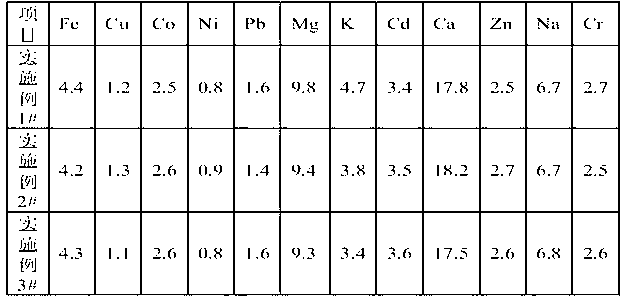
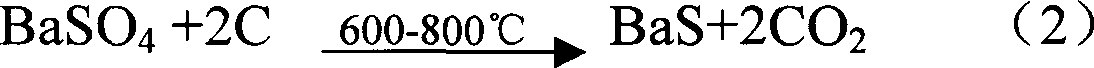Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Barium sulphide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Barium sulfide is the inorganic compound with the formula BaS. BaS is an important precursor to other barium compounds including BaCO3 and the pigment lithopone, ZnS/BaSO4.
Laser engraving methods and compositions, and articles having laser engraving thereon
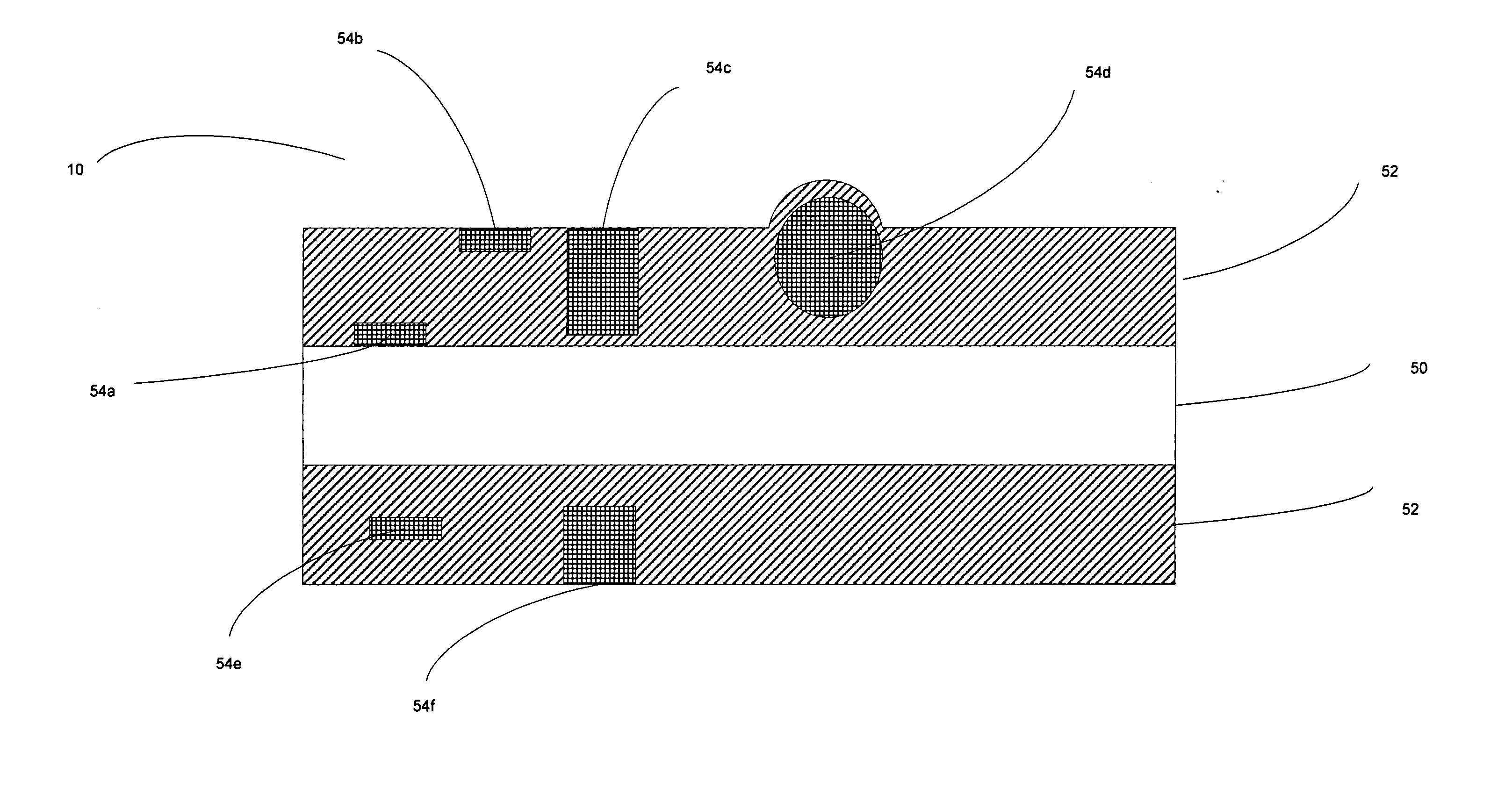
ActiveUS20050095408A1Not easy to counterfeitDifficult to alterRadiation applicationsDecorative surface effectsThioester synthesisBarium sulphide
The invention provides a composition having laser engraving properties, comprising a host material and an effective amount of a laser enhancing additive. The laser enhancing additive comprises a first quantity of least one of copper potassium iodide (CuKI3) or Copper Iodide (CuI), and a second quantity at least one substance selected from the group consisting of zinc sulfide (ZnS), barium sulfide (BaS), alkyl sulfonate, and thioester. The composition can be engraved with grayscale images by an Nd:Yag laser and can be added to laminates or coatings. The composition can be used during the manufacture of many articles of manufacture, including identification documents.
Owner:L 1 SECURE CREDENTIALING
Laser engraving methods and compositions, and articles having laser engraving thereon
InactiveUS20050003297A1Not easy to counterfeitDifficult to alterRadiation applicationsLayered productsThioester synthesisBarium sulphide
The invention provides a composition having laser engraving properties, comprising a host material and an effective amount of a laser enhancing additive. The laser enhancing additive comprises a first quantity of least one of copper potassium iodide (CuKI3) or Copper Iodide (CuI), and a second quantity at least one substance selected from the group consisting of zinc sulfide (ZnS), barium sulfide (BaS), alkyl sulfonate, and thioester. The composition can be engraved with grayscale images by an Nd:Yag laser and can be added to laminates or coatings. The composition can be used during the manufacture of many articles of manufacture, including identification documents.
Owner:L 1 SECURE CREDENTIALING
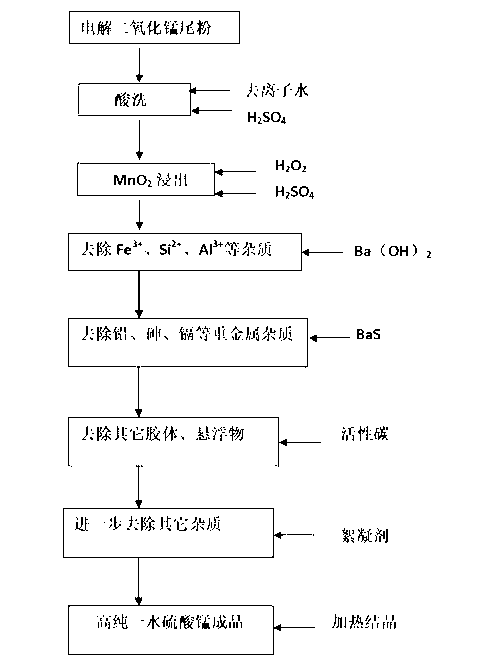
Method for high-purity manganese sulfate monohydrate
InactiveCN103342390ALess impuritiesQuality improvementManganese sulfatesElectrolysisChemical products
The invention relates to the preparation field of chemical products and particularly relates to a method for high-purity manganese sulfate monohydrate. The method specifically comprises the following steps of: taking tail powder of electrolytic manganese dioxide as a raw material, carrying out water washing and acid washing, then carrying out leaching and reducing on MnO2 by taking hydrogen peroxide as a reducing agent, adding barium hydroxide solution, removing Fe<3+>, Si<2+> and Al<3+>, adding barium sulfide solution into the obtained solution, heating the solution to 60-80 DEG C after uniform stirring, carrying out solid-liquid separation, and removing heavy metal elements such as Pb; adding activated carbon in the solution, carrying out stirring and solid-liquid separation at 60-80 DEG C, adding polyacrylamide as a flocculant into the solution after being subjected to activated carbon separation, standing for 24-36 hours after uniform stirring, and carrying out the solid-liquid separation; and carrying out high-temperature crystallization on the obtained solution so as to obtain a manganese sulfate monohydrate product. According to the method, the tail powder produced during the production of the electrolytic manganese dioxide is adequately utilized, the high-purity manganese sulfate monohydrate is produced at a relatively low cost, and then manganese sulfate for ternary materials applied to lithium battery industries is further obtained.
