Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Azacyclopropane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833549B2Reduce morbidityNot impart bitter tasteBiocidePowder deliveryNasal cavityAzelastine
Owner:MANNKIND CORP
Stable formulation components, compositions and laundry methods employing same
InactiveUS6903060B1Improve stabilitySuperior emission lifetimeIsocyanic acid derivatives preparationNon-ionic surface-active compoundsBleachOrganocatalysis
The present invention relates to formulation components, such as organic catalyst compounds having increased stability, compositions and laundry methods employing such organic catalyst compounds. More particularly, this invention relates to organic catalysts compounds such as quaternary imine bleach boosting compounds, quaternary oxaziridinium bleaching species, modified amines and amine oxides, compositions and laundry methods employing such organic catalyst compounds.
Owner:THE PROCTER & GAMBLE COMPANY
Method for performing ring-opening for cyclohexylaziridine by carboxylic acid
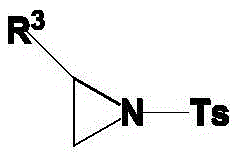
The invention discloses a method for performing ring-opening for cyclohexylaziridine by a carboxylic acid. The method includes that in a polar aprotic solvent system, alkali metal type inorganic base is utilized as a catalyst, a monocarboxylic acid is utilized as a nucleophilic reagent, and the cyclohexylaziridine which is activated by tosyl is subjected to a ring-opening reaction. The method has the advantages that the ring-opening reaction is simple in process, the reaction condition is mild, the solvent is environment-friendly, the carboxylic acid is utilized as the nucleophilic reagent, atom economical requirement of green chemistry is met, the catalytic agent is cheap, and the catalytic activity is high.
Owner:TAIYUAN UNIV OF TECH
Preparation of taurine and derivatives thereof
InactiveCN101255126AEasy to manufactureHigh purityOrganic chemistryOrganic compound preparationThiocarboxylic acidAziridine
The invention provides a novel preparation method for taurine, substituted taurine and N-acylated derivates. The method comprises taking aziridine and substituted aziridine as raw material, obtaining N-acylated taurine derivates by opening loop and oxygenizing, hydrolyzing N-acylated taurine derivates to obtain taurine or substituted taurine. The raw material of the preparation method is simple and easy to get and prepare, especially convenient to separate and purify, and can be used for the preparation of optical activity substituted taurine and N-acyl group substituted taurine. The obtained compounds can be used as nutrient substance, medicament, enzyme inhibitors, antibacterial agents, surface active agents, plant growth regulators and raw materials for preparing sulfonopeptides.
Owner:PEKING UNIV
Water solubility high-speed grinding fluid for machining settlement bearing and preparation method thereof
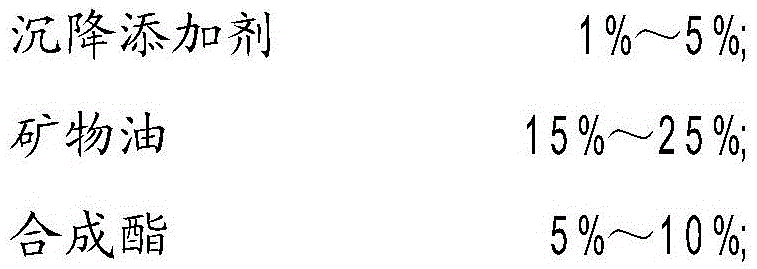
ActiveCN105542931ARaise the pHImprove the lubrication effectLubricant compositionSolubilityHigh-speed grinding
The invention discloses water solubility high-speed grinding fluid for machining a settlement bearing. The water solubility high-speed grinding fluid comprises the following components by weight percent: 1 to 5 percent of settlement additive; 15 to 25 percent of mineral oil, 5 to 10 percent of synthetic ester, 2 to 5 percent of organic acid, 8 to 15 percent of organic amine, 5 to 10 percent of surface active agent, 15 to 20 percent of antirust additive, 2 to 5 percent of coupling agent and the balance of distilled water. The settlement additive is a mixture of fatty amine polyoxyethylene ether, aziridine and dibenzyl ether. The water solubility high-speed grinding fluid has excellent rust resistance, wettability, lubricity and grinding ash settling performance. Meanwhile, the invention provides a preparation method of the water solubility high-speed grinding fluid for machining the settlement bearing.
Owner:QUAKER CHEM CHINA
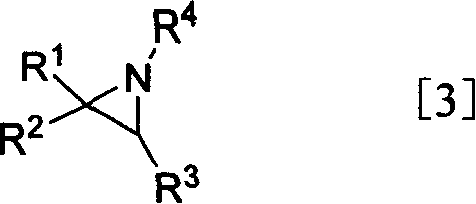
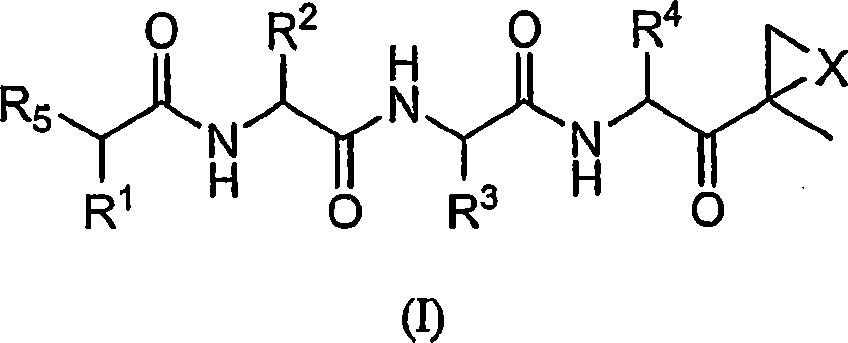
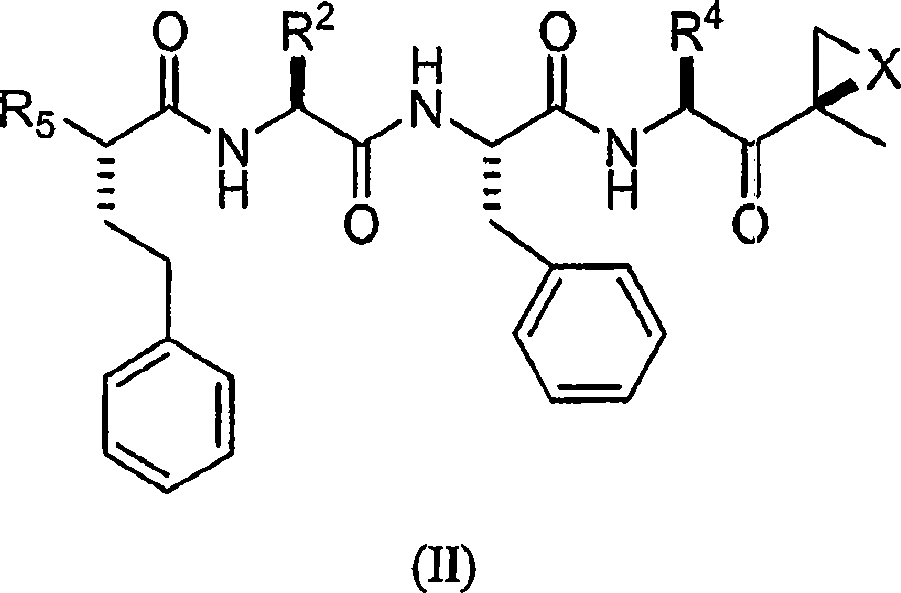
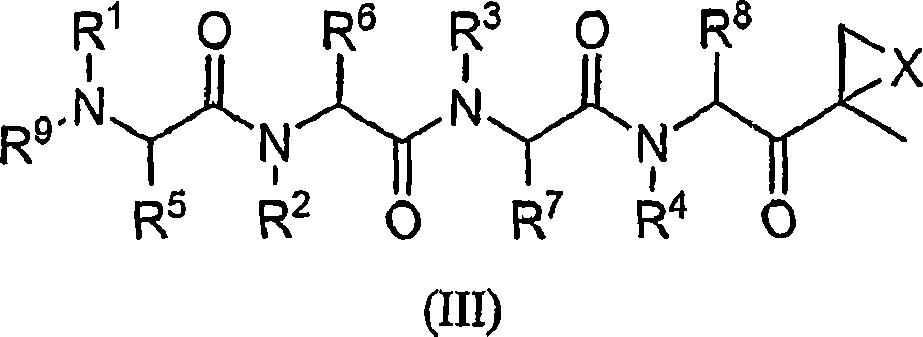
Compounds for proteasome enzyme inhibition
Peptide-based compounds including heteroatom-containing, three-membered rings efficiently and selectively inhibit specific activities of N-terminal nucleophile (Ntn) hydrolases. The activities of those Ntn having multiple activities can be differentially inhibited by the compounds described. For example, the chymotrypsin-like activity of the 20S proteasome may be selectively inhibited with the inventive compounds. The peptide-based compounds include at least three peptide units, an epoxide or aziridine, and functionalization at the N-terminus. Among other therapeutic utilities, the peptide-based compounds are expected to display anti-inflammatory properties and inhibition of cell proliferation.
