 2.5% of alpha-aminoketone derivative, 5 to 8% of auxiliary agent, 12 to 18% of pigment and 0.3 to 0.5% of graphene. The ink disclosed by the invention is cured to form an ink film, the ink film has good adhesion and flexibility, cross-cut test can reach 5B, a post-print processing requirement can be met, and the ink film cannot crack under 50Nm punching; furthermore, after the ink is printed to a plain sheet base material and irradiated by a low-energy LED-UV lamp, an accumulated light quantity value can reach 15mJ / cm<2>, instant full curing can be achieved, and a drying speed is quick.
Owner:深圳美联兴科技股份有限公司
Catalytic system for synthesizing alpha-hydroxy ketone by alkynol hydration reaction
ActiveCN104437621AHigh catalytic activityWide applicabilityOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by C-C triple bond hydrationHydration reactionAcetylenic alcohol
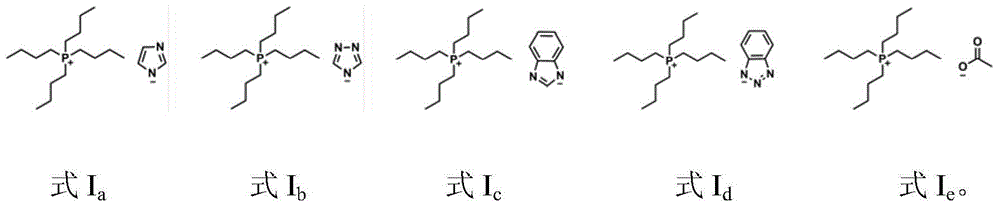
The invention discloses a catalytic system for synthesizing alpha-hydroxy ketone by alkynol hydration reaction. The catalytic system consists of an ionic liquid and a CO2 gas, wherein the ionic liquid is selected from at least one of ionic liquids as shown in formulas Ia-Ie described in the specification. In the catalytic system, the amount of substances of the ionic liquid is 0.01-0.5mol, and the pressure of the gas is 0.1-8MPa. The invention further provides a method of preparing alpha-hydroxy ketone compounds by utilizing the catalytic system. The catalytic system is suitable for a reaction system for catalyzing alkynol hydration reaction to synthesize alpha-hydroxy ketone compounds. The catalytic system is relatively high in catalytic activity, mild in reaction condition, and free of metals. The main catalyst ionic liquid is easy to synthesize and recycle, and has a relatively strong application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Alkali soluble transparent resin composition
InactiveCN102520585AHigh light transmittancePrinted circuit detailsPrinted circuit manufactureCarboxyl radicalTransmittance
An alkali soluble transparent resin composition is provided to have excellent transmittance of whole rays in visible light area, and yellowing resistance. An alkali soluble transparent resin composition comprises a carboxy group-containing photosensitive resin (A), [alpha] - hydroxyketone based photopolymerization initiator (B), a diluents (C), and an epoxy compound (D).
Owner:TAMURA KK
Mono/bis-methyl cinnamate photoinitiators as well as preparation method and application thereof
ActiveCN110563589AEasy to understandPreparation from carboxylic acid halidesOrganic compound preparationUltravioletMethyl cinnamate
The invention relates to methyl cinnamate compounds in a formula (I), wherein each variable in the compounds in the formula (I) is defined in the description. The invention further relates to a methodfor preparing the methyl cinnamate compounds, including an esterification reaction of a compound in a formula (II) and a compound in a formula (III). The methyl cinnamate compounds can be used as photoinitiators, can absorb radiant heat in the range of 200-400 nm and have good stability and yellowing resistance; and more importantly, the initiators greatly solve the problems of foreign odors andhigh migration as compared with existing alpha-hydroxyketone photoinitiator, besides, the photoinitiators have ultraviolet (UV) absorption red shift to about 300-400 nm and are applicable to UV-LED light source curing. The invention also relates to application of mono / bis-methyl cinnamate compounds as photoinitiators.
