Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46results about How to "Non-infectious" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Foot and mouth disease virus-like particle, preparation method and application thereof
ActiveCN101914501AImproving immunogenicityImprove biological activityInactivation/attenuationAntiviralsEnzyme digestionStructural protein
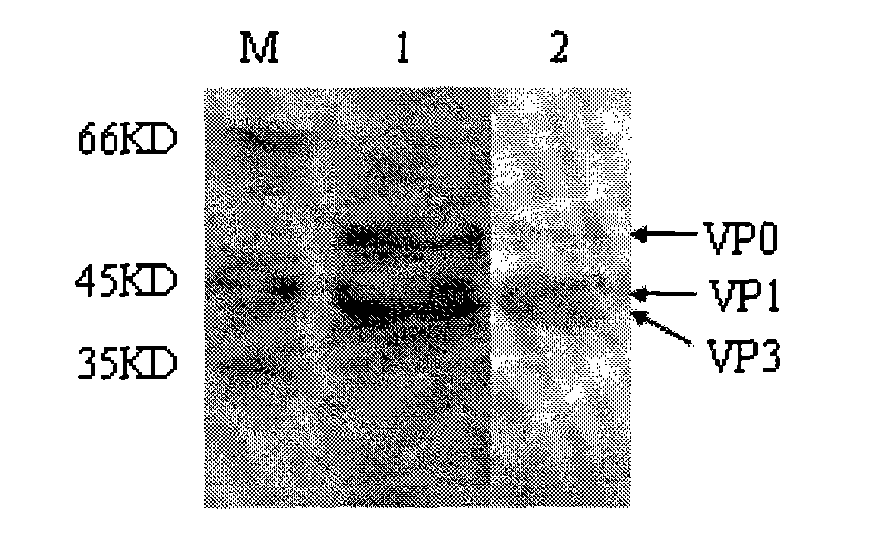
The invention discloses an Asia I type foot and mouth disease virus-like particle, a preparation method and an application thereof. The Asian I type foot and mouth disease virus-like particle comprises structural proteins of VP0, VP3 and VP1 of Asia I type foot and mouth disease virus, wherein the gene sequence of VP0 is shown in SEQ 1, the gene sequence of VP3 is shown in SEQ 3, and the gene sequence of VP1 is shown in SEQ 2. The preparation method of the Asian I type foot and mouth disease virus-like particle has the following steps: performing amplification to obtain the VP0, VP3 and VP1, performing enzyme digestion to obtain a recombinant expression vector, performing enzyme digestion on fusion protein by small ubiquitin-like modifier (SUMO), and carrying out in-vitro assembling to obtain the foot and mouth disease virus-like particle.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
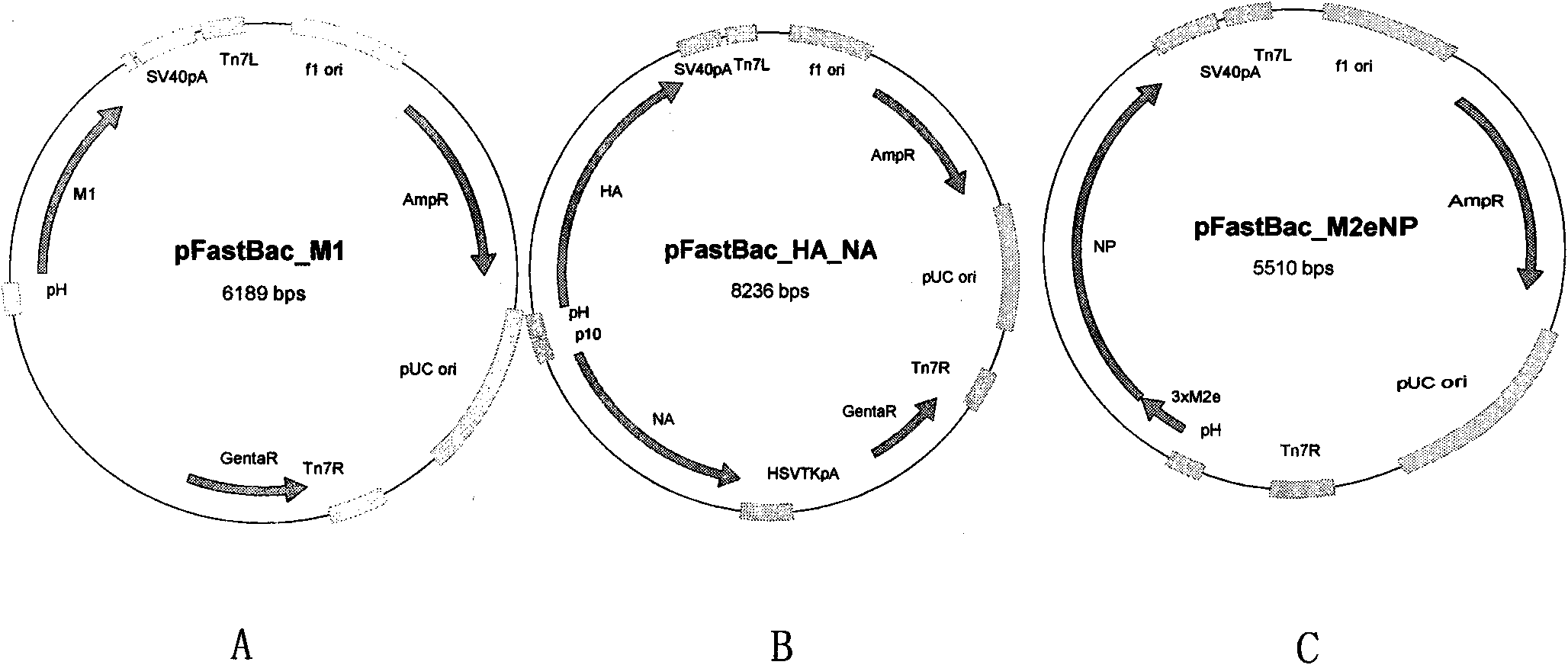
Broad-spectrum safe anti influenza A virus vaccine for animals
ActiveCN101643721AImprove immunityAnti-leakageMicroorganism based processesAntiviralsCell membraneMutant
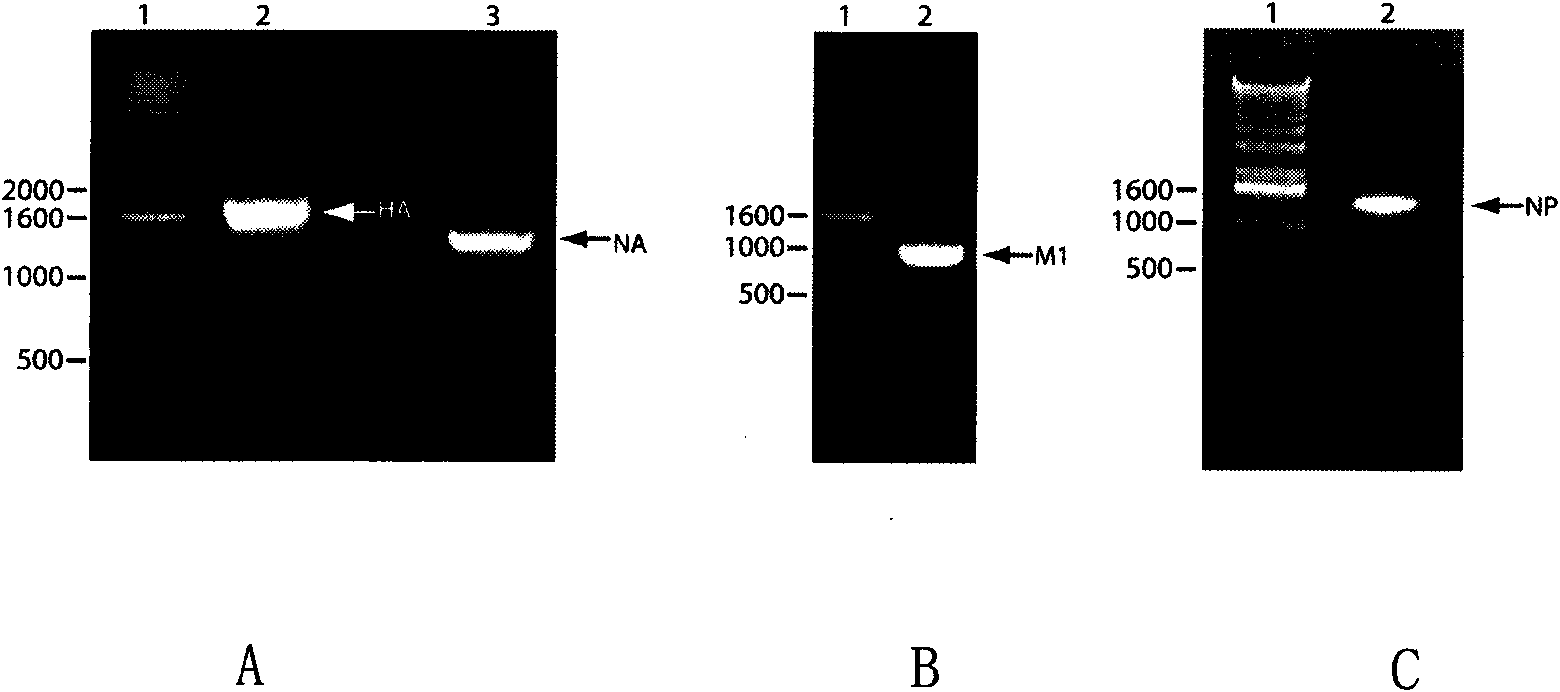
Recombinant virus-like particle contains influenza A virus matrix protein M1, surface film proteins HA and NA and M2eNP fusion protein or protein obtained by modification of mutant of at least one protein and the rest proteins, and the recombinant virus-like particle is non-replicative; wherein the M2eNP fusion