Owner:GUANGXI NANNING SHENGRUI METALLURGICAL & CHEM TECH
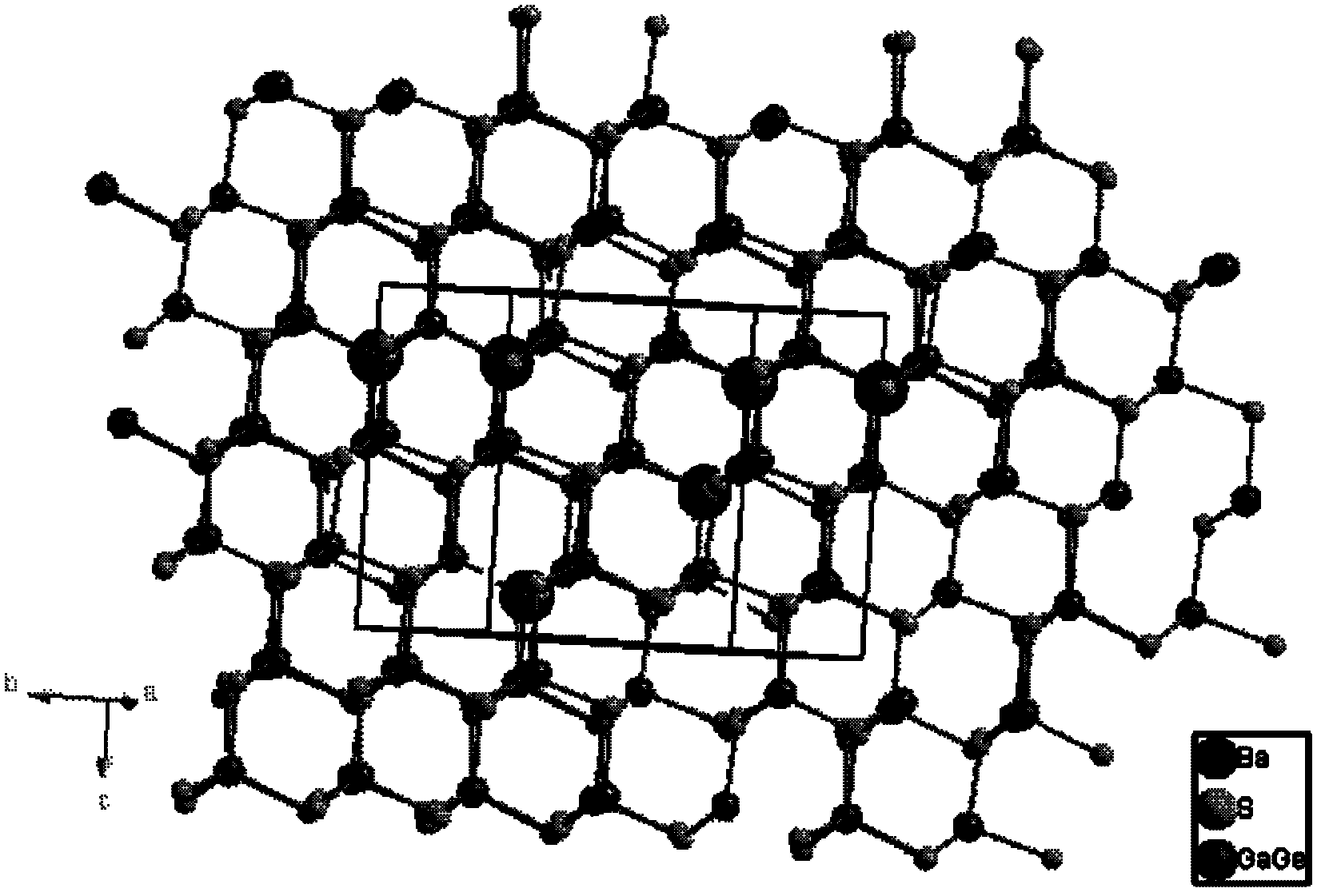
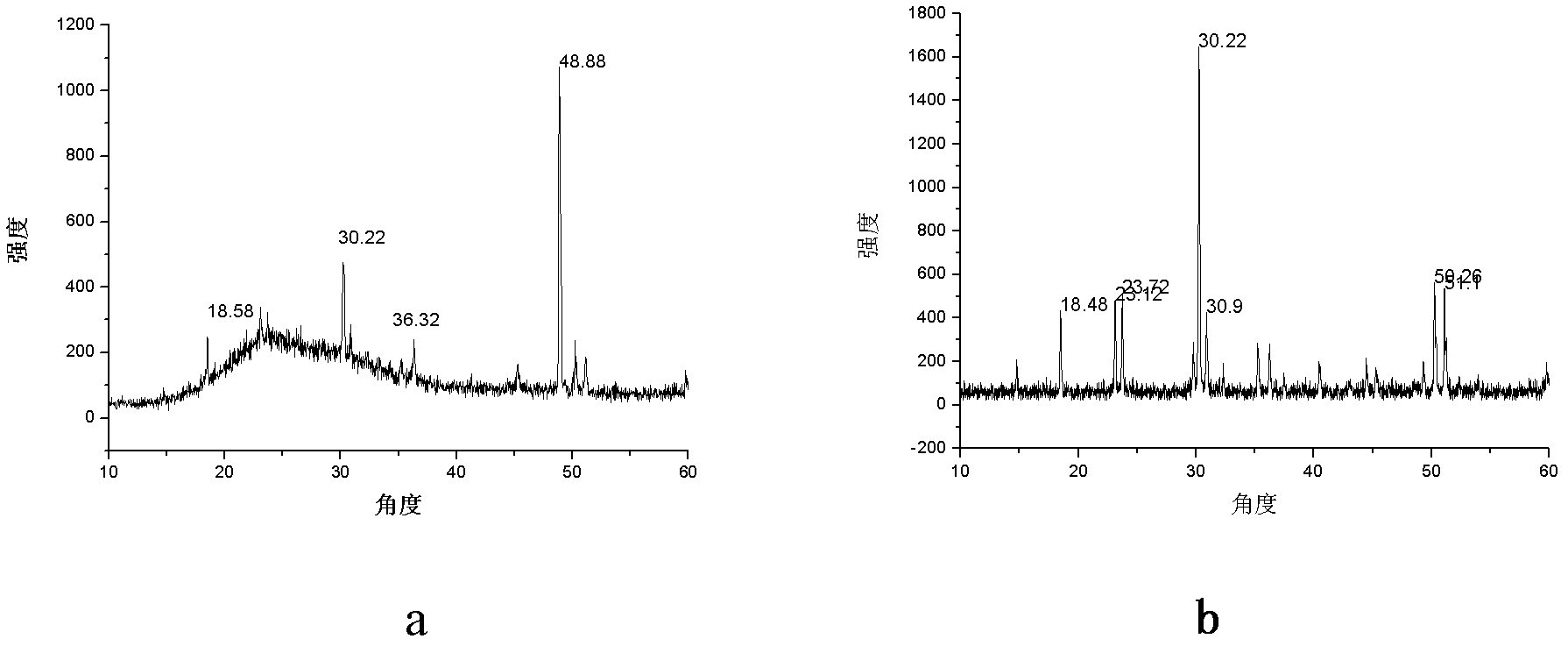
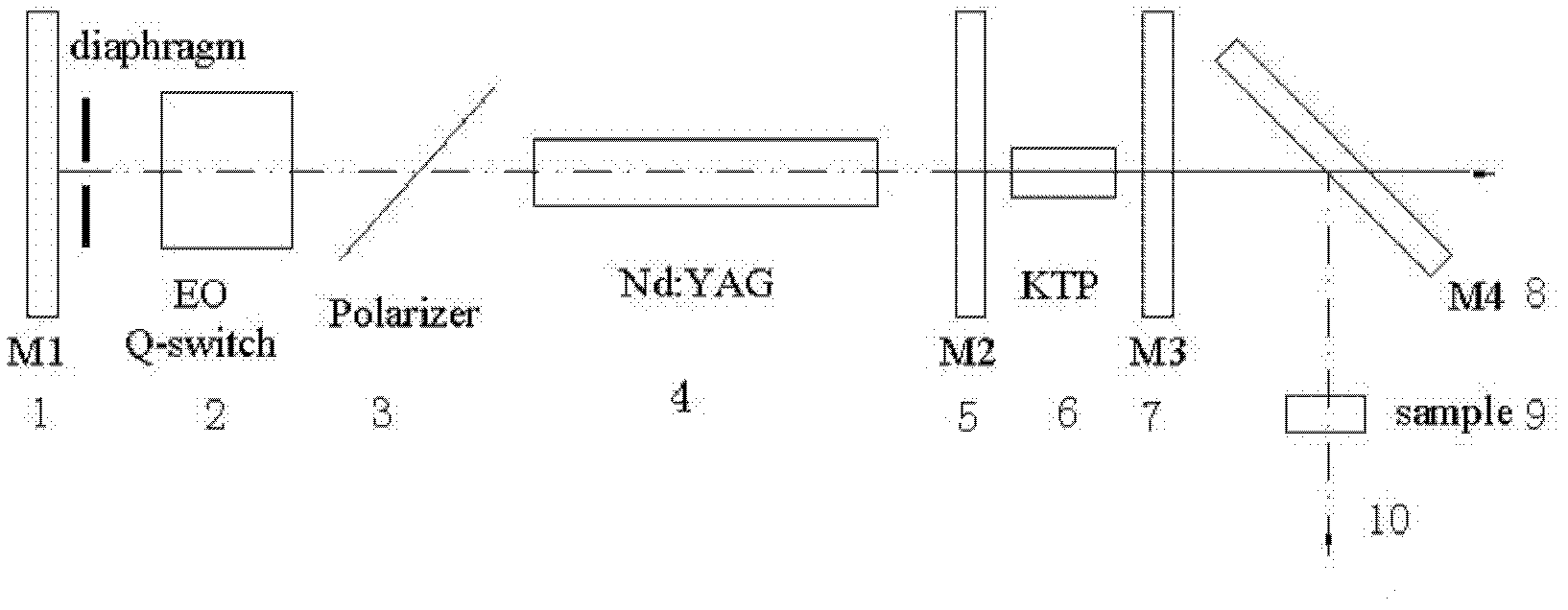
Novel non-linear optical crystal gallium germanium barium sulfide, and growing method and application thereof
ActiveCN102383196AHigh hardnessImprove mechanical propertiesPolycrystalline material growthFrom frozen solutionsNonlinear optical crystalMiddle infrared
The invention provides novel non-linear optical crystal gallium germanium barium sulfide, and a growing method and application thereof. The chemical formula is BaGa2GeS6 (short for BGGS). The novel non-linear optical crystal gallium germanium barium sulfide has the chemical formula of BaGa2GeS6, has an asymmetrical center structure and belongs to a trigonal crystal system, wherein the space group is R3; and cell parameters are that: a=9.5967(5) Angstrom, b=9.5967(5) Angstrom, c=8.6712(7) Angstrom, alpha=90 degrees, beta=90 degrees, gamma=120 degrees, Z=1, and V=691.62 Angstrom3. The clock multiplier factor of the BaGa2GeS6 is 0.8 time that of AgGaS2. The BGGS compound is obtained by sintering at high temperature through a solid phase synthesis method. The BGGS monocrystal can grow successfully by a crucible descending method. The BaGa2GeS6 has a non-linear optical effect, is not dissolved in dilute acid, has high chemical stability, can be widely applied in various non-linear optical fields, and can develop non-linear optical application of a middle infrared band.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Preparation method of electronic grade high-purity manganese sulfate monohydrate
The invention discloses a method for preparing manganese sulfate monohydrate by taking electrolytic manganese and industrial sulphuric acid as raw material; the method comprises the following steps: (1) the electrolytic manganese is ground and sieved to obtain manganese powder; the manganese powder is added in the industrial sulphuric acid and is heated to 80-90 DEG C and reacts for 4-12h continuously under the stirring condition, so as to obtain manganese sulphate suspension; the heavy metallic salt content in the manganese sulphate suspension is measured by atomic absorption spectroscopy; (2) the pH of the suspension is adjusted to 3-4, barium sulphide is added and filtration is carried out, so as to obtain filtrate 1; (3) lime stone is added in the filtrate 1, after the pH value is adjusted to be 5-6 and is placed still, so as to obtain the filtrate 2; (4) sodium fluoride is added in the filtrate 2 for reacting 20-26 hours and then is filtered, so as to obtain manganese sulphate solution; (5) after the manganese sulphate solution is concentrated and recrystallized, centrifugal separation, washing, drying and crashing are carried out, thereby obtaining the manganese sulfate monohydrate; the method has simple production process, easy operation, no pollution and low equipment requirements.
