Preparation method for chiral pentabasic bicyclic guanidine based on aziridine
A technology for aziridine and bicyclic guanidine, which is applied in the field of preparation of chiral five-membered bicyclic guanidine, can solve the problems of high risk factor, complicated operation, large energy consumption and the like, and achieves good reaction temperature and simple method. , the effect of low risk factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
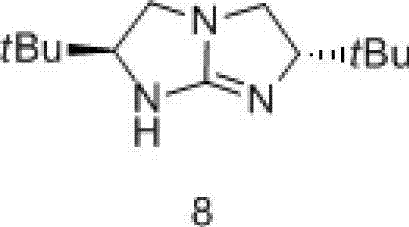
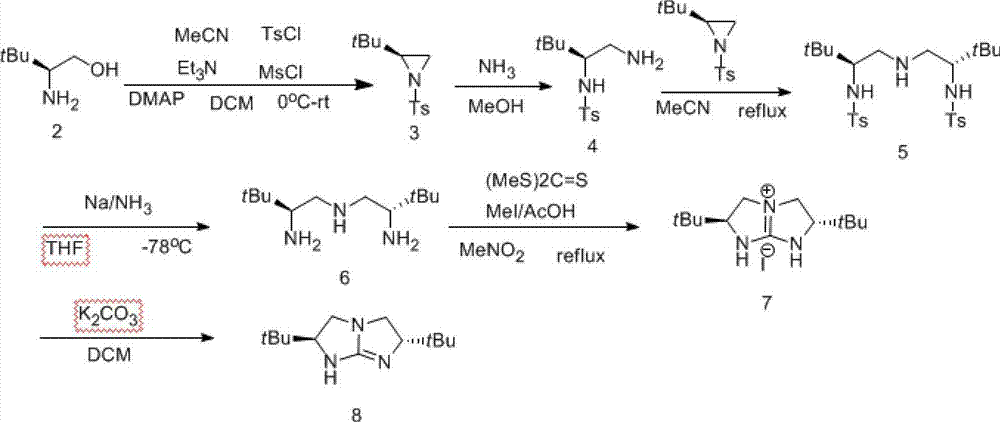
[0039] Example 1: The synthesis flow chart is shown in Formula I.
[0040]
[0041] Step 1: Synthesis of amino alcohol 2 from amino acid 1:
[0042] Add (83.04g, 4eq) sodium borohydride, 2L dried tetrahydrofuran, and 120g of L-tert-leucine to a 5L three-necked flask. Slowly add (231g, 1eq) iodine 760mL tetrahydrofuran solution dropwise in an ice bath at 0°C under the protection of a nitrogen balloon to make it fully react. Methanol 300ml clarified the mixture. Stir for 30min, remove the solvent to leave a white paste, and then dissolve it in 800mL 20%KOH. solution. The solution was stirred at room temperature for 4 h and extracted three times with 1.5 L of dichloromethane each time. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated by rotary evaporation to obtain 97 g of a white semi-solid with a yield of 90%.
[0043] Step 2: Synthesis of aziridine 3 from aminoalcohol 2:
[0044] Add 97 g of L-tert-leuci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com