Owner:ONYX THERAPEUTICS
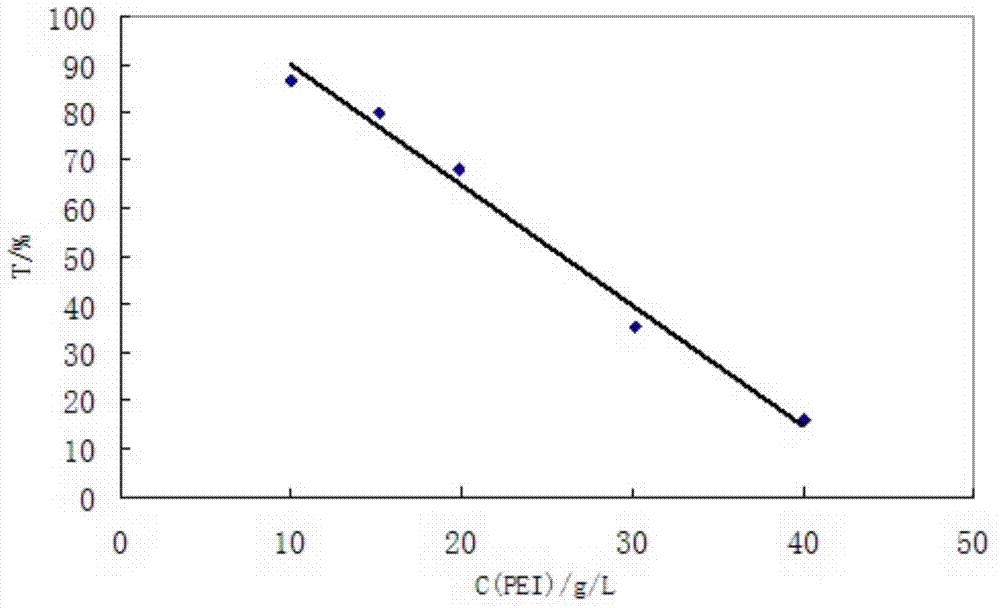
Environment-friendly high strength profile control water shut-off agent and preparation method thereof
The invention discloses an environment-friendly high strength profile control water shut-off agent and a preparation method thereof, and aims to solve the problems that conventional water shut-off agents are pollutant, and long-term use of the conventional water shut-off agent will pollute the environment and harm the human body. The water shut-off agent is composed of high polymers, a cross-linking agent, a catalyst, an antioxidant, and water. Through the organic cooperation among high polymers, a cross-linking agent, and a catalyst, a high strength, environment-friendly, and non-toxic profile control water shut-off agent is prepared, and the water shut-off agent can be widely applied to water shut-off operations of various oil reservoir profile controls. Polyaziridine is taken as the cross-linking agent, and the prepared gel has the advantages of deeper processing ground layer, higher strength, better stability, environment-friendliness, and no toxicity. Experiment results have proven that the water shut-off agent can well improve the profile performance and the water shut-off performance, is capable of being applied to the oil fields, and can improve the development effect of oil field water injection. The water shut-off agent is compounded by high polymers, a cross-linking agent, an antioxidant, and the like, and can be widely applied to water shut-off operations of various oil reservoir profile controls.
Owner:浙江仁智股份有限公司
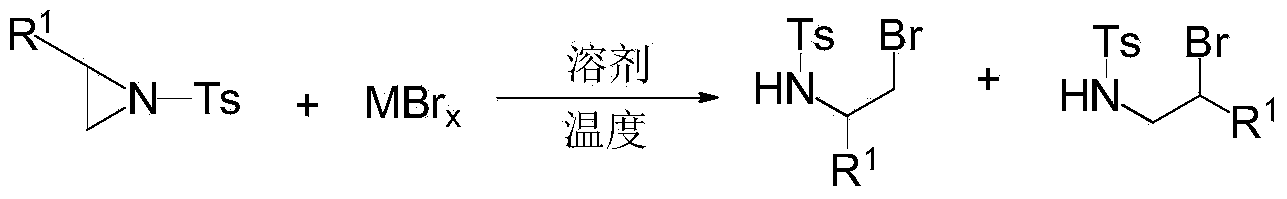
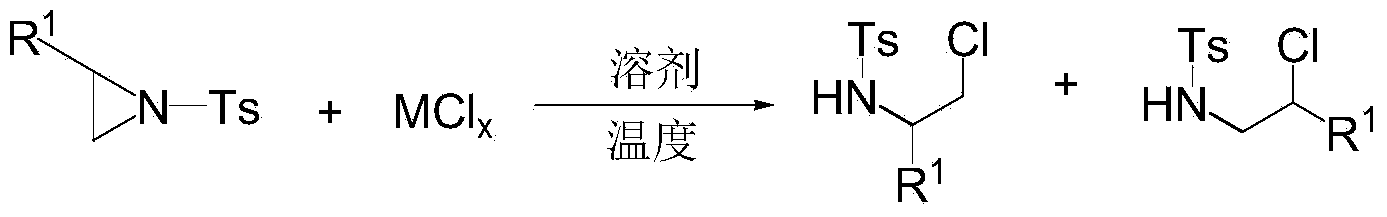
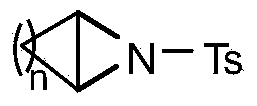
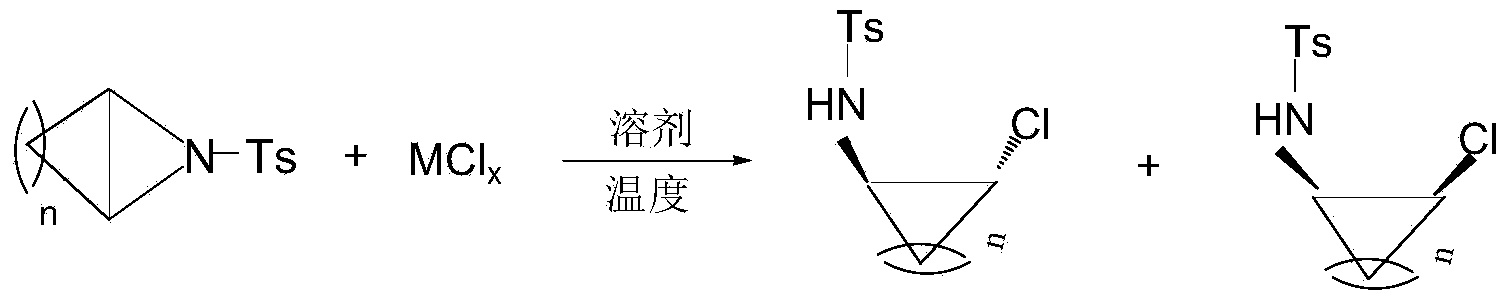
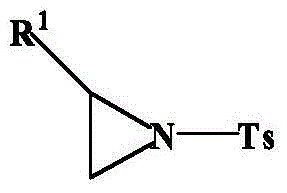
Ring opening method of aziridine compounds
InactiveCN103910657ALow priceReduce equivalence ratioSulfonic acid amide preparationAmino group formation/introductionOrganic synthesisAziridine
The invention discloses a ring opening method of aziridine compounds, particularly a ring opening method of aziridine compounds in different structures by using metal bromide, belonging to the technical field of organic synthesis. The aziridine compound is subjected to ring opening reaction by using tosyl-activated aziridine compounds as the initial raw material, dichloromethane as a solvent and bromine in the metal bromide as a nucleophilic reagent. The method has the advantages of simple reaction process, mild conditions and wide applicability, can obtain higher yield for aziridine compounds in different structures, has very high regioselectivity, and can form a single isomer from most aziridine compounds.
Owner:TAIYUAN UNIV OF TECH
Modification of the surface chemistry of macromolecular species in the presence of a conjugated guanidine
ActiveCN102046708AEasy accessOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCyclic etherAziridine
The present invention relates to a method for preparing macromolecular species with a modified surface, comprising a step (e) in which macromolecular species (M), initially carrying -OH and / or -SH functions, are brought into contact with: a catalyst (C) carrying at least one conjugated guanidine function; and reactive species (E), comprising reactive groups including: (i) at least one group including an alpha,beta-unsaturated carbonyl group C=C-C=O and / or an alpha, beta-unsaturated thiocarbonyl group C=C-C=S; and / or (ii) at least one heterocyclic group comprising from 3 to 5 ring members, the group being selected from cyclic ethers, cyclic thioethers and aziridine rings; and / or (iii) at least one group selected from isocyanate -N=C=O or thioisocyanate -N=C=S groups, and trivalent groups of formula >C=CZ-, where Z is an electron-withdrawing group. The invention also relates to the macromolecular species with a modified surface that are obtained in this context.
Owner:伯利莱斯
Light-sensitive silver halide photographic film material and radiographic intensifying screen-film combination
InactiveUS6472137B1Increase speedSuitable characteristicX-ray/infra-red processesRadiation applicationsSilver iodideIodide
A light-sensitive silver halide photographic film material has been provided, said film material comprising a transparent support and on both sides thereof at least one light-sensitive emulsion layer having spectrally and chemically sensitized tabular silver halide grains rich in silver bromide, further having silver iodide in an amount of less than 3 mole % based on silver, with two flat parallel {111} crystal faces, said grains accounting for a total projective surface of said parallel crystal faces in said emulsion of at least 50%, further having an average aspect ratio of at least 2:1, a grain thickness of from 0.05 up to 0.15 mum, a site-directing azacyanine compound satisfying the general formulae disclosed herein in an amount of not less than 1x10-4 mole per mole of silver halide coated and one or more J-aggregating spectrally sensitizing dye(s), wherein a molar ratio amount between said site directing compound and said J-aggregating spectrally sensitizing dye(s) is at least 1:6 for a grain coverage of said {111} tabular grains exceeding 50%.A radiographic screen / film combination has also been described comprising said light-sensitive silver halide photographic film material and two supporting or self-supporting X-ray intensifying screens) comprising luminescent phosphors, wherein by contacting the film material with a sandwich of a pair of said intensifying screens and exposing said combination to X-rays, emission of radiation by said luminescent phosphors in the wavelength range for which said material has been made spectrally sensitive provides a black-and-white diagnostic image after processing of said exposed radiographic film material.