Owner:HUBEI GURUN TECH CO LTD
Bis carbonyl indole compound and synthesis method
ActiveCN104817483AEasy to manufactureHigh yieldOrganic chemistryLuminescent compositionsSynthesis methodsStrong acids
The present invention discloses a method for double carbonylation of indole, wherein alpha-hydroxyketone and indole are adopted as reaction raw materials, oxygen is adopted as an oxidizing agent, and a reaction is performed in benzene at a temperature of 80 DEG C under the effect of a copper catalyst to obtain the bis carbonyl indole compound represented by a formula (III). According to the present invention, advantages of high efficiency of the reaction, high yield, application of the oxygen as the oxidizing agent, mild reaction conditions, no requirement of strong acid and strong alkali, catalysis with the cheap metal, easy reaction substrate preparation, and reaction amplification achieving are provided.
Owner:EAST CHINA NORMAL UNIV
Thermosensitive recording medium
To provide a thermosensitive recording medium, containing: a support; a thermosensitive recording layer; and a surface layer, where the thermosensitive recording layer and the surface layer are provided over the support, wherein the thermosensitive recording layer contains a binding agent, a coloring agent, and a color developer, and wherein the surface layer contains polyester (meth)acrylate having at least three (meth)acryloyl groups, and an α-hydroxyketone-based polymerization initiator having a melting point of 80° C. or higher.
Owner:RICOH KK
Application of a Chiral Tridentate Phosphine Nitrogen Oxygen Ligand and Its Related Ligands in Asymmetric Catalytic Reactions
ActiveCN105732725BEasy to controlImprove responseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric hydrogenationNitrogen
Owner:WUHAN CATALYS TECH CO LTD
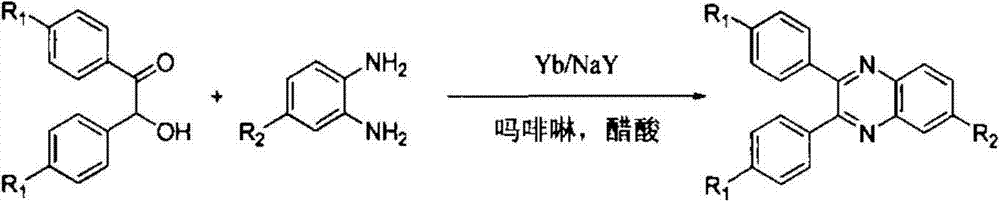
Method for synthesizing quinoxaline compound under catalysis of Yb/NaY molecular sieve catalyst
InactiveCN103787991AEasy to storeKeep intactOrganic chemistryMolecular sieve catalystsChromatographic separationQuinoxaline
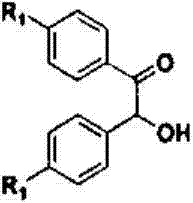
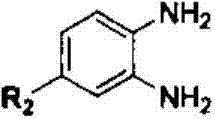
The invention relates to a method for synthesizing a quinoxaline compound under catalysis of a Yb / NaY molecular sieve catalyst. The method specifically comprises the following steps of (a) mixing alpha-hydroxyketone, o-phenylenediamine, morpholine and the Yb / NaY molecular sieve catalyst, adding a solvent, and performing stirring and refluxing for 4 to 6 hours at 75 to 85 DEG C; (b) filtering the mixture obtained in the step (a), recycling a filter residue which is the Yb / NaY molecular sieve catalyst, and performing column chromatography separation on filtrate to obtain the quinoxaline compound. Compared with the prior art, a method for synthesizing the Yb / NaY molecular sieve catalyst is simple and metal load rate is high; the catalyst is high in thermal stability and easy to store and recycle; device characteristic detection shows that integrated molecular sieve pore passages and crystal structures of the catalyst are maintained; during condensation reaction between the alpha-hydroxyketone and the o-phenylenediamine under the catalysis of Yb / NaY, reaction conditions of are mild, simplicity in operation is ensured, the yield of a target product is 84 to 93 percent, and reduction in the cyclic catalysis efficiency of Yb / NaY is avoided.