Owner:GUANGDONG GUANGHUA SCI TECH
Method for preparing cell-grade high-purity manganese sulfate by low-grade manganese ore high-pressure crystallization
The invention discloses a method for preparing cell-grade high-purity manganese sulfate by low-grade manganese ore high-pressure crystallization. The method comprises the following steps of grinding low-grade pyrolusite and pyrite into powder particles, preparing a manganese sulfate solution, mixing the manganese sulfate solution and the pyrolusite powder particles to obtain preliminary pulp, mixing the preliminary pulp, concentrated sulfuric acid and the pyrite powder particles according to a certain ratio, carrying out heating, stirring and manganese impregnation on the mixture, adding a neutralizer into the mixture to adjust a pH value, adding barium sulphide and sodium dimethyldithiocarbamate into the mixture to remove impurities so that a pure manganese sulfate solution is obtained, carrying out heating pressurization stirring on the pure manganese sulfate solution, discharging a supernatant to obtain a manganese sulfate crystal-containing solution, carrying out standing precipitation of the manganese sulfate crystal-containing solution at a normal temperature, carrying out filtration to obtain a high-concentration manganese sulfate solution, adding the high-concentration manganese sulfate solution into a high-pressure autoclave, carrying out crystallization at a high temperature under high pressure, discharging a supernatant after crystallization to obtain manganese sulfate crystal-containing magma, separating the manganese sulfate crystal-containing magma to obtain high-purity manganese sulfate crystals, and drying and crushing the high-purity manganese sulfate crystals to obtain the cell-grade high-purity manganese sulfate.
Owner:CENT SOUTH UNIV +1
Process for producing superfine barium carbonate and strontium carbonate
InactiveCN1504411ALow costIncrease productivityCalcium/strontium/barium carbonatesStrontium carbonateStrontium sulfide
A novel process for preparing ultra-fine barium carbonate and strontium carbonate, wherein barium sulphide solution or barium hydrate solution are used as raw material to be carbonized by carbon dioxide in helical channel type revolving bed, obtaining precipitate, then ultramicro fine barium carbonate is obtained through washing, dewatering and drying, and strontium sulfide solution or strontium hydroxide solution are used as raw material to be carbonized by carbon dioxide in helical channel type revolving bed, obtaining precipitate, then ultramicro fine strontium carbonate is obtained through washing, dewatering and drying.
Owner:HENGYANG JINYUAN NANO TECH
Method for natural gas desulphurization and resource utilization of desulphurization waste solution
InactiveCN102703149AReduce consumptionImprove labor productivityCalcium/strontium/barium carbonatesGaseous fuelsSodium bicarbonateSodium hydrosulfide
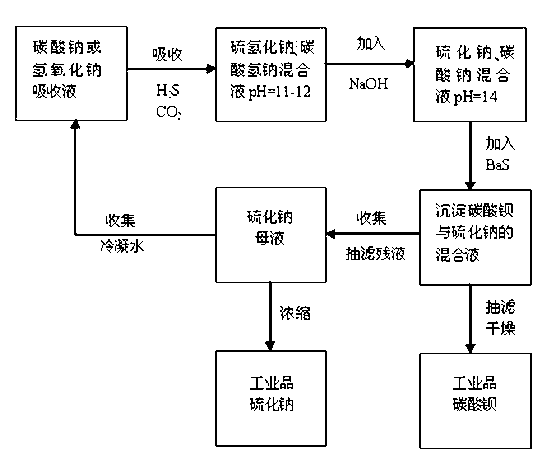
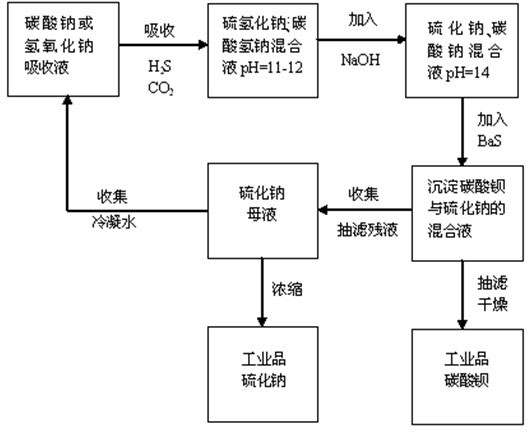
The invention provides a method for natural gas desulphurization and resource utilization of desulphurization waste solution. The method comprises the following steps: determining liquid sodium hydroxide or sodium carbonate as absorption liquid for natural gas to absorb H2S and CO2 in the natural gas, ensuring that the absorption reaction reaches an end point by adjusting the pH value, converting the absorption liquid into absorption liquid of a sodium sulfhydrate or sodium bicarbonate system, adding sodium hydroxide solution, further converting the absorption liquid into the absorption liquid of the sodium sulfide and sodium carbonate system, then adding liquid barium sulfide, and extracting industrial products barium carbonate and sodium sulfide after reaction. The method is totally different from the conventional desulphurization technology, realizes clean production without three-waste pollutant discharged in the whole technological process, simply and thoroughly solves the problem that the existing natural gas desulphurization method pollutes the environment, and simultaneously improves the labor productivity, reduces the energy consumption, and has high added value in obtained resource products, simple process flow and obvious environmental and economic benefits.
Owner:赵志军
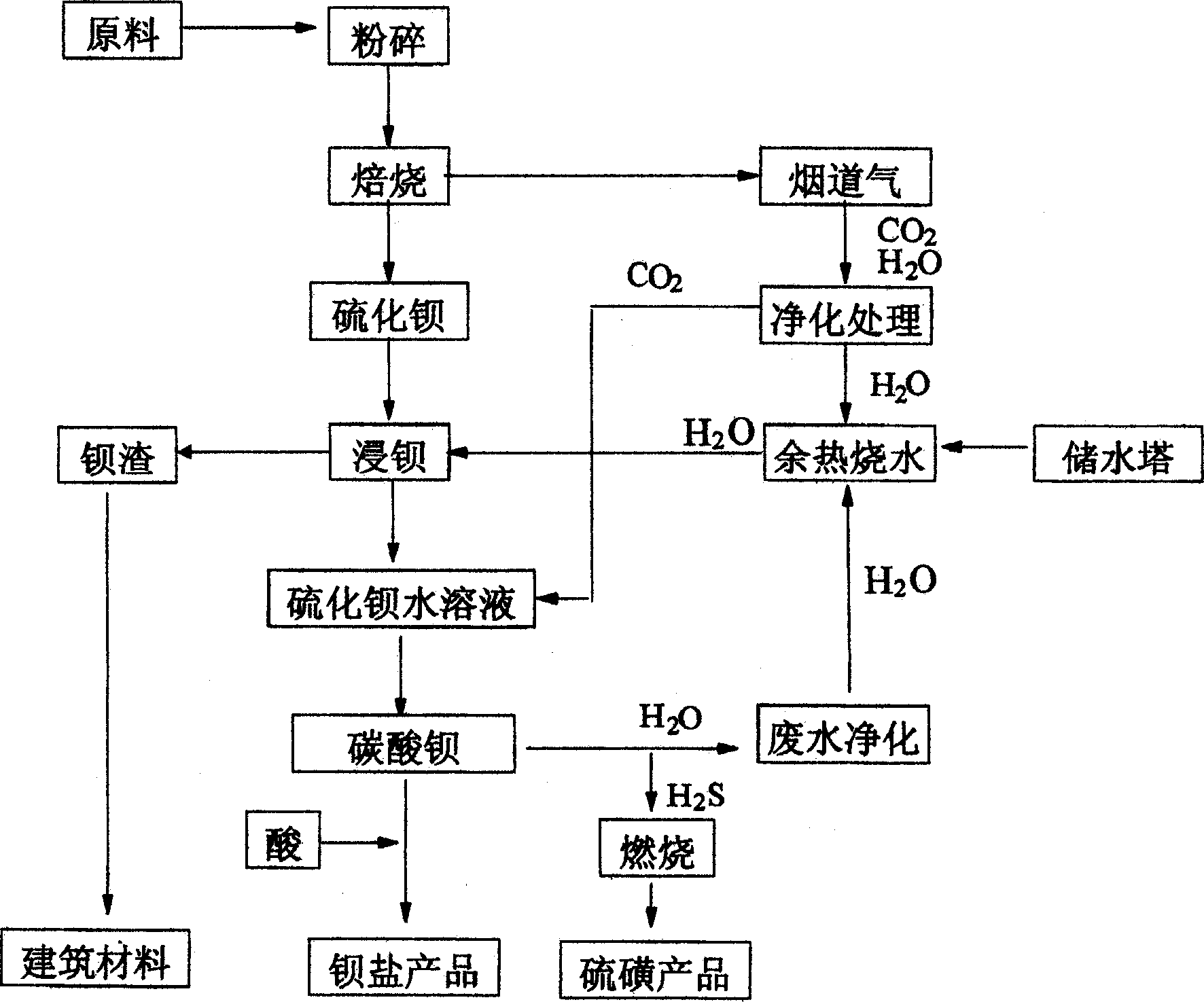
Method of producing barium sulfide and flue gas by calcining coal and barite to synthesize barium carbonate
A process for preparing barium carbonate from coal and barite includes such steps as pulverizing coal and barite, mixing in ratio of 1: 3, calcining in rotary furnace to generate barium sulfide and fume containing CO2 and ash, immersing barium sulfide in water to become aqueous solution, cleaning the fume to obtain CO2 gas, filling it in said aqueous solution of barium sulfide, and reaction to obtain barium carbonate.