Owner:AGFA NV
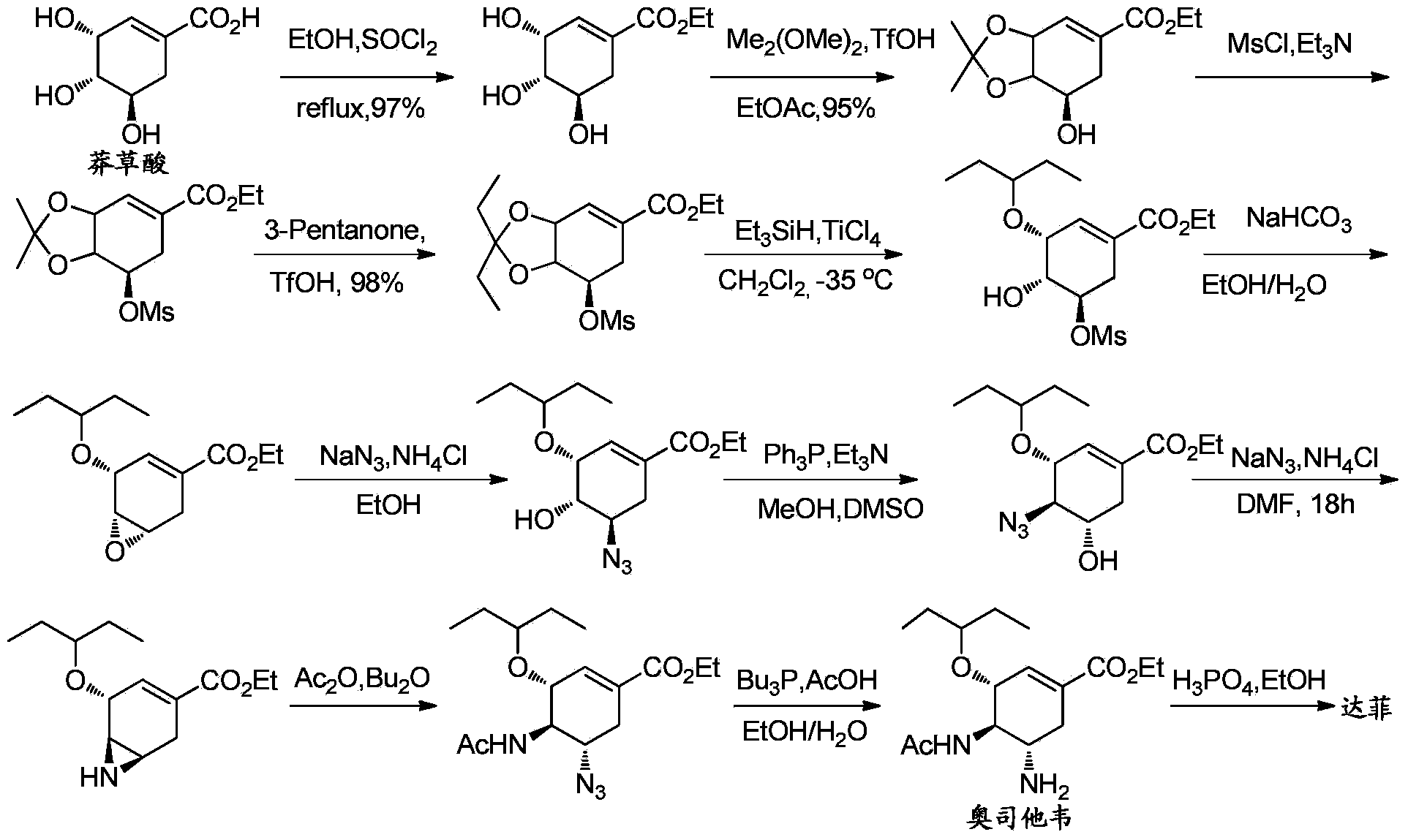
Synthesis method of oseltamivir
InactiveCN103833570AHigh yieldEasy to operateOrganic compound preparationCarboxylic acid amides preparationBird fluSynthesis methods
The invention discloses a synthesis method of oseltamivir. The synthesis method of oseltamivir comprises the following steps: starting from a compound 1,3-butadiene-3-amyl ether and compound 3-nitro-ethyl acrylate, carrying out Diels-Alder reaction, then reacting at room temperature in acetonitrile in the presence of a copper catalyst and PhI-NNs to prepare an aziridine compound in a one-pot method, wherein the mole ratio of the 1,3-butadiene-3-amyl ether to 3-nitro-ethyl acrylate to the copper catalyst is 1.1: 1: 0.025-0.1; and finally synthesizing the oseltamivir for preventing bird flu through the aziridine ring opening, nitryl and p-nitrobenzene sulfonyl removal, acetylation and hydrogenation. The method comprises short steps, the used reagent is cheap and easily available, the operation is simple, the total yield is up to 40%, and the method is a simple and efficient synthesis method of oseltamivir.
Owner:ZHEJIANG NORMAL UNIVERSITY
Electrochemical catalytic synthesis method of aziridine compounds
ActiveCN103436911ALower internal resistanceImproving electrochemical synthesis methodsElectrolysis componentsElectrolytic organic productionSupporting electrolyteN-aminophthalimide
The invention relates to an electrochemical catalytic synthesis method of aziridine compounds. The method comprises the following steps: by employing N-aminophthalimide and styrene or cycloolefin as raw materials in a single-room electrolytic tank, in electrolyte, employing halogenated tetra-alkylamine or alkali halide as an electrocatalyst, by employing lithium perchlorate or triethylamine / acetic acid as an supporting electrolyte, electrolyzing in the presence of alkali, wherein the reaction temperature is 0-40 DEG C, the current density is 4-12 mA / cm<2>, and thus obtaining the aziridine compounds after being electrified with the electric quantity of 2.5-3.5 F / mol. According to the electrochemical catalytic synthesis method of aziridine compounds, an indirect electrolysis method of electrochemical catalysis which is simple to operate is firstly employed to synthesize the aziridine compounds, the single-room electrolytic tank is used, constant-current electrolysis is employed, and glassy carbon electrodes are used as working electrodes, so that the conversion of the double-room electrolytic tank to the single-room electrolytic tank is achieved, and meanwhile, as the working electrodes are changed to be the glassy carbon electrodes in stead of previously used expensive platinum electrodes, the cost is greatly reduced, and the operation is much simpler, thus being more suitable for industrial production.
Owner:BEIJING UNIV OF TECH
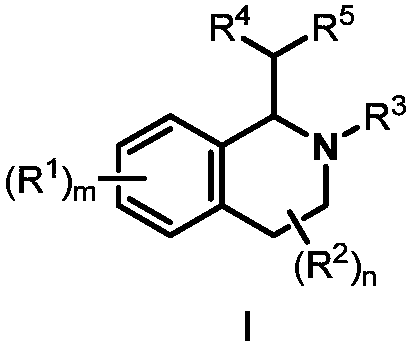
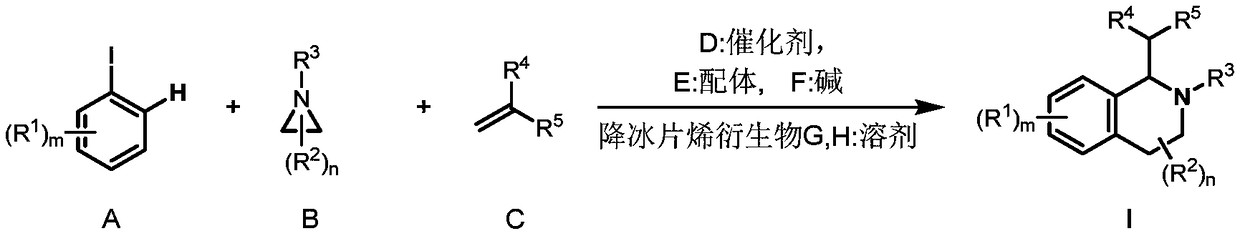
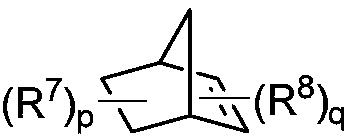
Preparation method of 1,2,3,4-tetrahydroisoquinoline derivative
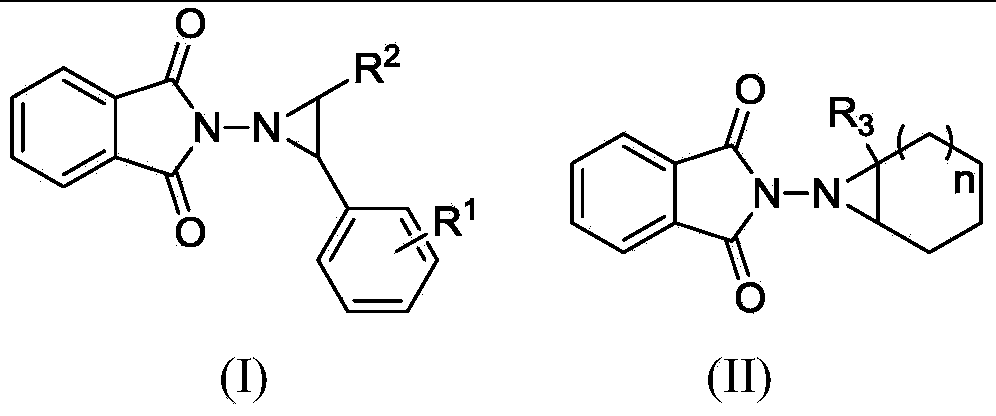
The invention discloses a preparation method of a 1,2,3,4-tetrahydroisoquinoline derivative. Aromatic iodide, aziridine compounds and olefin compounds are used as initial materials and stirred to react at 30-120 DEG C under the action of a catalyst, a ligand, a norbornene derivative and alkali, separation and purification are performed after the reaction, and the 1,2,3,4-tetrahydroisoquinoline derivative can be obtained. The 1,2,3,4-tetrahydroisoquinoline derivative can be synthesized efficiently, economically and greenly with the method. The method is mild in condition, good in substrate universality and high in yield, and the prepared 1,2,3,4-tetrahydroisoquinoline derivative can be widely applied to the fields of medicinal chemistry and organic chemistry.