Owner:TONGJI UNIV
Processes for production of alpha-aminooxyketones and alpha-hydroxyketones
InactiveUS20070055081A1Easy to getHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAnticarcinogenOxygen
The present invention provides a method for easily obtaining α-aminooxyketone compound which is a synthetic equivalent for monosaccharide and pentoses, and a equivalent of α-hydroxyketone compound that can be synthetic intermediates of various physiologically active materials, in high yield; to pave the way for the synthesis of monosaccharide and furthermore of oligosaccharide from the resulting α-hydroxyketone compound induced from α-aminooxyketone compound; and to open new possibilities for the synthesis of various sugar medicines such as anticancer agents, antithrombogenic agents, anti-viral agents, anti-HIV agents, inhibitors of cholesterol synthesis, verotoxin neutralizing agents. According to the invention, a carbonyl compound is allowed to react with a nitroso compound to produce an α-aminooxyketone compound using a catalyst containing a heterocyclic compound shown in the general formula (I) (wherein X1, X2 and X3 independently represent nitrogen, carbon, oxygen or sulfur; and Z represents a substituted or unsubstituted 5- to 10-membered ring).
Owner:JAPAN SCI & TECH CORP
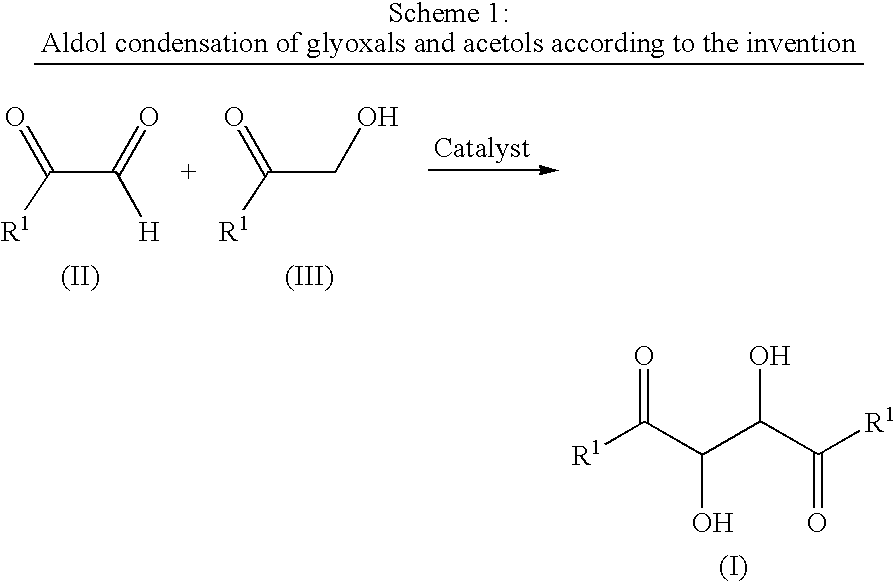
Process for the preparation of 1,4-dialkyl-2,3-diol-1,4-butanedione
The present invention relates to a process for the preparation of compounds of a 1,4-dialkyl-2,3-diol-1,4-butanedione by a catalytic aldol condensation between an alkyl glyoxal and an α-hydroxy ketone.
Owner:FIRMENICH SA
Method for preparation of alpha-hydroxy ketone from ethynylation reaction byproduct
ActiveCN110511127AImprove conversion rateHigh selectivityOrganic compound preparationCatalyst activation/preparationRutheniumAlpha hydroxyketone
The invention provides a method for preparation of alpha-hydroxy ketone from an ethynylation reaction byproduct. The ethynylation reaction byproduct is adopted as the raw material, and loaded metal ruthenium is adopted as the catalyst for Meyer-Schueter rearrangement reaction on alkynediol substance in the raw material, thus obtaining alpha-hydroxy ketone. The product has high selectivity, the ethynylation reaction byproduct is comprehensively utilized, the product value is improved, and the method adapts to the industrial production requirements.