Owner:陆巧芳
A method for cultivating special aroma-generating functional bacterial liquid in pit mud and its culture medium
ActiveCN102260639ASolve the problem of lack of compound aroma in wineBacteriaBiotechnologyBarium sulphide
The invention discloses a culture method and culture medium of a cellar mud bacterium liquid with special flavor-producing function. The culture medium comprises the following components in percentage by weight: 3-5% of waste fermented grains, 8-10% of high-quality cellar mud, 10% of bottom fermented grain immersion liquid, 0.2-0.5% of K2HPO4, 0.1-0.3% of NaAC, 0.005-0.01% of MgSO4, 2-4% of 95% alcohol and 5-10% of barium sulfide solution. The cellar mud bacterium liquid with special flavor-producing function, which is cultured by the culture medium, can provide a hexanoic acid bacteria / methane bacteria / nitrate reducing bacteria compound flora which takes effect on producing compound fragrance of a wine body, and solves the problem of lack of compound fragrance of the wine produced in thepresent cellar which serves for a short time.
Owner:SICHUAN MIANYANG FORGOOD DISTILLERY
Preparation method of barium hydroxide
InactiveCN1962451ASimple methodClean manufacturingCalcium/strontium/barium oxides/hydroxidesBarium sulphideSlag
The invention discloses a preparing method of barium hydroxide, which comprises the following steps: (1) reducing barite into barium sulphide through coal; (2) immersing barium sulphide through washing water to obtain mother liquid of barium sulphide; adjusting the temperature of mother liquid of barium sulphide to 30-70 Deg C and density at 50-150g / L; washing filter slag through water; immersing barium sulphide through washing water; (3) oxidizing barium sulphide to produce barium hydroxide, sulfur and manganese monoxide through manganese dioxide; filtering to obtain barium hydroxide mother liquid; obtaining the filter slag with sulfur and manganese monoxide; (4) condensing barium hydroxide; crystallizing; centrifuging; separating to obtain barium hydroxide octahydrate; (5) separating filter slag in the step (3); recycling sulfur; oxidizing manganese monoxide into manganese dioxide through air to circulate in the step (3).
Owner:李守德
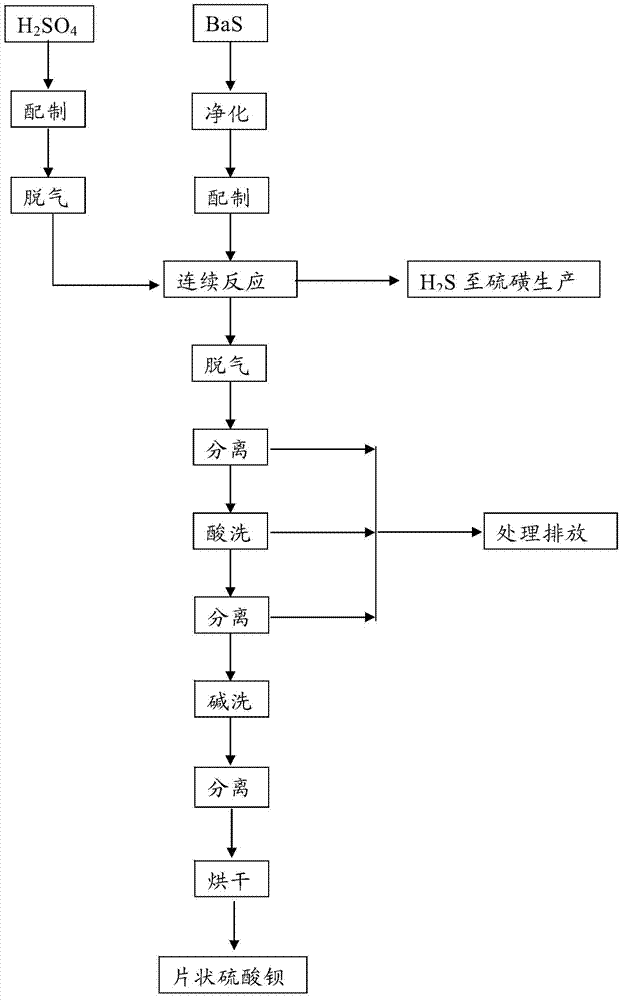
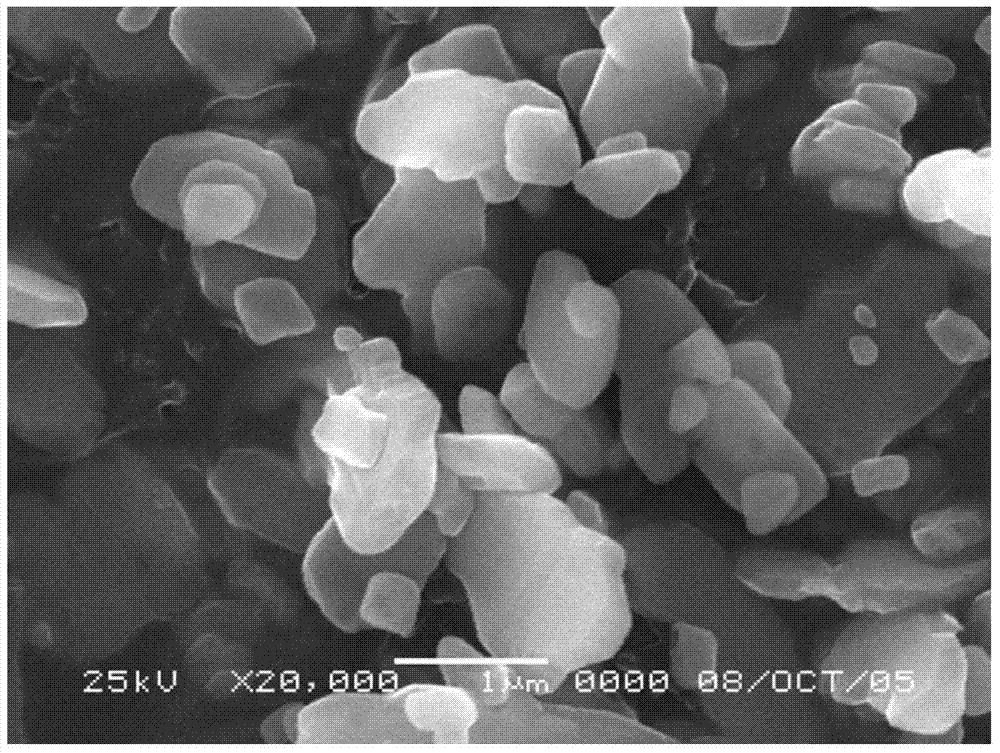
Submicron flake-shaped barium sulfate and preparation method thereof
ActiveCN103693671ASolve technical problems of preparationHigh purityCalcium/strontium/barium sulfatesFiltrationSulfur
The invention discloses a submicron flake-shaped barium sulfate and a preparation method thereof. The preparation method comprises the following steps: (1) pretreating barium sulfate, namely, preparing sulfur into a sulfur aqueous solution with the concentration of 4.0+ / -0.5mol / L, degassing under stirring, and subsequently cooling and filtering so as to obtain pretreated sulfuric acid; (2) pretreating barium sulfide, namely, performing hot filtration on the barium sulfide solution with the concentration of 60-90g / L so as to obtain pretreated barium sulfide; (3) continuously synthesizing, namely, stirring to react the obtained BaS solution with the sulfuric acid obtained in the step (1) in the flow of 600+ / -50L / h, and keeping the concentration of the sulfuric acid in the reaction solution be greater than 3.0g / L in the reaction process so as to obtain reaction slurry; (4) performing an aftertreatment procedure; and (5) performing a finished product procedure so as to obtain the barium sulfate product. The barium sulfate is flake-shaped, the barium sulfate content is within 99.4-99.5%, the whiteness is greater than 99.5%, and D50 is within the range of 0.70-0.80 micron.
Owner:HONGXING XINHUANG EXACT CHEM
Method for treating copper-containing waste acid water
ActiveCN1765764ATake advantage ofWide range of usesWater/sewage treatmentSodium hydrosulfideChemical products
The treatment method for copper-containing acid sewage comprises: treating the sewage with barium sulphide or sodium sulfide to prepare a plurality of chemical products, such as barium sulfate, and can recycle acid liquid with H+ content more than 16% as iron rust remover. This invention has no pollution and well social and economic benefits.