Owner:WUHAN UNIV
Ring opening method of aziridine compounds
InactiveCN103910655ALow priceReduce equivalence ratioSulfonic acid amide preparationOrganic synthesisAziridine
The invention discloses a ring opening method of aziridine compounds, particularly a ring opening method of aziridine compounds in different structures by using metal chloride, belonging to the technical field of organic synthesis. The aziridine compound is subjected to ring opening reaction by using tosyl-activated aziridine compounds as the initial raw material, dichloromethane as a solvent and chlorine in the metal chloride as a nucleophilic reagent. The method has the advantages of simple reaction process, mild conditions and wide applicability, can obtain higher yield for aziridine compounds in different structures, has very high regioselectivity, and can form a single isomer from most aziridine compounds.
Owner:TAIYUAN UNIV OF TECH
Multi-component boiler deoxidant
InactiveCN1854083AWith residual hardnessHas anti-scaling effectWater/sewage treatmentSulfite saltSodium sulfite
A multi-component boiler deaerization agent consists of catalytic sodium sulfite 70-80wt%, 2,3-diamino-piperidine 10-20wt% and azacyclopropane 5-10wt%. It can remove dissolved oxygen, residues and scale.
Owner:SHANGHAI WANSEN WATER TREATMENT CO LTD
Insulation-coated electroconductive particles
ActiveCN1836295AGood solvent resistanceImprove reliabilityPrinted circuit assemblingNon-insulated conductorsAnisotropic conductive adhesiveSolvent
In order to impart excellent solvent resistance and conduction reliability to the insulating coated conductive particles suitable for anisotropic conductive adhesive conductive particles, the surface of the conductive particles is coated with a carboxyl group using a polyfunctional aziridine compound. The insulating resin layer of the insulating-coated electrically-conductive particle covered with the insulating resin layer formed of insulating resin is surface-treated. As the aziridine compound, for example, trimethylolpropane-tri-β-aziridine propionate, tetramethylolmethane-tri-β-aziridine propionate, or N,N-hexamethylene-1,6-bis-1-aziridine carboxamide, etc. The insulating resin layer is preferably composed of an insulating resin having an acrylic monomer unit or a methacrylic monomer unit. Specifically, an acrylic-styrene copolymer is preferable.
Owner:DEXERIALS CORP
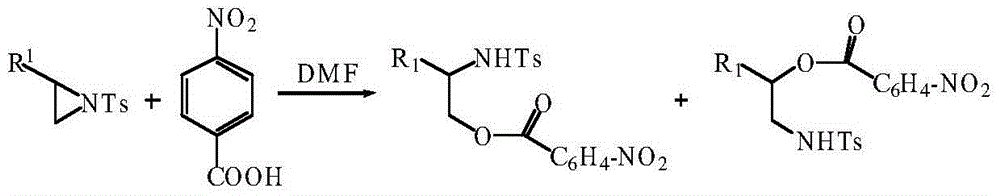
Method using carboxylic acid for ring opening of aziridine compound
ActiveCN104311457ALow costResponse to environmental protectionSulfonic acid amide preparationRegioselectivityAziridine
The invention discloses a method using carboxylic acid for ring opening of an aziridine compound; a tosyl activated aziridine compound is used as a starting material, in catalyst-free conditions, ring opening of the aziridine compound is performed in N, N-dimethyl formamide solvent by use of carboxylic acid as a nucleophilic reagent. The method is simple in reaction process, uses no catalyst, is mild in conditions, good in environmental protection property, and the method for ring opening has wide universality, and has higher yield and good regioselectivity on the aziridine compounds and carboxylic acid with different structures.
Owner:TAIYUAN UNIV OF TECH
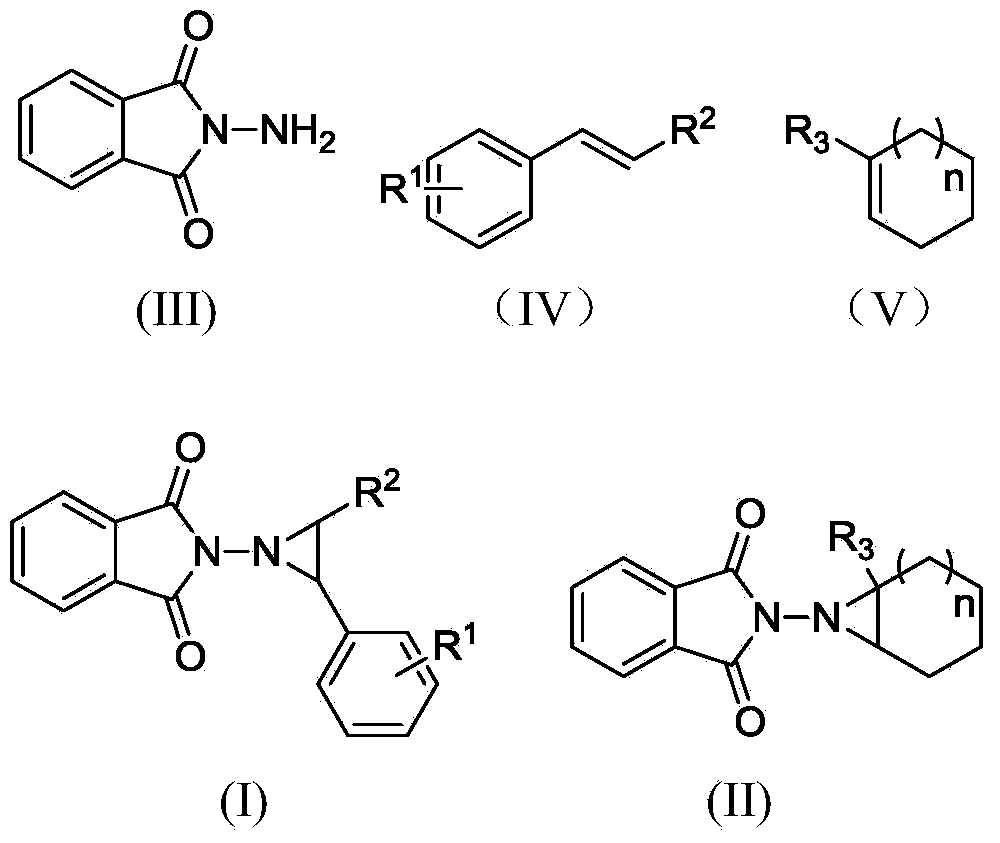
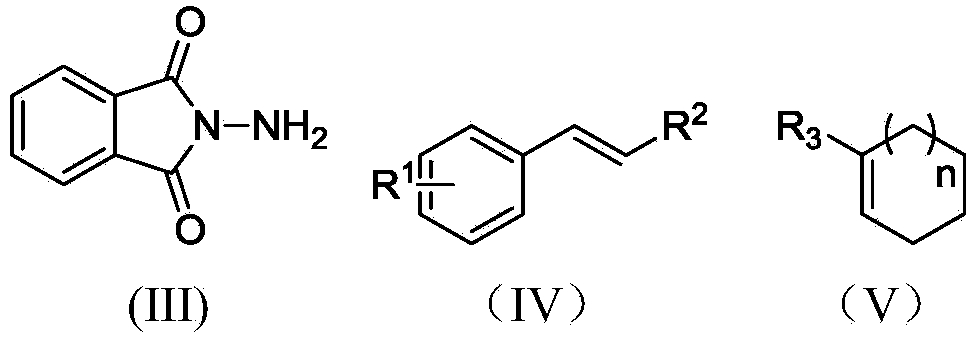