Owner:WANHUA CHEM GRP CO LTD
Active energy ray curable offset ink composition
The present invention addresses the problem of providing an active energy ray curable offset ink composition which is more suitable for offset printing while also exhibiting favorable curability, and low toxicity and low migration properties for use as toy or food packaging. An active energy ray curable offset ink composition containing a coloring pigment, a photoinitiator composition (A), a photoinitiator composition (B), a tetrafunctional or higher polymerizable acrylate monomer, and a resin oligomer having a polymer group, the active energy ray curable offset ink composition being characterized in that (A) is an α-aminoalkylphenone photoinitiator having a number average molecular weight of 350-1000, inclusive, and (B) is an α-hydroxyketone photoinitiator having a number average molecular weight of 320-1000, inclusive.
Owner:DAINIPPON INK & CHEM INC
UV-LED curing initiation system, sealing glue, and preparation method and application of sealing glue
ActiveCN107964057AIdeal three-dimensional structureMeet the use requirementsNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesSurface layerHydrogen
The invention relates to a UV-LED curing initiation system, a sealing glue, and a preparation method and an application of the sealing glue, and belongs to the technical field of chemical materials. The UV-LED curing initiation system is prepared mainly from the following raw materials in parts by weight: 2-8 parts of a cracking type free radical photoinitiator and 2-5 parts of a hydrogen abstracting type free radical photoinitiator; the cracking type free radical photoinitiator is at least one of alpha-hydroxyketone derivatives and acyl phosphine oxides; the hydrogen abstracting type free radical photoinitiator is at least one of thioxanthone or thioxanthone derivatives. The UV-LED curing initiation system is used with combination of short-wave photoinitiators and long-wave photoinitiators, can ensure the curing efficiency of a surface layer and an inner layer of the glue, ensures the curing quality and solves an oxygen polymerization inhibition phenomenon. Moreover, the UV-LED curinginitiation system itself is OK to acid and alkali tolerance, and cannot affect the tolerance of the sealing glue to acids and alkalis, so as to meet the use requirements of the sealing glue in a glass thinning process.
Owner:广州惠利电子材料有限公司
Interior decoration coating for automobile lamp with good adhesion force
The invention relates to an interior decoration coating for an automobile lamp. The coating comprises the following components by weight: 25 to 35% of novolac epoxy resin, 20 to 30% of acrylic resin, 5 to 10% of polyester resin, 3 to 5% of TM resin, 2 to 7% of TP resin, 2 to 3% of an initiator, 3 to 4% of an auxiliary agent and 30 to 40% of a solvent, wherein the solvent is prepared from mixing propyl butyl ester and ethyl ester with a weight proportion of 10 to 20% of propyl butyl ester and 80 to 90% of ethyl ester; the initiator is one or two selected from the group consisting of benzophenone and alpha-hydroxy ketone; and the auxiliary agent is one or more selected from the group consisting of a drying promoter, a toughening agent, a defoaming agent, a leveling agent, a light stabilizer, etc.. The interior decoration coating for the automobile lamp is effectively regulated in an internal chemical structure and a molecular structure of the coating; and the molecular content of the coating is in a range of 15000 to 30000, so depainting, dark-moire and yellowing phenomenons of a modified resin coating in the prior art are changed.