protein is polymer formed by one M2e polypeptide or a plurality of M2e polypeptides atthe external end of cell membrane of matrix protein M2 or is formed by fusion of nucleoprotein NP and polymer formed by one M2e polypeptide through artificial modification or a plurality of M2e polypeptides after modification; and the nucleoprotein NP is coupled with recombinant matrix protein M1 after recombination expression and embedded in the recombinant virus-like particle. The vaccine produced by the recombinant virus-like particle can be directly applied to various animals for prevention of infection and spread of influenza A virus. The vaccine is safe in use and obvious in immune effect. Production period is short, technical operation is simple, and no purification is required, thus the vaccine is low in cost.
Owner:许雁
Magnetic targeting carrier capable of carrying gene and drug, preparation method and application thereof
InactiveCN101797387AFree from degradationNot easy to degradeGenetic material ingredientsInorganic non-active ingredientsControl releasePharmaceutical drug
The invention discloses a magnetic targeting carrier capable of carrying genes and drugs, a preparation method and an application thereof. The invention is a carrier which has stability, safety and targeting and has controlled release behavior for non-viral magnetic gene therapy and drug therapy. The carrier material of the invention is characterized in that the carrier material is a bunchy silica mesoporous material with magnetism; the length-diameter ratio is not less than 3; the loading capability is big; the material has a protective effect on loaded genes and carriers and superparamagnetism, is not easy to agglomerate, and can control release speed of genes and drugs in vitro; and the surface thereof is easy to modify various functional groups, thus having wide adaptability. The