Preparation method of 3, 5-disubstituted thiazolidine-2-thioketone compound
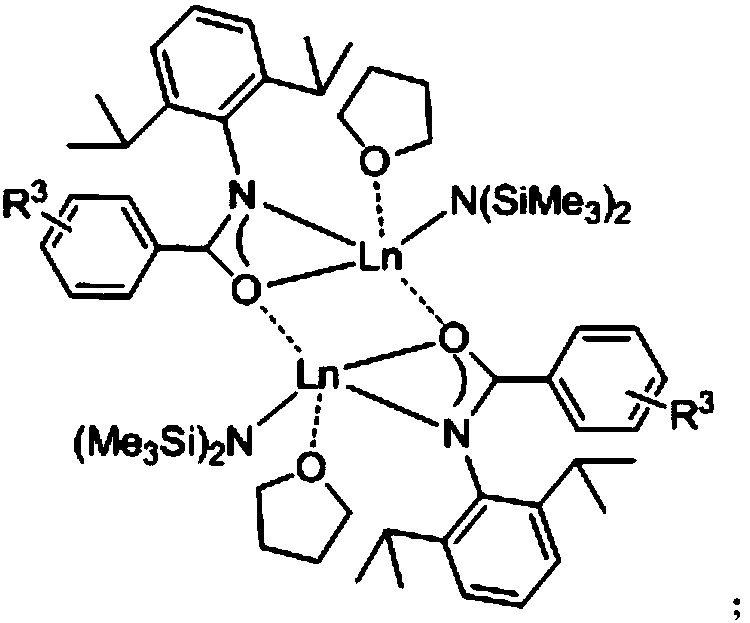
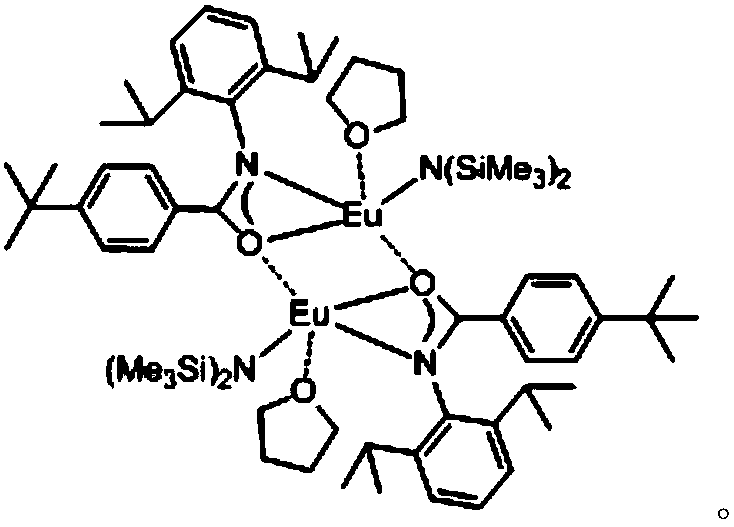
ActiveCN108276356AHigh catalytic activityHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsThioketoneRare earth
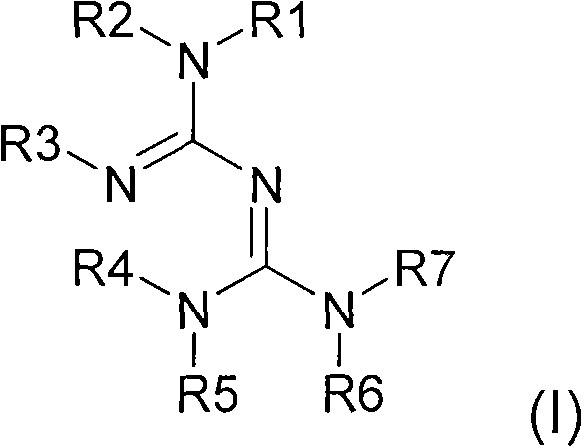
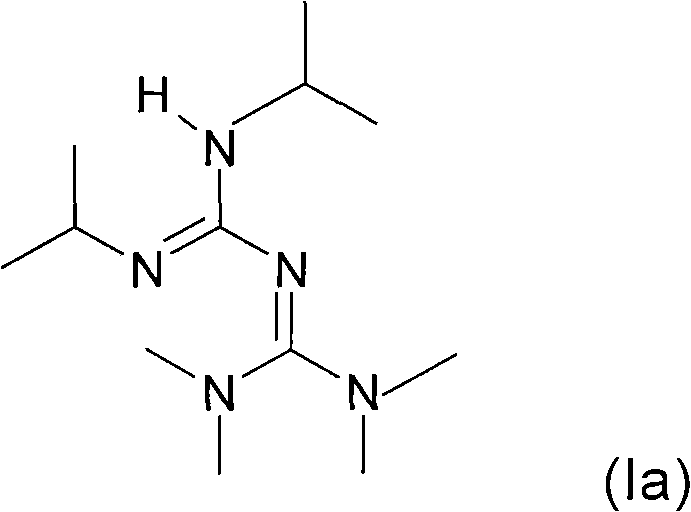
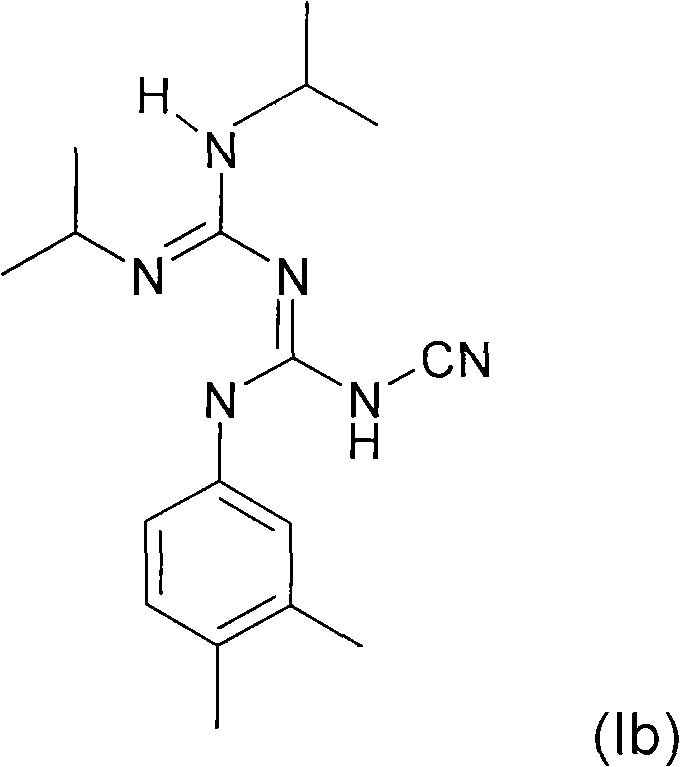
The invention relates to a preparation method of a 3,5-disubstituted thiazolidine-2-thioketone compound. The preparation method comprises the following steps of enabling an aziridine compound as shownin a formula (I) and carbon disulfide to be subjected to a reaction under the action of a catalyst, and obtaining the 3,5-disubstituted thiazolidine-2-thioketone compound as shown in a formula (II),wherein the catalyst is acylamino bivalent rare earth metal amide; the reaction is carried out in an anhydrous and oxygen-free inert atmosphere; the reaction route is as shown in the attached figure;wherein R1 is selected from hydrogen, C1 to C4 alkyl groups, an alkoxy group or halogen; the halogen is chlorine or bromine; R2 is selected from C1 to C4 alkyl groups, a benzyl group or a cyclohexyl group. The method provided by the invention is less in catalyst dosage, mild in reaction conditions, good in substrate universality and capable of synthesizing target products with high yield.
Owner:SUZHOU UNIV
An aziridine compound cyclizing method adopting a ketoxime
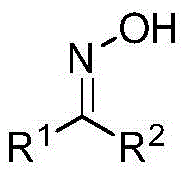
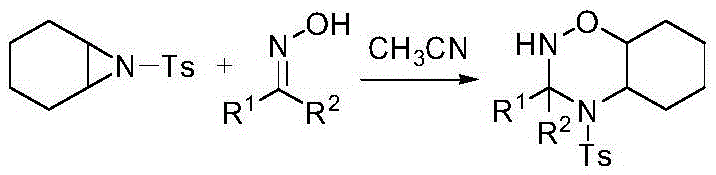
ActiveCN105461651ALower reaction costStrong toleranceOrganic chemistryPotassium hydroxideCycloaddition
The invention discloses an aziridine compound cyclizing method adopting a ketoxime. According to the method, an aziridine compound activated with tosyl is adopted as an initial raw material, the ketoxime is adopted as a nucleophilic reagent, and the aziridine compound is subjected to cycloaddition in acetonitrile or chloroform under the catalytic functions of potassium hydroxide, potassium carbonate, potassium tert-butoxide or triethylamine. Reaction processes of the method are simple. The method adopts the potassium hydroxide or the triethylamine as a catalyst and is environmental friendly, mild in conditions and wide in universality. High yields and good regioselectivity can be achieved for aziridine compounds with different structures and ketoximes with different structures.
Owner:TAIYUAN UNIV OF TECH
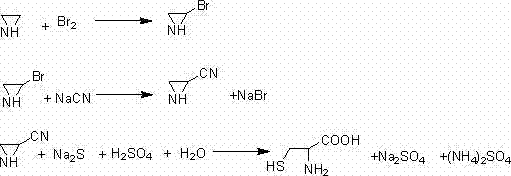
Method for synthetizing DL-cysteine
InactiveCN102531980ASufficient sourceMild reaction conditionsThiol preparationCyanideAcid hydrolysis
The invention discloses a method for synthetizing DL-cysteine, comprising the following steps: obtaining 2-bromine-azacyclopropane through the reaction of azacyclopropane and bromine water, obtaining 2-cyano-azacyclopropane through the reaction of the 2-bromine-azacyclopropane and cyanide, obtaining 2-sulfydryl-3-aminopropyl cyanide through a ring-opening reaction of 2-cyano-azacyclopropane and sodium sulfide and obtaining the DL-cysteine through the acid hydrolysis of the 2-sulfydryl-3-aminopropyl cyanide. The method disclosed by the invention has the advantages of mild reaction condition, high reaction yield, adequate raw material source, low production cost and simple production process.