Owner:江苏飞翔涂料有限公司
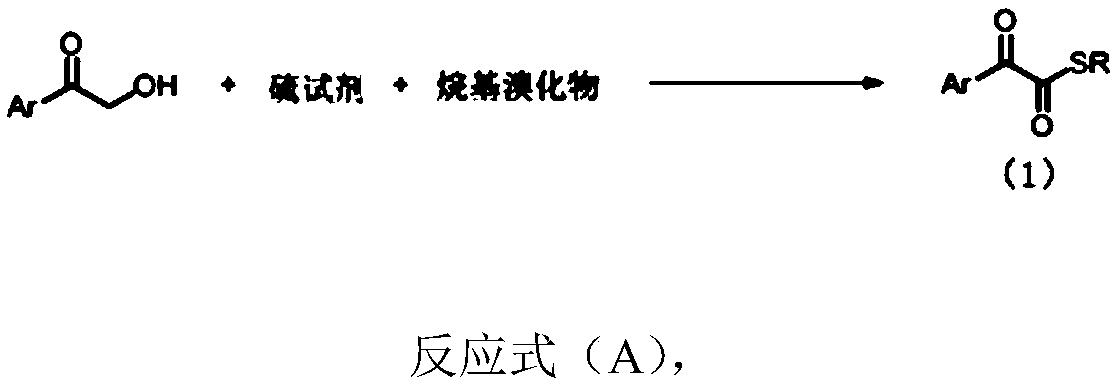
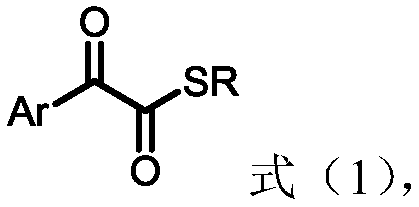
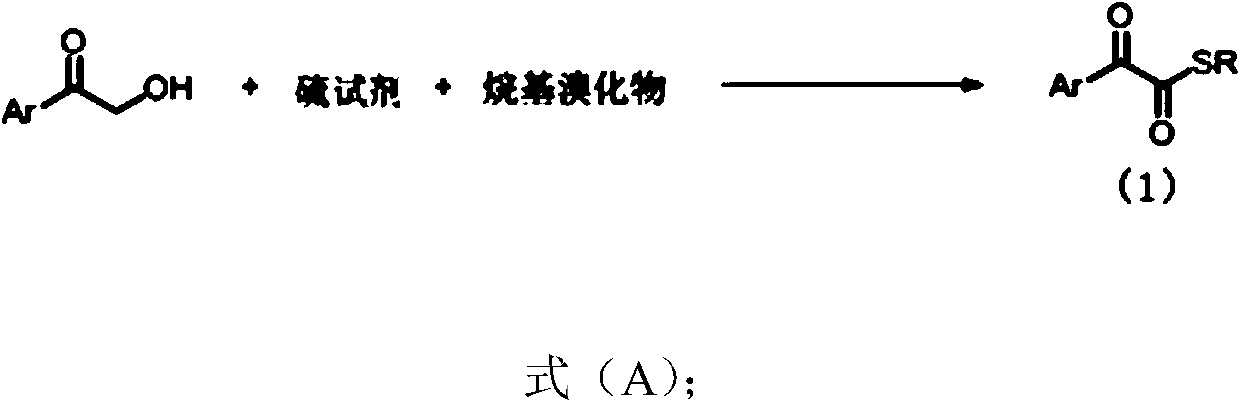
1,2-dicarbonyl compounds and synthesis method thereof
InactiveCN110015983AWide applicabilityThe synthesis method is simpleOrganic chemistrySynthesis methodsSulfur
The invention discloses a synthesis method of new 1,2-dicarbonyl compounds. The 1,2-dicarbonyl compounds are represented by a formula (1), and the synthesis method comprises the steps: adopting alpha-hydroxyketone, a sulfur reagent and alkyl bromide as reaction raw materials, and performing a reaction in a solvent under the action of alkali and additives so as to obtain a series of the new 1,2-dicarbonyl compounds. The 1,2-dicarbonyl compounds are synthesized in one step by using the sulfur reagent as a sulfur source under the condition of no metal catalysis, and the disadvantage that unstableacyl chloride is utilized to synthesize 1,2-dicarbonyl compounds in a conventional method is overcome; and the synthesis method is simple, the raw materials are cheap and easily available, the substrate has wide universality, and the yield of 45-86% is good.
Owner:EAST CHINA NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com