Owner:CHINA PINGMEI SHENMA GRP KAIFENG XINGHUA FINE CHEM
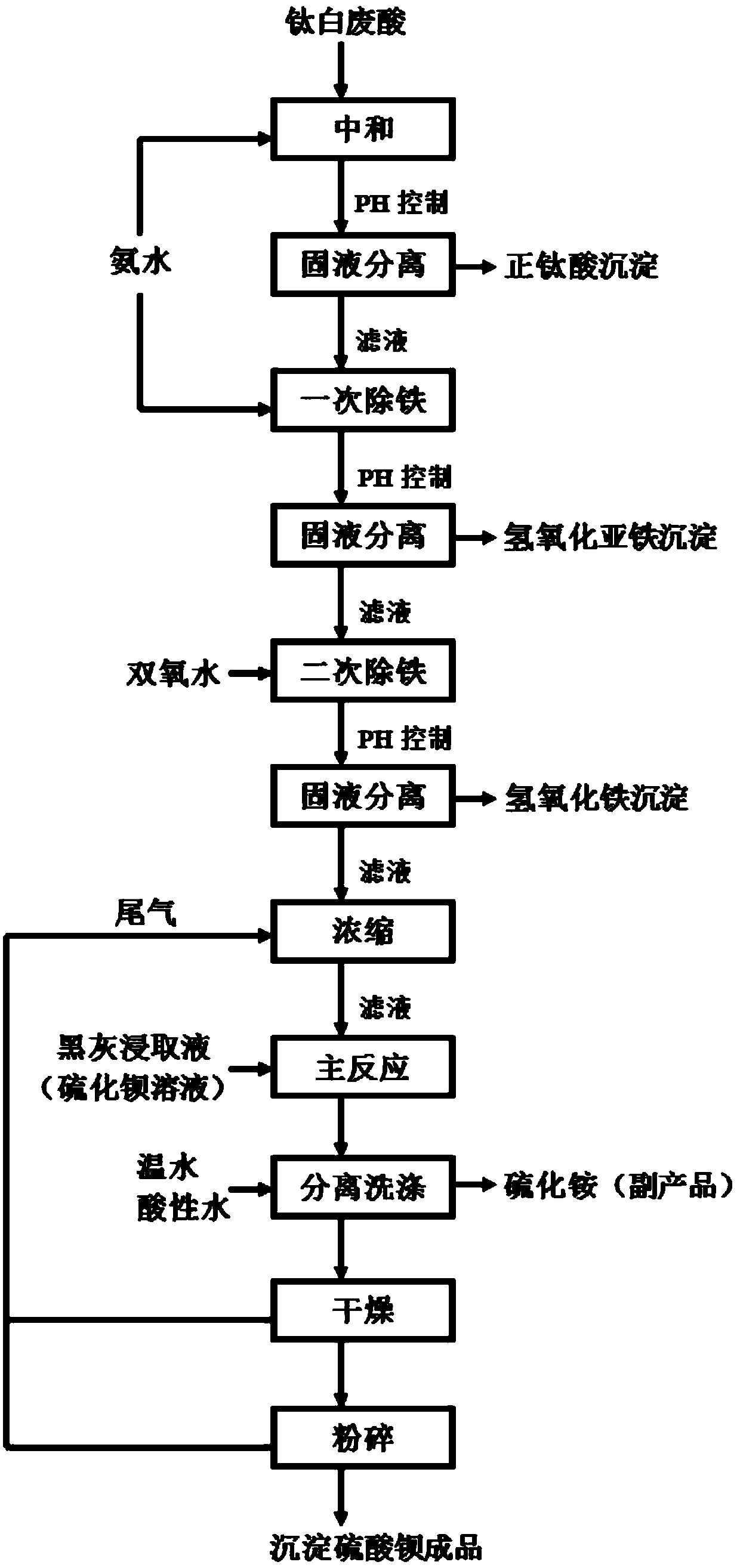
Method for preparing precipitated barium sulfate by utilizing titanium white waste acid
ActiveCN107720801AReduce cost pressureReduce pressure on environmental protectionAmmonium sulfides/polysulfidesCalcium/strontium/barium sulfatesFerric hydroxideSodium sulfate
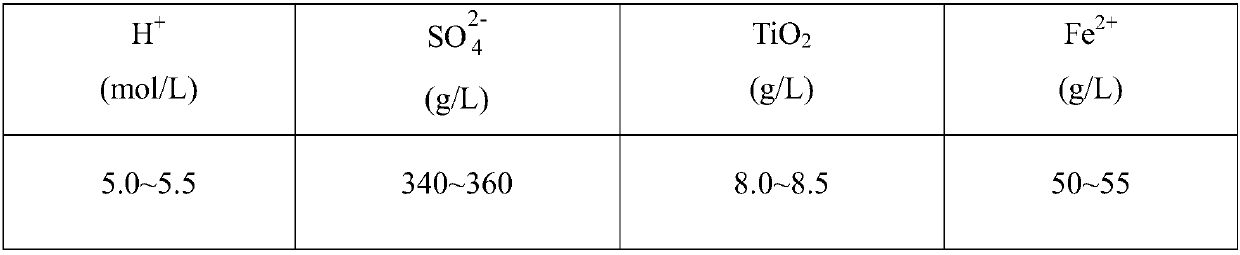
The invention discloses a method for preparing precipitated barium sulfate by utilizing titanium white waste acid, belonging to the technical field of comprehensive utilization of titanium white wasteacid and preparation of barium sulfate. The method comprises the following steps of adding ammonium hydroxide to neutralize with titanium white waste acid firstly, then generating ammonium sulfate, and controlling a systemic pH to separate out photodegredation sediment; then adding the ammonium hydroxide continuously, and controlling the pH to separate out ferrous hydroxide sediment; then addinghydrogen peroxide in proportion, oxidating residual Fe2<+> into Fe3<+>, and adjusting the pH to separate out ferric hydroxide sediment; condensing an ammonium sulfate solution, then replacing a mirabilite solution in a traditional precipitated barium sulfate production process, enabling the ammonium sulfate solution to generate replacement reaction with a barium sulfide solution, and washing, drying and smashing to prepare a precipitated barium sulfate product. According to the method, after neutralizing the titanium white waste acid with the ammonium hydroxide and performing purification andimpurity removal, a traditional sodium sulfate solution is replaced for producing the precipitated barium sulfate, meanwhile, recycled photodegredation sediment can be further used for titanium dioxide production, not only are resources saved, but also waste can be recycled, and the waste acid treatment costs of titanium dioxide plants and environment protection pressure are reduced.
Owner:ANHUI JXTB GRP
Method for preparing high-purity manganese sulfate through extracting low-grade pyrolusite by using rice straw
InactiveCN104195331AAlleviate resource shortagesEnsure sustainable developmentProcess efficiency improvementManganese sulfatesPyrolusiteDiammonium carbonate
The invention provides a method for preparing high-purity manganese sulfate through extracting low-grade pyrolusite by using rice straw. The method comprises the steps of adding a sulfuric acid solution and dry rice straw into pyrolusite, reacting at the temperature of 70-950 DEG C for 8-15h while continuously stirring, and filtering to obtain pyrolusite leachate; respectively removing heavy metal ions such as iron ions, Ni<2+> and Co<2+> as well as calcium and magnesium ions by using an ammonia solution, barium sulfide and ammonium fluoride to obtain an initial solution of manganese sulfate; adding ammonium carbonate to form a white sediment, filtering, mixing the obtained solid and 500-DEG C water, stirring for 10 minutes, filtering, mixing the obtained solid and 500-DEG C water, stirring for 10 minutes, and then, filtering to obtain solid manganese carbonate; and adding the sulfuric acid solution to completely dissolve the solid manganese carbonate, continuing to stir for 30 minutes, heating to concentrate the solution, crystallizing, centrifugally dehydrating, and drying in hot air to obtain high-purity solid manganese sulfate. By using the method, the process flow is simplified, the operation condition is improved, the energy consumption is greatly reduced, and the industrial application prospect is huge.