Owner:JIANGSU YUANYANG PHARMA
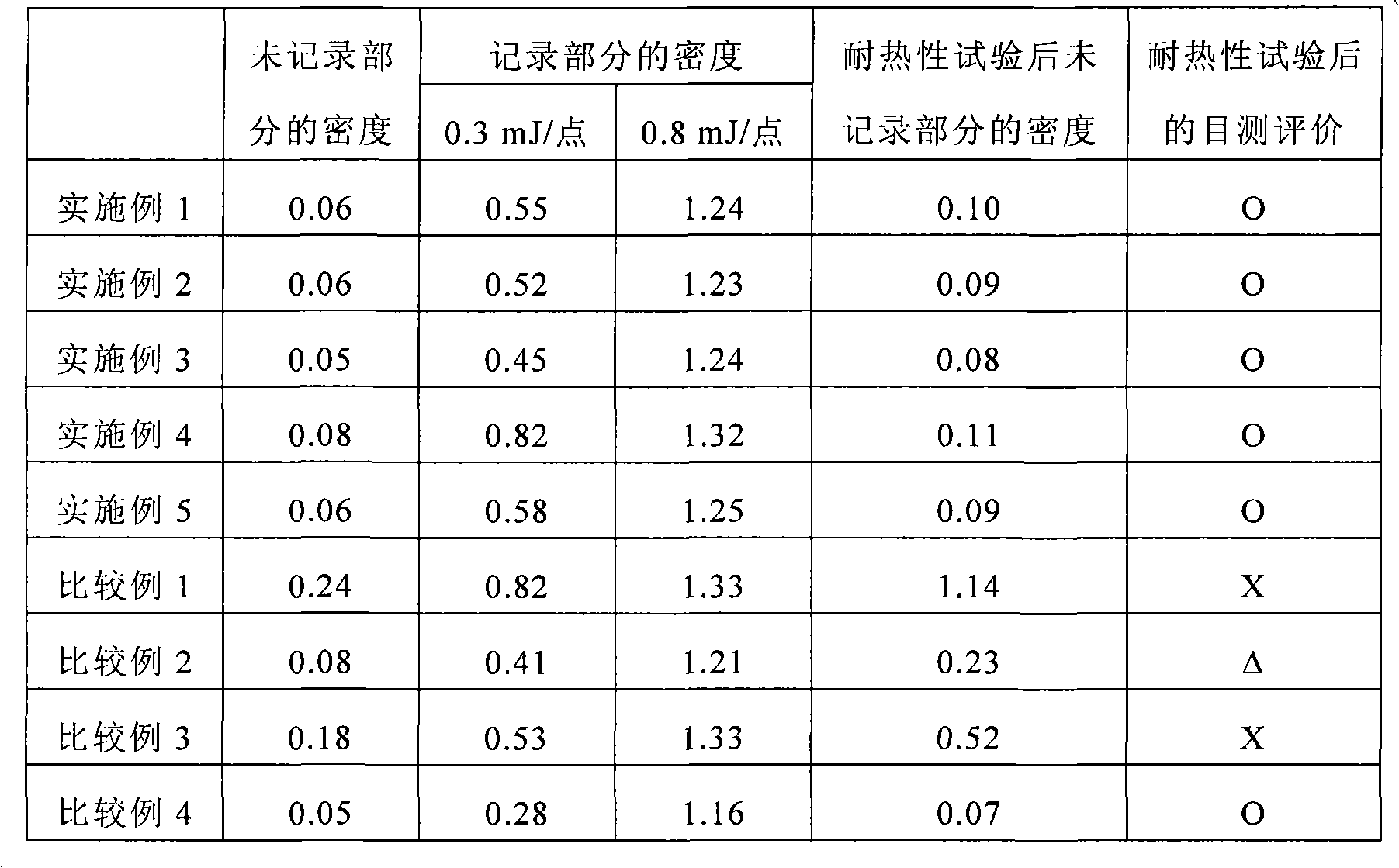
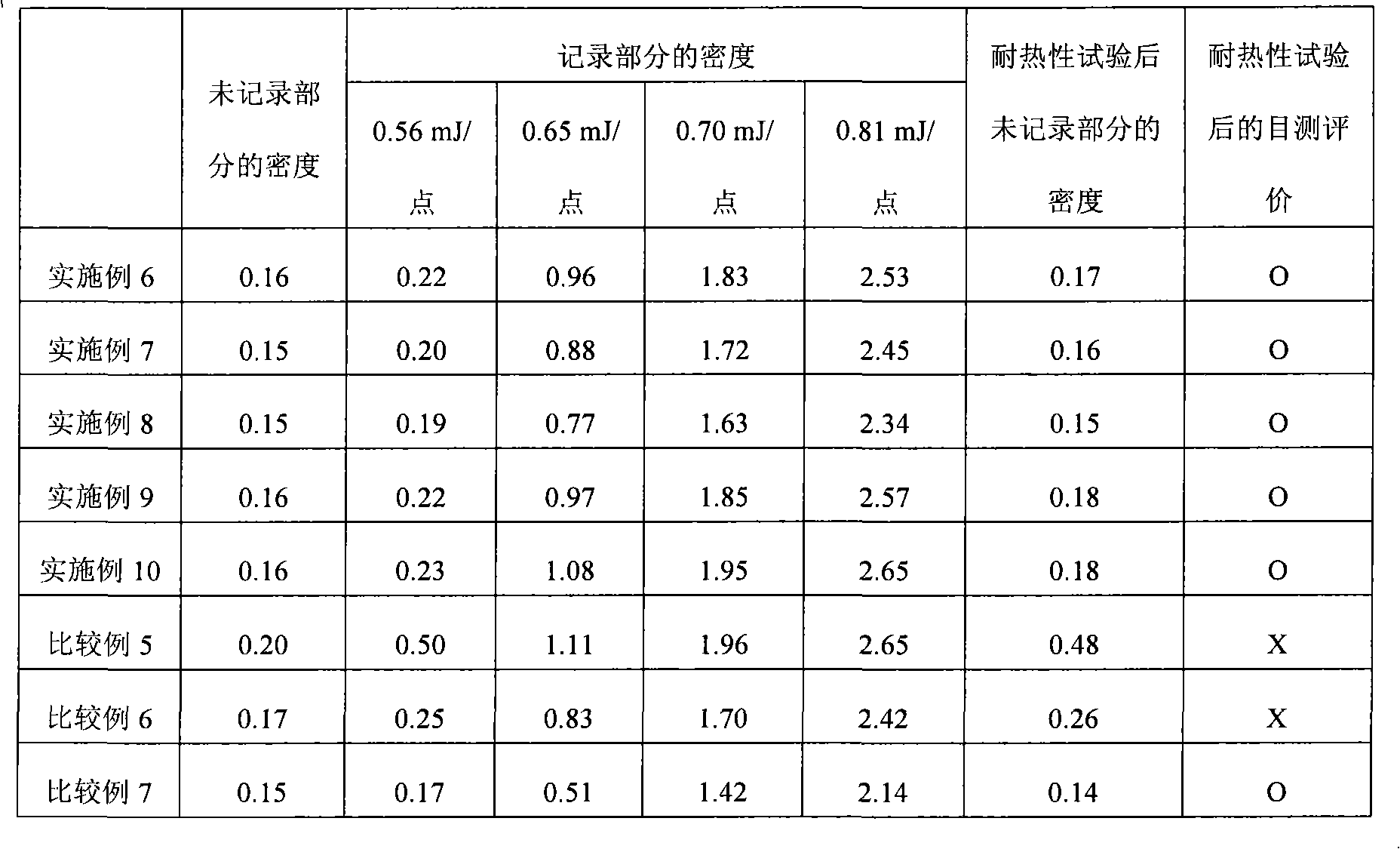
Heat-sensitive recording material
InactiveCN101657332AImprove heat resistanceExcellent recording sensitivityThermographyHeat sensitiveRecording layer
Disclosed is a heat-sensitive recording material comprising a supporting body and a heat-sensitive recording layer formed on the supporting body and containing a dye precursor-containing composite particle and a developer. This heat-sensitive recording material is characterized in that the dye precursor-containing composite particle is obtained by dissolving a solute containing a dye precursor into a solvent containing a polyvalent isocyanate compound-containing polymerization component, then emulsifying and dispersing the thus-obtained solution into an aqueous medium, and then performing a polymerization reaction of the polyvalent isocyanate compound-containing polymerization component in the presence of a polyethylene imine having a molecular weight of 200-1,500. Also disclosed is a method for producing such a heat-sensitive recording material.
Owner:OJI PAPER CO LTD
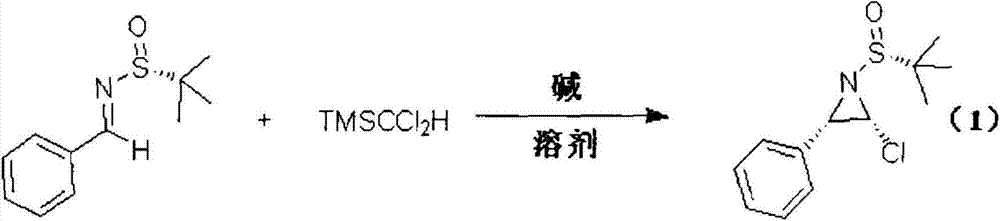
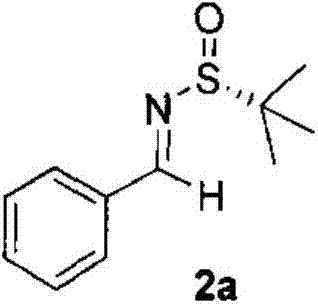
Method for preparing chiral alpha-chloroaziridine
The invention relates to a method for preparing chiral alpha-chloroaziridine. In an organic solvent, the chiral alpha-chloroaziridine is obtained by enabling chiral (Rs)-N-(tertiary butyl sulfonyl) imide, trimethyl (methyl dichloride) silane and alkali to react for 0.5-10h at the temperature of -80 DEG C-30 DEG C. Compared with the prior art, the chiral alpha-chloroaziridine prepared by the method provided by the invention is a potential bioactive molecular synthetic building block and can be used an important intermediate to synthesize a chiral nitrogen-containing compound, such as aziridine. According to the method for preparing the chiral alpha-chloroaziridine, provided by the invention, raw materials used for preparation are economic and easy to obtain, the preparation process conditions are mild, the method is efficient, and the optical purity of the alpha-chloroaziridine prepared by the method is high. The chiral alpha-chloroaziridine prepared by the method provided by the invention is expected to be applied to the fields of asymmetric synthesis and research and development of medicines.
Owner:SHANGHAI UNIV OF ENG SCI
Chromogenic formaldehyde adsorption material and method for preparing same
The invention discloses a chromogenic formaldehyde adsorption material and a method for preparing the chromogenic formaldehyde adsorption material. The chromogenic formaldehyde adsorption material is prepared from a substrate, an active component for formaldehyde, an auxiliary agent and a chromogenic agent of formaldehyde; the mass ratio of the active component for formaldehyde to the auxiliary agent is 1:0.03 to 1:0.2; the use amount of the chromogenic agent for formaldehyde is 0.1-1 per mill of the mass of the mixture of the active component for formaldehyde and the auxiliary agent; the active component of formaldehyde is polyethyleneimine or polyethylene polyamine; the chromogenic agent of formaldehyde is a mixture of 3-methyl-2-benzothiazolinonehydrazone hydrochloride hydrate (MBTH) and a ferric iron salt or 4-amino-3-hydrazine-5-mercapto-1,2,4-triazole (AHMT); and the mixed molar ratio of MBTH to the ferric iron salt is 1:1-1:20. The chromogenic formaldehyde adsorbent cannot cause secondary pollution, is safe to use and easy to prepare, is environmental friendly, and can be produced industrially easily.