invention also provides a preparation method of the carrier. When in use, therapeutic short chain DNA, siRNA or drugs enter in holes or are combined with surface modified functional genes by a soaking mode, then reach a targeted tissue by guidance of an applied magnetic field, and release the short chain DNA, siRNA or drugs carried thereby under the action of an alternating magnetic field, thus achieving the purpose of magnetic targeting controlled therapy.
Owner:CENT SOUTH UNIV
Norovirus RNA fragment-containing pseudoviral particle and preparation method thereof
InactiveCN102559616AGood stabilityEasy to store and transportInactivation/attenuationFermentationCoat ProteinsPolymerase chain reaction
The invention discloses a norovirus RNA fragment-containing pseudoviral particle and a preparation method thereof. The pseudoviral particle is a spherical RNA-protein complex which is prepared by coating a norovirus RNA with an MS2 bacteriophage coat protein. The pseudoviral particle is prepared by the following steps: designing and manually synthesizing a primer and obtaining a target gene MS2 by a by PCR (polymerase chain reaction) method; connecting the target gene MS2 to a plasmid pET-28b(+) to obtain a recombinant plasmid pET-28b / MS2; connecting the recombinant plasmid pET-28b / MS2 with a NV fragment to obtain a recombinant plasmid pET-28b / MS2 / NV; guiding the obtained recombinant plasmid pET-28b / MS2 / NV into escherichia coli for prokaryotic expression; and precipitating a virus-like particle by a polyethylene glycol method, wherein the obtained virus-like particle is the norovirus RNA fragment-containing pseudoviral particle. The pseudoviral particle can be used as a standard product and a quality control product for RT-PCR detection, is non-infectious, safe, reliable, and good in stability, and has an RNase-resistant characteristic.
