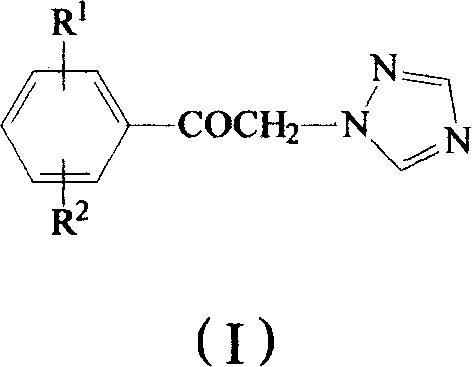
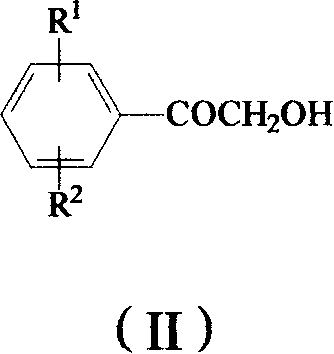
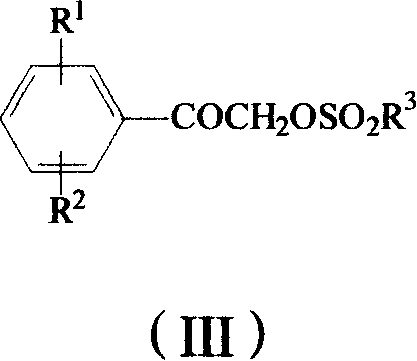
Method for preparing omega-(1H-1,2,4-triazol-1-yl)-arylethanone
A technology of hydroxyaryl ethyl ketone and aryl ethyl ketone, which is applied in the field of preparation of pesticide and pharmaceutical intermediate ω--aryl ethyl ketone, can solve the problems of low reaction yield and the like, achieves overcoming the low yield and is suitable for Wide range of effects with excellent yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation method of the ω-(1H-1,2,4-triazol-1-yl)-arylethanone of the structure of formula (I) described in the present invention can also further make the halogen-substituted ω-(1H-1, 2,4-triazol-1-yl)-arylethanone is converted into aryloxy-substituted ω-(1H-1,2,4-triazol-1-yl)-arylethanone, namely the method It can further include:
[0047] (c) A preparation method of ω-(1H-1,2,4-triazol-1-yl)-arylethanone of structure of formula (Ib)
[0048]
[0049] In formula (Ib), R 1 Same as the definition in general formula (I), R 2 is phenoxy or is composed of halogen, alkyl, alkoxy, cyano, nitro or CF 3 Substituted phenoxy group; Make the omega-(1H-1,2,4-triazol-1-yl)-arylethanone represented by the structure of formula (Ia)
[0050]
[0051] In formula (Ia), R 1 Same as defined in general formula (I); R 2 Is a halogen; further etherification reaction with potassium phenate or sodium phenate in an organic solvent, converted into the corresponding ether compo...
Embodiment 1
[0064] Preparation of ω-formyloxy-2,4-dichloroacetophenone
[0065] Add 7.0g (0.12mol) of anhydrous sodium formate and 0.5g of tetrabutylammonium bromide into a solution consisting of 22.5g (0.1mol) of ω, 2,4-trichloroacetophenone and 100mL of toluene under stirring . The resulting mixture was heated to reflux, and continued to stir under reflux conditions, and the chromatographic trace was followed until the conversion of ω, 2,4-trichloroacetophenone was basically complete, which took about 5 hours. After the reaction was completed, it was washed with water, the organic layer was dried with anhydrous Na2SO4, and the solvent was precipitated under reduced pressure. The residue was 20.0 g, which was light brown viscous liquid, and the content of HPLC analysis was 96.1%. 1 H NMR: δ 5.37(s, 2H), 7.26(d, J=7.4Hz, 1H), 7.40(s, 1H), 7.72(d, J=7.4Hz, 1H), 8.01(s, 1H).
Embodiment 2
[0067] Preparation of ω-acetoxy-2,4-dichloroacetophenone
[0068] With reference to Example 1, 10.0g (0.15mol) anhydrous sodium acetate and 1.0g tetrabutylammonium bromide are added under stirring by 22.5g (0.1mol) omega, 2,4-trichloroacetophenone and In a solution composed of 100mL toluene. The obtained mixture was heated to reflux for 5 hours, and after treatment, 21.6 g of a brown viscous liquid was obtained, and the HPLC analysis content was 98.0%. EIMS (m / z, %): 250(M+4, 0.3), 248(M+2, 0.6), 246(M, 1.0), 186(2), 175(65), 173(100), 145 (21), 109(18), 77(17), 15(17).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com