Owner:HUNAN UNIV OF SCI & TECH
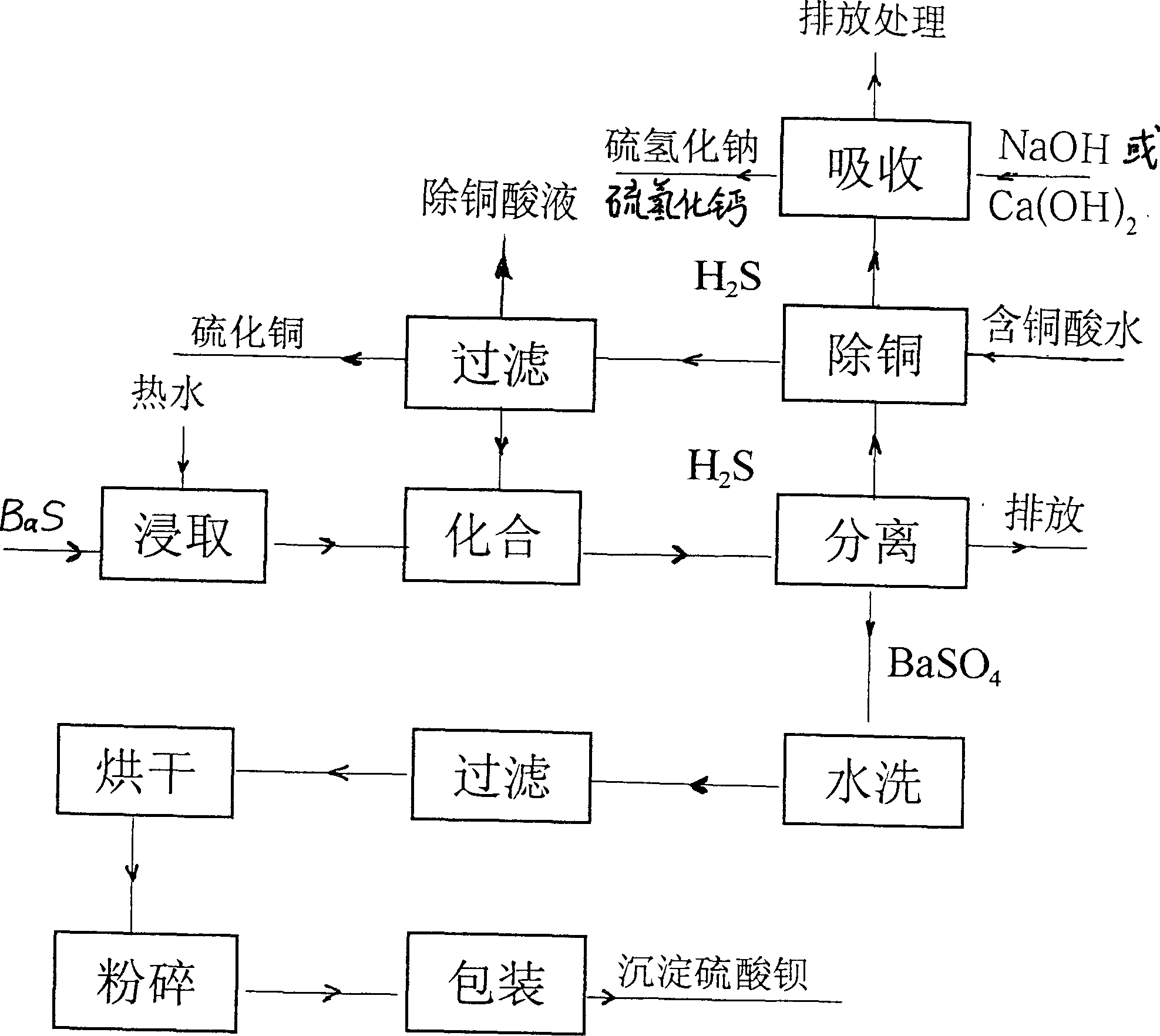
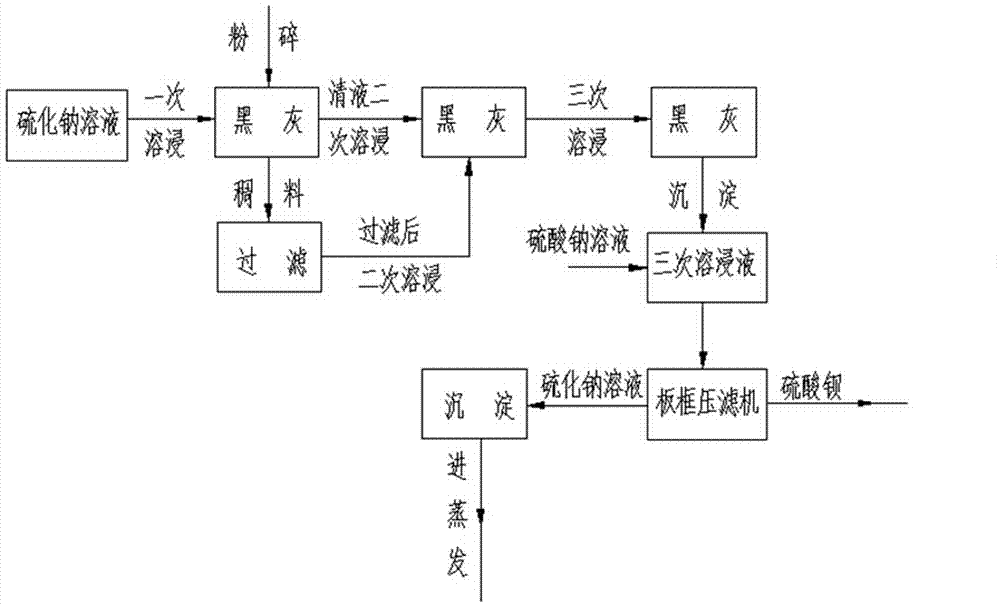
Method for improving sodium sulfide solution concentration by leaching black ash for three times
ActiveCN104326499AIncrease concentrationReasonable designCalcium/strontium/barium sulfatesAlkali metal sulfides/polysulfidesBarium sulphideEnvironmental engineering
The invention discloses industrialized application of a sodium-sulfide solution three-time black-ash (a product which is obtained by calcining barite and has the main composition barium sulfide) leaching technology to barium sulfate production industry, and concretely discloses a method for improving sodium sulfide solution concentration by leaching black ash for three times. The method comprises: using a sodium sulfide solution with the concentration of 4-5% to perform leaching on crushed black ash for three times, namely, performing precipitate separation on a solution subjected to first leaching to obtain a mother liquid I, using the mother liquid I to continue to perform secondary leaching on black ash, and performing secondary precipitate separation on the leached solution to obtain a mother liquid II, using the mother liquid II to performing third leaching, and performing precipitate separation on a solution subjected to third leaching to obtain a mother liquid III, using the mother liquid III to react with a sodium sulfate solution to generate barium sulfate and a sodium sulfide solution which has the concentration of 8-10% after three times of leaching, performing solid-liquid separation on the sodium sulfide solution, and then performing precipitation and clarification, so as to enable the sodium sulfide solution to enter a next process of evaporation. The method is reasonable in design, helps to improve the concentration of the sodium sulfide solution and reducing the evaporation amount by performing leaching on black ash for three times, and realizes the purpose of reducing production cost of 60 alkali (an alkali sulfide with the sodium sulfide content of 60%).
Owner:南风化工(运城)集团有限公司
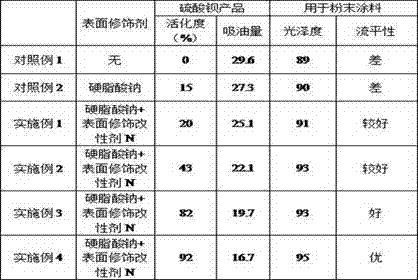
Preparation method of barium sulfate for powder coating
InactiveCN106976900AGood compatibilityImprove bindingCalcium/strontium/barium sulfatesPowdery paintsLipophilicityImpurity
The invention discloses a preparation method of barium sulfate for powder coating. The method comprises the following steps of enabling barite and a mirabilite solution from which calcium, magnesium and other impurities are removed to react to prepare barium sulfate, wherein the barite is calcined and reduced into barium sulfide; modifying the surface of a newly-generated barium sulfate crystal nucleus via surface modifying and a dispersant M, and further modifying the surfaces of barium sulfate particles by a surface modifier N, so that the particles can be effectively combined with organic macromolecules in the powder coating, and well dispersed in the coating. The prepared barium sulfate has the advantages of low oil absorbing amount, good lipophilicity, uniform particle dispersing and the like. The powder coating for production has the advantages of high whiteness, good fluidity, uniform dispersing and the like.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
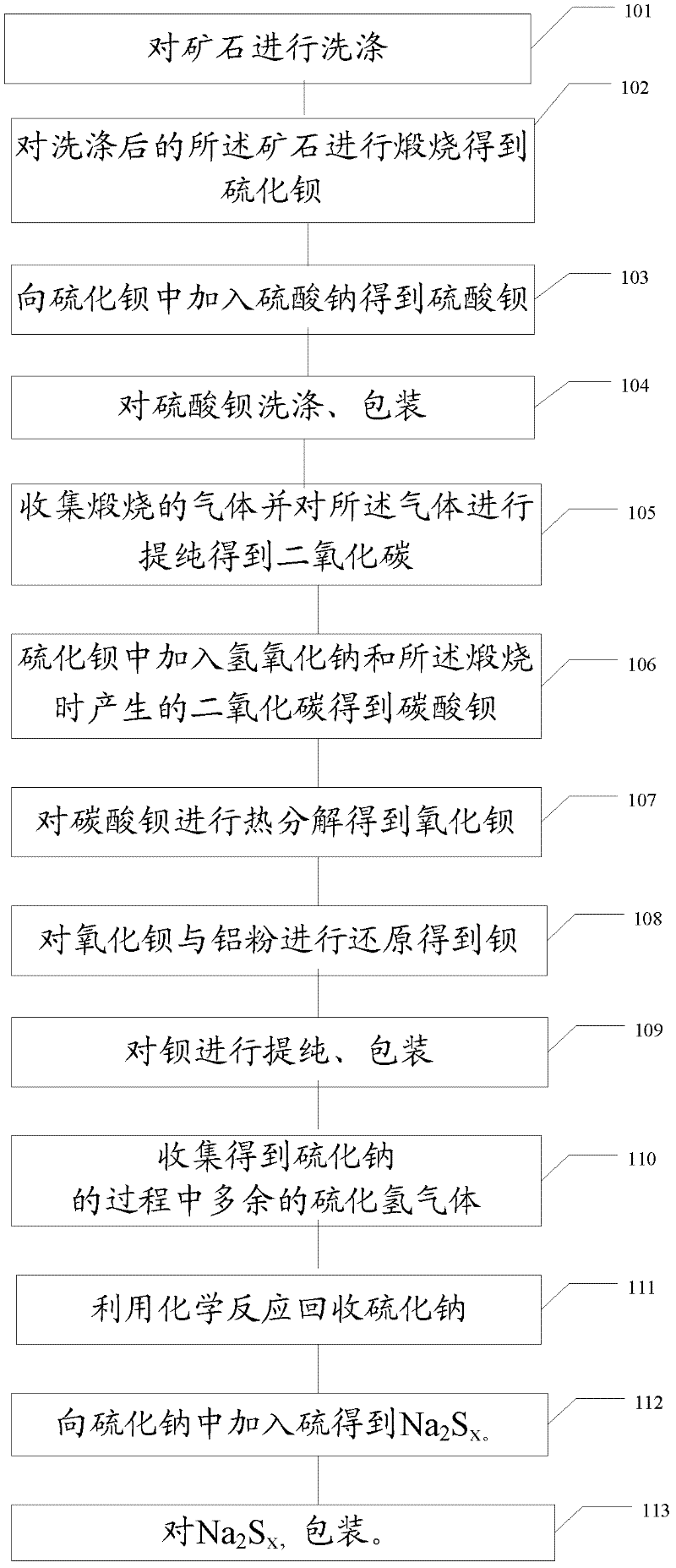
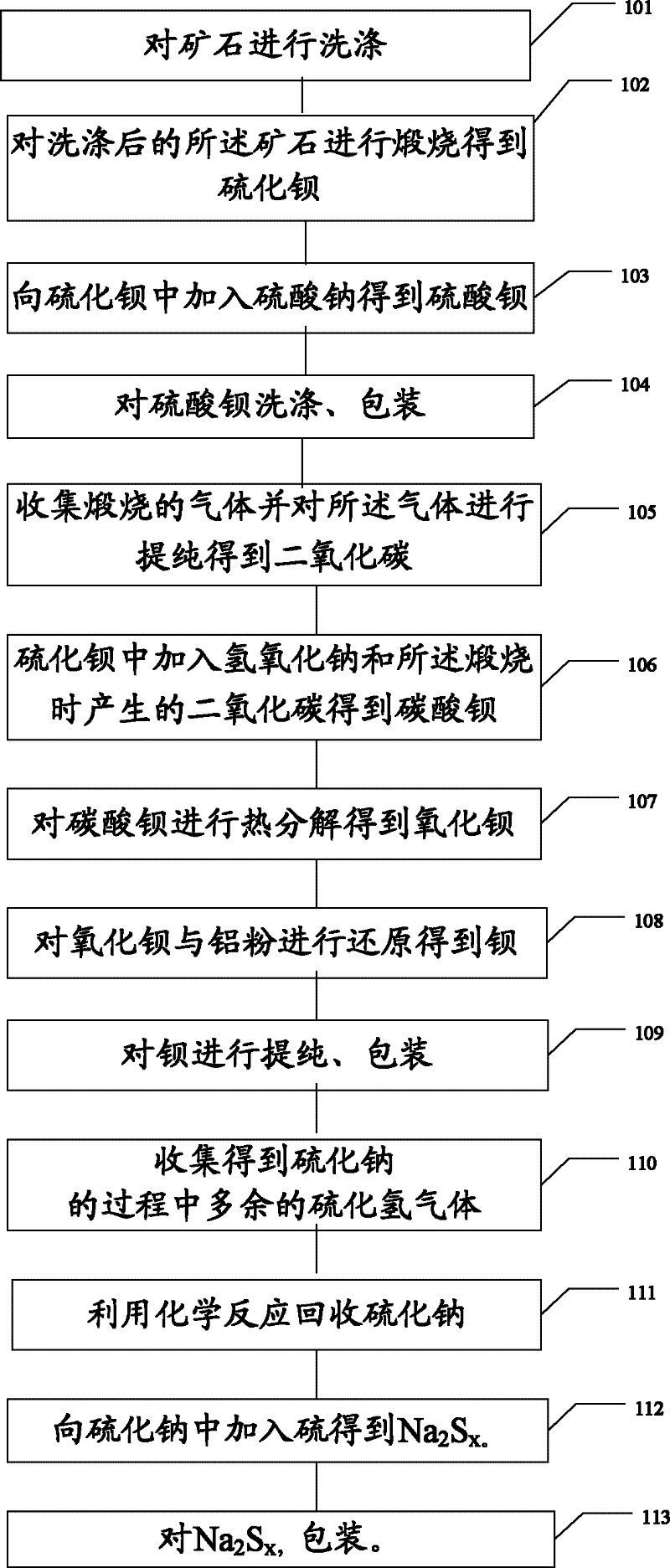
Method and device for extracting barium and barium salt from ore
InactiveCN102505082ATake advantage ofSave resourcesCalcium/strontium/barium compoundsProcess efficiency improvementChemical industryBarium sulphide
The invention relates to the field of chemical industry, and particularly to a method and a device for extracting barium and barium salt from ore. The method comprises the steps of: washing the ore; roasting the washed ore, obtaining barium sulfide; processing the barium sulfide to obtain barium salt, wherein the barium salt includes barium sulfate and / or barium sulfide and / or barium carbonate; and processing the barium carbonate to obtain barium. The device comprises a washing device for washing the ore; a roasting device for roasting the washed ore so as to obtain barium sulfide; a first reacting device for processing the barium sulfide so as to obtain barium salt, wherein the barium salt includes barium sulfate and / or barium sulfide and / or barium carbonate; and a second reacting devicefor processing the barium carbonate so as to obtain barium. The method and the device for extracting barium and barium salt from the ore provided by the invention can make full use of ore resources to obtain barium and barium salt.