Owner:邯郸派瑞电器有限公司
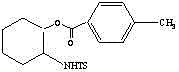
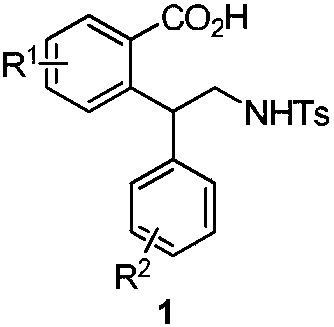
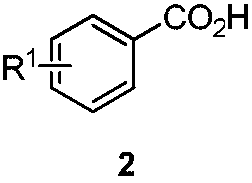
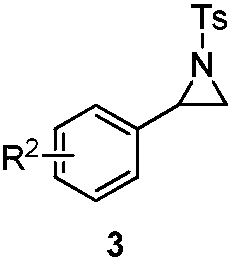
Preparation method for chiral pentabasic bicyclic guanidine based on aziridine
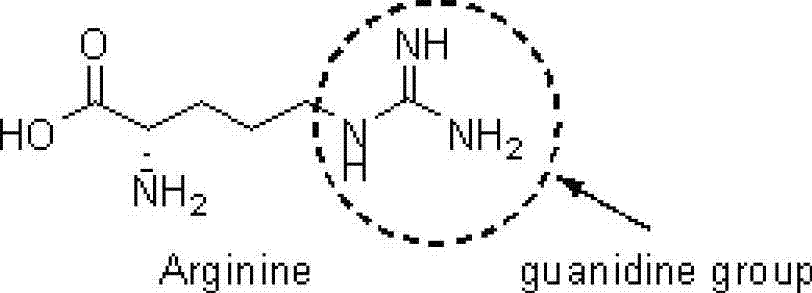
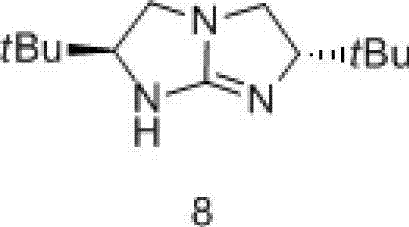
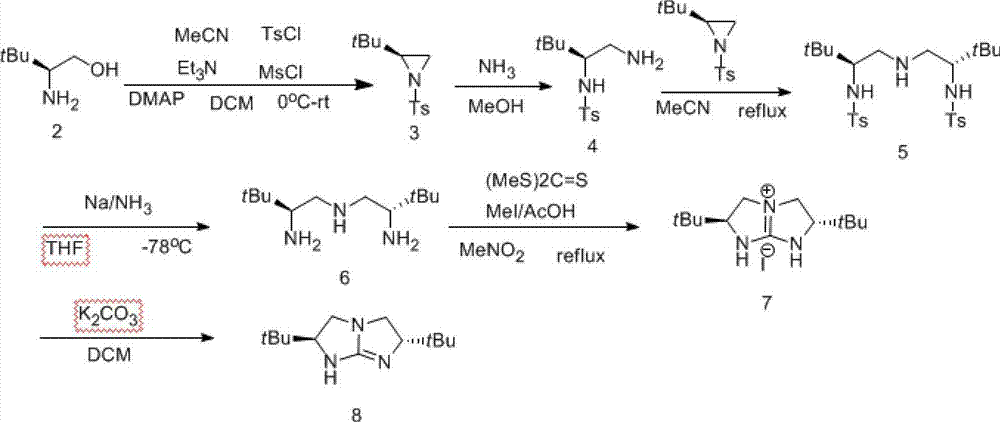
The invention discloses a preparation method for chiral pentabasic bicyclic guanidine based on aziridine. The method comprises the following steps: (1) using amino acid to synthesize alkamine; (2) using the alkamine to synthesize the aziridine; (3) using the aziridine to synthesize-NHTs triamine; (4) removing protection to obtain triamine; and (5) cyclizing to obtain the pentabasic bicyclic guanidine. The method overcomes the defects of high cost of the purifying technique, complexity in operation, -78 DEG C of low temperature reaction, high danger coefficient, expensive prices of raw materials, high cost and difficulty in industrial production in the original technique; and the method has the advantages of simplicity, feasibility, excellent reaction temperature, cheap raw materials, simple operation, reduced cost and improved feasibility of industrial production.
Owner:RAFFLES PHAMRMATECH CO LTD
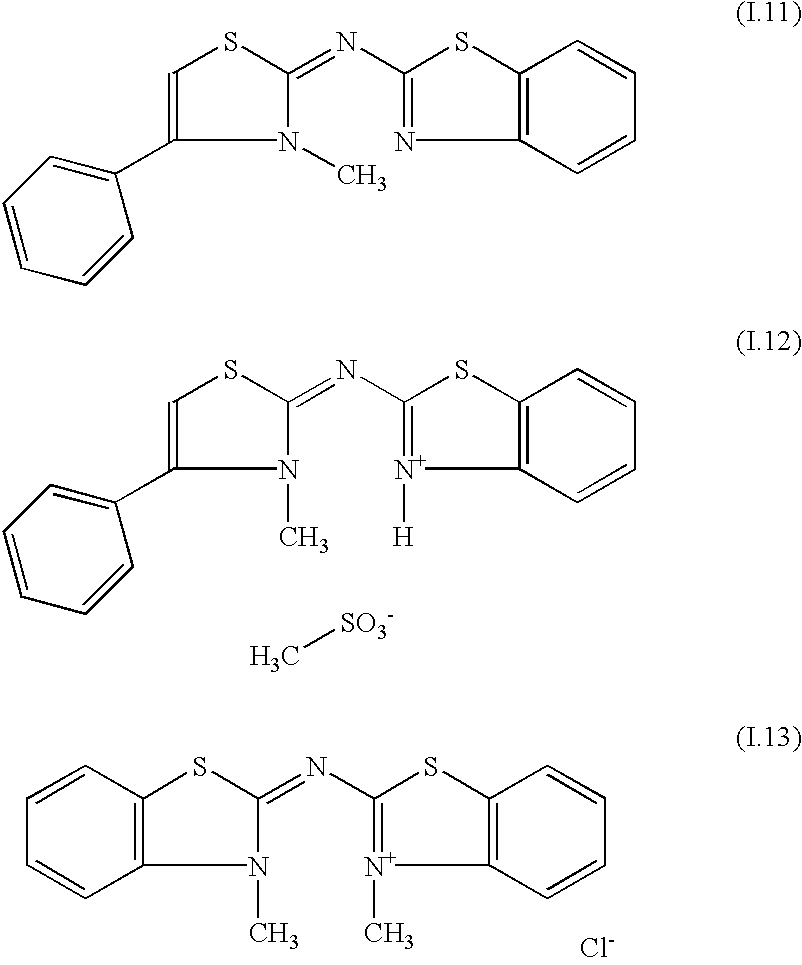
Optical recording medium and azacyanine dye
InactiveUS20100173114A1High-density recordingOrganic chemistryMethine/polymethine dyesRecording layerLaser beams
An optical recording medium on which high-density recording and reading of optical information can be conducted by a short-wavelength light such as a blue laser beam is provided.In an optical recording medium comprising at least a substrate and a recording layer thereon that can record or read information by irradiation with light, the recording layer contains an azacyanine dye represented by general formula [I]:wherein R1 and R2 each independently represent a hydrogen atom or an optionally substituted linear or branched alkyl group having one to four carbon atoms; R3 represents a hydrogen atom or a hydrocarbon group; R4 represents a hydrogen atom or a linear or branched alkyl group having one to four carbon atoms; R5 represents an optionally substituted aromatic ring group or an optionally substituted unsaturated heterocyclic group; R4 and R5 may be combined together to form a ring; X− represents a counter anion; and the benzene ring A may be optionally substituted.