Owner:HENAN UNIV OF SCI & TECH
Virus-like particle recombinant protein of virus variation strain VP2 gene of infectious bursal disease
InactiveCN101624421ANon-infectiousImproving immunogenicityViral antigen ingredientsVirus peptidesVp2 geneVirus-like particle
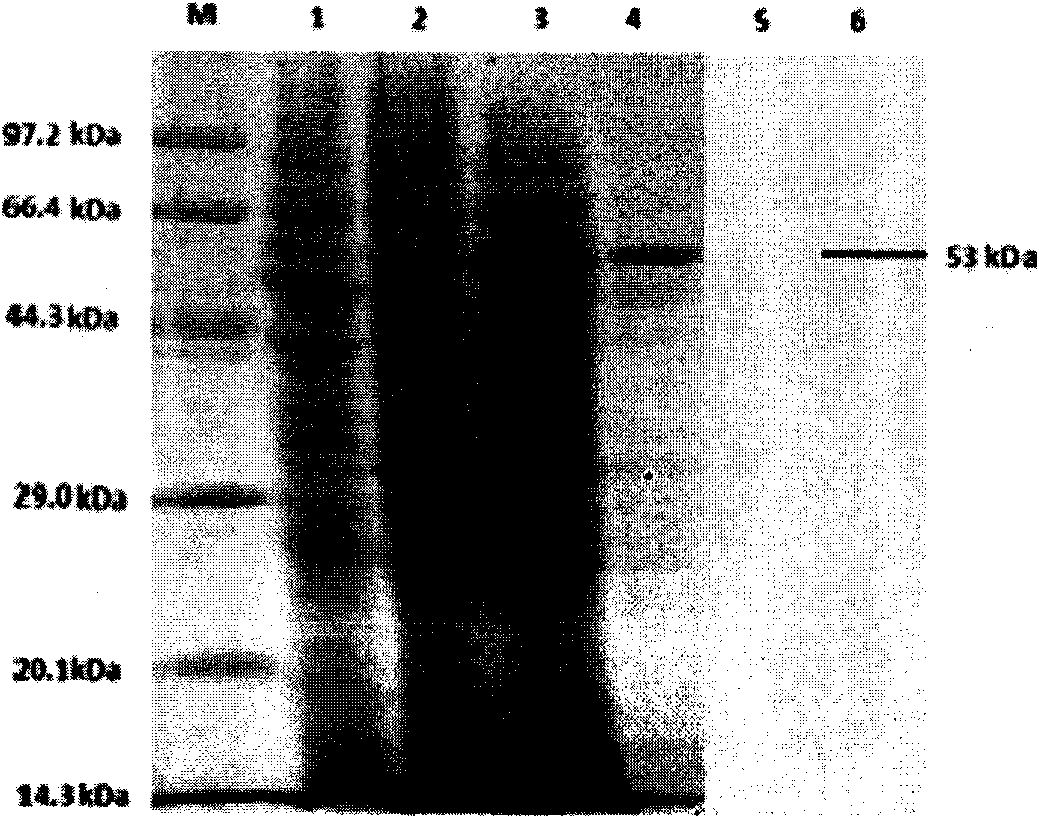
The invention relates to virus-like particle recombinant protein of a virus variation strain VP2 gene of an infectious bursal disease, belonging to the field of biologic pharmacy. The IBDV variation strain (AH1) VP2 gene is cloned, converted and transfected to obtain a recombinant baculovirus vBac-VP2; an infected Sf9 insect cell has specific fluorescence, and the antigen valence of the infected Sf9 insect cell is above 1.6*10<3>; the molecular weight of a recombinant VP2 protein is 53kDa, and the recombinant VP2 protein is in the state of virus-like particles; an indirect ELISA detection method established for an envelope antigen by the purified recombinant VP2 protein has good specificity and sensibility; an immune chicken can resist IBDV virulent attack, and the protection ratio achieves 100 percent. The novel virus-like particle recombinant VP2 protein prepared by the IBDV variation strain VP2 gene has high pertinence on the immune prevention of a current prevalent IBDV virulent strain and good practical value and popularization prospects.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Primer for amplifying novel coronavirus and application of primer
InactiveCN111270016ASensitiveStrong conservativeMicrobiological testing/measurementAgainst vector-borne diseasesPrimary screeningOligonucleotide Primer
The invention relates to a primer for amplifying novel coronavirus and an application thereof. The primer comprises at least one oligonucleotide primer capable of recognizing a specific region on a novel coronavirus N gene, and the sequence range of the specific region on the novel coronavirus N gene comprises a region sequence of a novel coronavirus reference strain genome 28778-28971. By adopting the primer, novel coronavirus nucleic acid can be rapidly detected from RNA extracted from samples such as blood, saliva, sputum and air; the method has the advantages of simplicity, convenience, quickness and no need of training of large instruments and professionals, can be used as a primary screening and environment detection method for novel coronavirus infected suspected pneumonia cases, and can also be used as an important reference for clinical diagnosis.