Owner:欧阳隆章 +1
Wear-resistant automotive brake pad
InactiveCN104847818AImprove wear resistanceNo noiseOther chemical processesFriction liningAcrylonitrileAluminum silicate
A wear-resistant automotive brake pad is characterized by comprising, by weight, 5 to 10 parts of copper powder,4 to 8 parts of calcined coke, 3 to 6 parts of graphite, 7 to 16 parts of absolute ethyl alcohol, 3 to 7 parts of wetting agent, 6 to 12 parts of aluminum silicate fiber, 2 to 7 parts of potassium titanate whisker, 6 to 10 parts of butadiene-acrylonitrile rubber, 3 to 9 parts of boron phenolic resin, 6 to 10 parts of calcium sulfate whisker, 5 to 8 parts of heavy calcium carbonate, 10 to 16 parts of glass fiber, 4 to 8 parts of molybdenum disulfide, 1 to 2 parts of talcum powder, 8 to 16 parts of melamine resin, and 4 to 7 parts of barium sulfide. The wear-resistant automotive brake pad is excellent in wear resistance, noise free and longer in service life.
Owner:青岛千帆高新技术有限公司
Preparation method of special electrolytic manganese dioxide for automobile power battery
ActiveCN103555940AGood effectRelieve stressElectrolysis componentsProcess efficiency improvementElectrolysisElectrical battery
The invention belongs to the field of battery material processing and specifically discloses a preparation method of a special electrolytic manganese dioxide for an automobile power battery. The preparation method comprises the following steps: performing manganese sulfate leaching, neutralizing and removing iron, removing impurities for heavy metals, electrolyzing and performing post-processing; manganese oxide mineral powder, pyrite powder and industrial sulphuric acid are soaked in the presence of SO2 to prepare crude manganese sulfate liquor; iron is removed by ammonium bicarbonate liquor; heavy metals are removed by utilizing barium sulphide; a manganese dioxide semi-finished product is obtained at a positive electrode through periodic electrolysis; the special electrolytic manganese dioxide for the automobile power battery is finally obtained by post-processing. According to the preparation method disclosed by the invention, the production process is simplified, the production process efficiency is improved, and the performance of obtained products is excellent, and finally, the production cost is lowered.
Owner:广西百色市德柳锰业有限公司
Process for preparing hydrogen sulfide
The invention discloses a process for preparing hydrogen sulfide through the reaction between sulfuric-acid-containing exhausted liquid (by product of the production process for preparing chemical product and intermediate product by means of chemical reactions) and barium sulphide water soluble liquid phase (prepared from barium sulphide calcined member through water leaching), the produced hydrogen sulfide can be further used in preparing other chemical products, such as sodium hydroxide, sodium hydrogen sulfide, ammonium sulfide, ammonium hydrosulfide, sulfourea, methyl hydrosulfide, ethyl mercaptan and dimethyl sulfur ether.
Owner:唐培堃
Hydrogenation catalyst modified by using solid-phase modifier and application of hydrogenation catalyst
ActiveCN110639613AAvoid Toxic ResiduesGood for repeated applicationHydrocarbon by hydrogenationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPalladium catalyst
The invention discloses a hydrogenation catalyst modified by using a solid-phase modifier and an application of the hydrogenation catalyst. The hydrogenation catalyst is a mixture of a supported palladium catalyst and the solid-phase modifier, or a metal palladium material supported by using the solid-phase modifier as a carrier; when the hydrogenation catalyst is the mixture of the supported palladium catalyst and the solid-phase modifier, the mass ratio of the solid-phase modifier to the supported palladium catalyst is (0.1-500):1; and when the hydrogenation catalyst is the metal palladium material supported by using the solid-phase modifier as the carrier, the loading amount of metal palladium is 0.1-20 wt%, wherein the solid-phase modifier is polyphenylene sulfide or a metal sulfide, and the metal sulfide is at least one selected from the group consisting of silver sulfide, barium sulfide, cadmium sulfide, cerium sulfide, ferrous sulfide, ferrous disulfide, lithium sulfide, sodiumsulfide, nickel sulfide, manganese sulfide, molybdenum sulfide, selenium sulfide, tungsten sulfide, zinc sulfide, copper sulfide and titanium sulfide. The hydrogenation catalyst provided by the invention has high catalytic activity in selective hydrogenation of alkynes, and can effectively improve the catalytic selectivity of a target olefin product.