Owner:VERBATIM CORPORATION
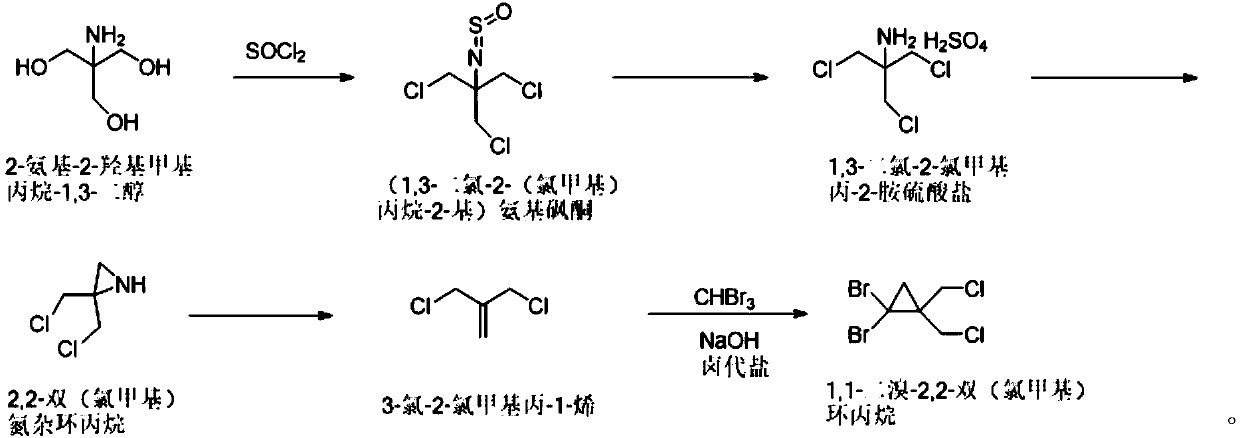
Synthetic method of 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane
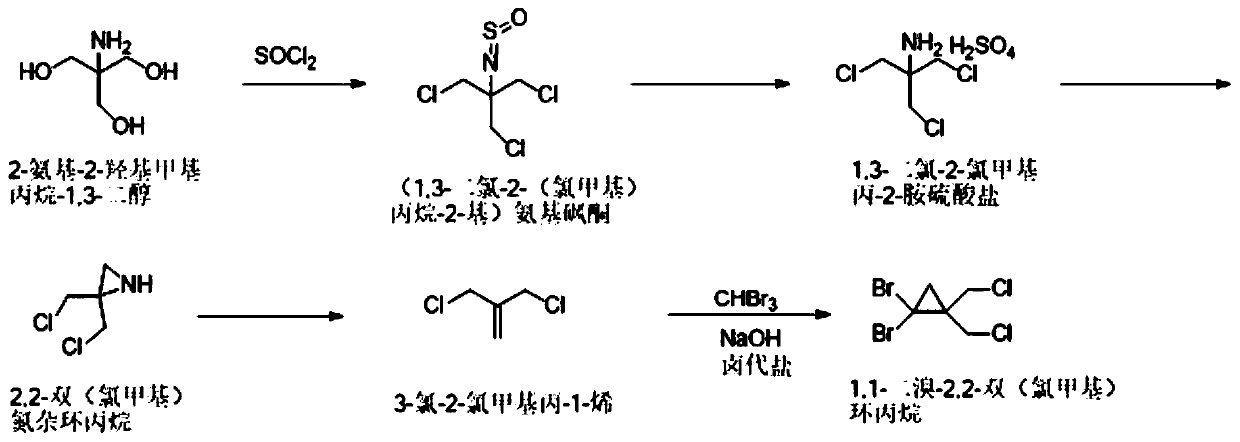
ActiveCN110759840AReduce instant heat releaseGuaranteed reaction rateOrganic compound preparationAmino compound preparationAziridinePropylamine
The invention discloses a synthetic method of 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane. The method comprises the following steps that chlorine substitution reaction happens to 2-amino-2-hydroxylmethylpropane-1,3-diol to obtain (1,3-dichloro-2-(chloromethyl)propane-2-group)amino-sulfone-ketone; the (1,3-dichloro-2-(chloromethyl)propane-2-group)amino-sulfone-ketone is hydrolyzed through a concentrated sulfuric acid aqueous solution to obtain 1,3-dichloro-chloromethylpropyl-2-amine-sulfate; the 1,3-dichloro-chloromethyl-propyl-2-amine-sulfate is subjected to salt dissolution on the alkaline condition, and is subjected to cyclization to form 2,2-dual(chloromethyl)aziridine; reaction happens to 2,2-dual(chloromethyl)aziridine and sodium nitrite to form 3-chloro-2-chloromethyl-propyl-1-alkene; and the 3-chloro-2-chloromethyl-propyl-1-alkene reacts with bromoform on the alkaline condition to generate 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane. The synthetic method of 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane is simple and can be used for enlarged production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Polysubstituted benzoic acid and a synthesis method thereof
ActiveCN109867613AGood functional group diversityRaw materials are easy to getOrganic compound preparationSulfonic acid amide preparationBenzoic acidSynthesis methods
The invention discloses a synthetic method of polysubstituted benzoic acid. Benzoic acid which is simple and easy to obtain and aziridine which is easy to prepare are used as raw materials, synthesisof polysubstituted benzoic acid is achieved in one step, and the obtained polysubstituted benzoic acid can be further converted into a functional product. The method has the advantages of easily available raw materials, simple operation, mild synthesis reaction conditions, high reaction efficiency, and diversity of functional groups.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
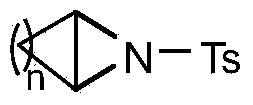
(1Z, 4Z, 5Z) ¿C 6 N alkyl ¿C 6 ¿C aza ¿C2 ¿C oxo ¿C 3 ¿C oxa ¿C 4 ¿C methoxy ¿C dicyclo [3, 1, 0] hexane and preparation method
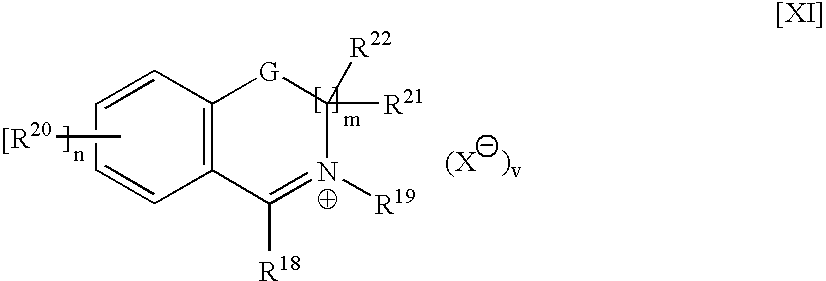
InactiveCN101092419AEnhanced inhibitory effectHigh yieldOrganic active ingredientsOrganic chemistryAziridineColon cancer cell
This invention relates to aziridine derivatives, i.e., (1Z, 4Z,5Z)-6-N-n-hexadecyl / octadecyl-6-aza-2-oxo-3-oxa-4-methoxy-bicyclo[3.1.0]hexane, as shown in chemical formulae I and II. This invention also provides a method for preparing the aziridine derivatives. The preparation method comprises: synthesizing 5-methoxy-3-bromo-2(5H)-furanone, reacting with hexadecylamine / octadecylamine, and Et3N in DMSO to obtain the aziridine derivatives. The aziridine derivatives have good inhibitive effects on colon cancer cells, human gastric cancer cells and human ovarian cancer cells, and can be used in drugs for treating colon cance, gastric cancer and ovarian cancer. The preparation method avoids traditional phase transfer catalysis and water / oxygen-free operation, and adopts room temperature homogeneous catalytic condition, thus has such advantages as simple operation, low cost and high target compound yield. Besides, another diasteromer can be obtained.
Owner:BEIJING NORMAL UNIVERSITY
Macromonomer containing lactam structure and application thereof in preparation of polymer polyol
The invention relates to a preparation method of a macromonomer containing a lactam structure. The preparation method comprises the following steps: 1), aziridine is subjected to reaction with polyester polyol or polyether polyol at / in a molar ratio of 0.8:1-1.2:1 at a temperature of 100-150 DEG C; 2), the compound shown in the formula (II) is subjected to reaction with the product in the step 1)at the temperature of 60-180 DEG C, and the macromonomer containing the lactam structure is prepared. The invention also relates to the macromonomer containing the lactam structure. The macromonomer has the structure shown in the description, wherein R is a polyester chain segment or a polyether chain segment, and R1 and R2 are independently selected from H or C1-C3 alkyl. The invention also relates to an application of the macromonomer containing the lactam structure in preparation of polymer polyol. The macromonomer containing the lactam structure has the advantages of low viscosity and lowusage and is suitable for being used as a dispersion stabilizer of polymer polyol, and obtained polymer polyol has low viscosity.
Owner:WANHUA CHEM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

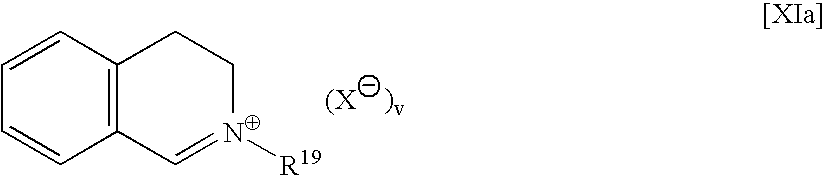












![(1Z, 4Z, 5Z) ¿C 6 N alkyl ¿C 6 ¿C aza ¿C2 ¿C oxo ¿C 3 ¿C oxa ¿C 4 ¿C methoxy ¿C dicyclo [3, 1, 0] hexane and preparation method (1Z, 4Z, 5Z) ¿C 6 N alkyl ¿C 6 ¿C aza ¿C2 ¿C oxo ¿C 3 ¿C oxa ¿C 4 ¿C methoxy ¿C dicyclo [3, 1, 0] hexane and preparation method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d6dfcd9-94ca-44c8-9b20-656438f9dcc4/A20061009013200131.PNG)
![(1Z, 4Z, 5Z) ¿C 6 N alkyl ¿C 6 ¿C aza ¿C2 ¿C oxo ¿C 3 ¿C oxa ¿C 4 ¿C methoxy ¿C dicyclo [3, 1, 0] hexane and preparation method (1Z, 4Z, 5Z) ¿C 6 N alkyl ¿C 6 ¿C aza ¿C2 ¿C oxo ¿C 3 ¿C oxa ¿C 4 ¿C methoxy ¿C dicyclo [3, 1, 0] hexane and preparation method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d6dfcd9-94ca-44c8-9b20-656438f9dcc4/A20061009013200061.PNG)
![(1Z, 4Z, 5Z) ¿C 6 N alkyl ¿C 6 ¿C aza ¿C2 ¿C oxo ¿C 3 ¿C oxa ¿C 4 ¿C methoxy ¿C dicyclo [3, 1, 0] hexane and preparation method (1Z, 4Z, 5Z) ¿C 6 N alkyl ¿C 6 ¿C aza ¿C2 ¿C oxo ¿C 3 ¿C oxa ¿C 4 ¿C methoxy ¿C dicyclo [3, 1, 0] hexane and preparation method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d6dfcd9-94ca-44c8-9b20-656438f9dcc4/A20061009013200071.PNG)