Owner:ZHEJIANG SUPERIOR TECH CORP LTD
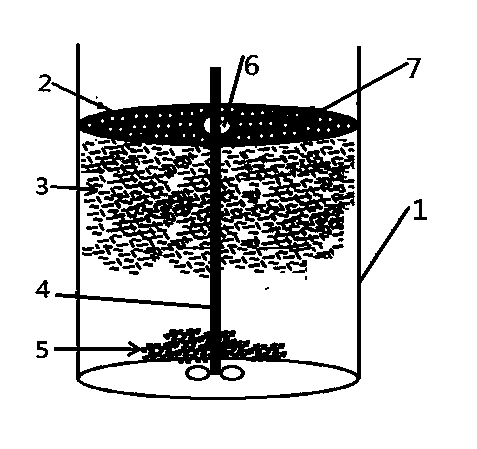
Process for producing barium sulfide
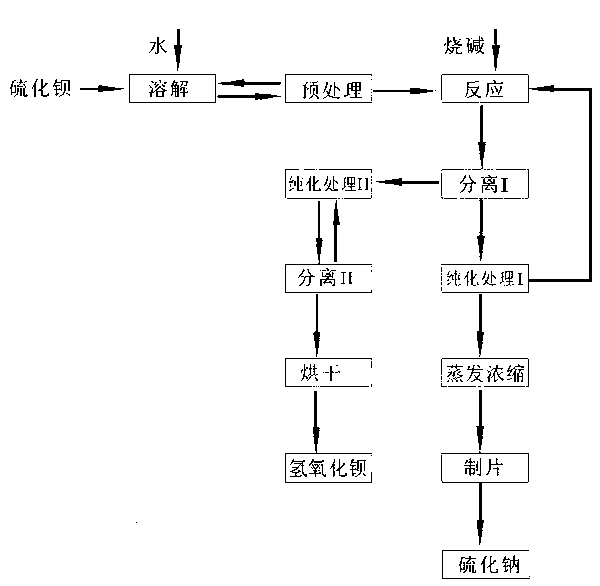
InactiveCN1876564AReduce coal consumptionImprove insulation effectMagnesium/calcium/strontium/barium sulfides/polysulfidesTunnel kilnBarium sulphide
Owner:马武权
Sewage treatment agent
InactiveCN106044895ANothing producedNo corrosionSpecific water treatment objectivesWater/sewage treatmentEthylenediaminePhosphate
The invention relates to a sewage treatment agent, which is composed of the following components: cocoic acid diethanolamide, sodium dioctyl sulfosuccinate, aminotrimethylene phosphonic acid, pyrrolidine, glycolic acid, cinnamaldehyde, barium sulfide, piperazine Pyridine, sodium cellulose, polyaluminum chloride, polyferric chloride, sodium edetate, ammonium aluminum sulfate, polyaluminum sulfate, montmorillonite powder, attapulgite powder, sodium tartrate, sodium borate, amyl acetate , ether, citric acid, sodium cumene sulfonate, o-tert-butylphenol, sorbitan oleate, hydroxyethylethylenediamine, diacetone acrylamide, phosphate, trimethylolpropane, tetratitanate Butyl esters, hydroxyethylcellulose, deionized water. The product of the present invention has a fast reaction speed and no toxic and harmful gases are produced during the process; the product after the reaction is stable and will not be decomposed into toxic substances; it is highly efficient and non-toxic, safe for the human body before and after the reaction, and has no corrosion on objects; it is aimed at sewage treatment efficient.
Owner:李云海
Soilless cultivation nutrient formula of peonies and application thereof
InactiveCN104945162ASynchronous absorptionIncrease resistanceAgriculture gas emission reductionCultivating equipmentsAdditive ingredientNutrient solution
The invention discloses a soilless cultivation nutrient formula of peonies. The soilless cultivation nutrient formula is composed of, by weight, 13-16.5 parts of diphenylamine, 11.5-12.5 parts of ammonium ferric sulfate, 2-20 parts of auxin, 4-5 parts of methionine, 0.4-0.8 part of barium sulfide, 0.1-0.3 part of barium vanadate, 0.1-0.3 part of iodine pentafluoride, 0.05-0.2 part of glycerol phosphate calcium and the balance water. The soilless cultivation nutrient formula is made by weighing and evenly mixing the ingredients according to the ratio. By the adoption of the nutrient liquid, the seepage force and diffusivity of the nutrient liquid are greatly enhanced in the breeding process, so that the seeding stage is greatly shortened, the emergence rate is increased, the quality of emergence is improved, and meanwhile the cost is lowered; the nutrient liquid is more suitable for high-altitude low-temperature seedling cultivation.
Owner:汤在英
Boron fertilizer and its preparing process
The invention discloses a boron fertilizer, which comprises the following parts: 5-50% boric acid, 20-80% sodium tetraborate, 2-10% diboron trioxide and 5-15% boron potahydride, wherein the total weight rate of raw material and catalyst is 100:1-5; each one percent of catalyst is composed of 0.1-0.35% gadolinium chloride, 0.1-0.2% neodymium oxalate, 0.05-0.1% calcium chloride, 0.1- 0.2% barium sulphide, 0.1-0.3% sodium aluminate, 0.1-0.3% sodium phosphate and 0.05-0.2% palladium carbonate.
Owner:HAINAN ZHENGYE ZHONGNONG HIGH TECH
One-step production of barium hydroxide and sodium sulfide by using barium sulfide and sodium hydroxide
ActiveCN102718245AAlkali metal sulfides/polysulfidesCalcium/strontium/barium oxides/hydroxidesBarium sulphidePhysical chemistry
The invention provides a one-step production of barium hydroxide and sodium sulfide by using barium sulfide and sodium hydroxide, that is, industrial barium sulfide is utilized to directly react with an alkaline liquor to produce a barium hydroxide product and a byproduct sodium sulfide. The method comprises the following steps: leaching barium sulfide raw materials, carrying out slag removal on the leachate, then heating and preserving heat, determining the amount of barium ions, adding an alkali lye with the amount of caustic soda being 1-3 times of the amount of the barium ions, and separating the obtained product to obtain barium hydroxide and sodium sulfide. According to the invention, the final product can be obtained by only one step; compared with traditional process routes, the process provided by the invention is more economic, saves equipment cost and production time, omits the step of using hydrochloric acid to process barium sulfide in traditional processes, discharges without harmful gas hydrogen sulfide, is environmentally friendly, fully utilizes raw materials, and realizes atom economy.
Owner:李易东 +2
Method for preparing battery-grade manganese sulfate by separating nickel, cobalt, lithium and manganese from battery black powder
PendingCN113104897AAchieve separationReduce alkali consumptionProcess efficiency improvementManganese sulfatesManganese sulphateLithium
The invention relates to a method for preparing battery-grade manganese sulfate by separating nickel, cobalt, lithium and manganese from battery black powder. The method comprises the following steps: 1) slurrying the battery black powder, adding concentrated sulfuric acid, performing first-stage agitation leaching, controlling the pH value to be less than 1.5 and the temperature to be 85-95 DEG C, and adding a reducing agent; (2) carrying out filter pressing on the first-stage leachate, removing iron and aluminum from filtrate by adjusting the pH value, and then carrying out extraction treatment to separate manganese, cobalt, nickel and lithium, and taking filter residues as raw materials for second-stage leaching; 3) carrying out second-stage reduction leaching on filter residues produced by the first-stage filter pressing, adding the reducing agent, controlling the pH value to be less than 1.5, and controlling the temperature to be 85-95 DEG C; (4) carrying out filter pressing on the leachate, and washing filter residues to obtain graphite residues; 5) adding barium sulfide into the filtrate subjected to second-stage filter pressing, controlling the temperature to be 55-70 DEG C and the pH value to be 3.5-4.5, and reacting for 1-3 hours; (6) carrying out filter pressing on the nickel and cobalt removed solution, wherein the filtrate is a manganese sulfate solution; and 7) adding sodium dimethyl dithiocarbamate and sodium fluoride into the manganese sulfate solution, and carrying out filter pressing to obtain the battery-grade manganese sulfate solution.
Owner:ZHEJIANG TIANNENG NEW MATERIAL CO LTD
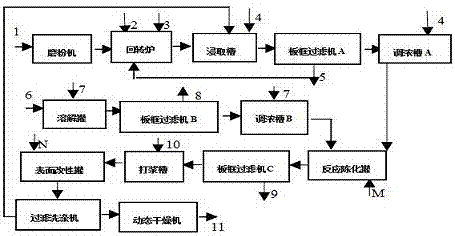
Method for preparing silica modified barium sulfate powder
ActiveCN107746068AHigh activitySmall sizeMaterial nanotechnologyCalcium/strontium/barium sulfatesBarium sulfideSodium silicate
A method for preparing silica modified barium sulfate powder comprises the following steps: adding a barium sulfide solution into a sodium carbonate solution, performing stirring reacting at 20-95 DEGC for 0.5-4 h, filtering the obtained solution, adding the obtained filter cake into a sodium sulfate solution, performing high-shearing stirring reacting at 20-95 DEG C for 0.5-8 h, adding sodium silicate accounting for 0.1-10% of the mole of the barium sulfide, adding a sulfuric acid solution according to a molar ratio of sulfuric acid to the sodium silicate being (1.1-1.2):1, continuously performing stirring reacting for 1-2 h, and sequentially carrying out filtration, water washing and 100-200 DEG C drying to obtain the silica modified barium sulfate. The method has the advantages of simple process, less device investment, and realization of large-scale industrialization.
Owner:CHINA RES INST OF DAILY CHEM IND
Preparation method of manganous-manganic oxide
InactiveCN110697786AAvoid pollutionHealth hazardManganese oxides/hydroxidesManganese(II) carbonateBarium sulphide
The invention discloses a preparation method of manganous-manganic oxide, and belongs to the technical field of manganese compound synthesis. The preparation method comprises the following steps: leaching, namely grinding manganese carbonate ore into powder, reacting with manganese dioxide and sulfuric acid, and filtering; neutralizing, namely adding manganese dioxide, adjusting the pH value to 6-7 with lime, and filtering; removing heavy metals, namely adding barium sulfide, and filtering; removing calcium and magnesium, namely adding ammonium fluoride into a solution obtained after heavy metal removing, and filtering; purifying, namely filtering by using a chemical manganese dioxide filter layer; carrying out hydrolysis precipitation, namely slowly adding the purified solution into ammonia water, continuously stirring, carrying out suction filtration, and carrying out vacuum drying on a filter cake; and carrying out oxidation,namely preparing a suspension from the dried filter cake,introducing hot air, dropwise adding ammonia water, carrying out suction filtration, and carrying out vacuum drying to obtain manganous-manganic oxide. According to the method, the high-purity manganous-manganic oxide is prepared by removing impurities through a chemical method, purifying and then directly oxidizing, so that the manganous-manganic oxide is directly prepared from manganese carbonate ore, the process is simple, the preparation cost is low, and the prepared manganous-manganic oxide is high in purity and stable in quality.
Owner:四川中哲新材料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com