Owner:LUDONG UNIVERSITY
Bovine viral diarrhea virus nano-PCR detection kit and preparation method thereof
InactiveCN107058618AAccurate detectionImprove featuresMicrobiological testing/measurementBovine Viral Diarrhea VirusesNanoparticle
The invention discloses a bovine viral diarrhea virus nano-PCR detection kit and a preparation method thereof, relates to the field of bovine viral diarrhea virus detection, and solves the problems that BVD infection frequently has cross infection and is easily misdiagnosed, and that existing detection methods waste much time and labor. The kit comprises an Mighty Amp enzyme, a 2xBuffer Mix buffer liquid, gold nanoparticle sol, sterile double distilled water, a pair of specific primers and bovine viral diarrhea virus positive reference plasmid; the pair of specific primers include P1(5'-GGTAGCAACAGTGGTGAGTTC-3') and P2(5'-CTCAGGTTAAGATGTGCTGTG-3'). The bovine viral diarrhea virus positive reference plasmid is pMD18-T recombinant plasmid formed by a nucleotide fragment containing 130 basic groups, and the sequence of the plasmid is as shown in SEQ ID NO.2. The kit is high in sensitivity, simple in operation and low in cost.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Stem cell factor microvesicle preparation coming from human umbilical cord mesenchymal stem cells and preparation method thereof
ActiveCN110179826AAvoiding Medical Ethics IssuesImprove stabilityPowder deliveryCell dissociation methodsUmbilical cord tissueFreeze-drying
The invention relates to a stem cell factor microvesicle preparation coming from human umbilical cord mesenchymal stem cells and a preparation method thereof. The method uses discarded umbilical cordtissue after clinical delivery of pregnant woman as a material to obtain human umbilical cord mesenchymal stem cells with medical value and stem cell factor microvesicles coming from the human umbilical cord mesenchymal stem cells, the product uniformity is good, the production efficiency is high, the large-scale production is facilitated, and the medical ethics problem is avoided. The method strictly operates processes in the culture and amplification process of the human umbilical cord mesenchymal stem cells, the whole process is carried out in a megascale cell laboratory without using antibiotic, the possible stem cell character change and the influence on the quality of the stem cell factor microvesicles coming from the human umbilical cord mesenchymal stem cells are avoided, and tissue anaphylactic reaction is caused; the optimized serum-free human umbilical cord mesenchymal stem cell culture medium is used to avoid human tissue allergy caused by mixing animal source blood components into suspension; and the freeze-dried powder prepared from the microvesicle suspension is convenient to store and provides convenience for subsequent product preparation.
Owner:武汉五州润达生物医药科技有限公司
Construction, expression and application of tumor-targeted peptide F3 modified cowpea chlorotic mottle virus-like particles
InactiveCN108558990ADoes not affect self-assemblyNon-infectiousSsRNA viruses positive-senseAntibody mimetics/scaffoldsIodideTumor target
The invention provides a construction and application of tumor-targeted peptide F3 modified cowpea chlorotic mottle virus-like particles. The F3-CCMV virus-like particles are constructed by insertingthe tumor-targeted peptide F3 into CCMV capsid protein CP whose amino terminal 1-26 amino acid residues are knocked out. The F3-CCMV virus-like particles serve as targeted drug delivery carriers to encapsulate near-infrared dye IR780 iodide, thereby preparing F3-CCMV-IR780 nanoparticles; and the F3-CCMV virus-like particles is capable of targeting and recognizing tumor cells, killing the tumor cells by a photothermal effect, and exerting a biological immune-targeting therapeutic effect.
Owner:ECOLOGY INST SHANDONG ACAD OF SCI
Bovine respiratory syncytial virus nano-PCR detection kit and preparation method thereof
InactiveCN107058619AAccurate detectionImprove featuresMicrobiological testing/measurementMicroorganism based processesHigh probabilityNanoparticle
The invention discloses a bovine respiratory syncytial virus nano-PCR detection kit and a preparation method thereof, relates to the field of bovine respiratory syncytial virus detection, and solves the problems that an existing bovine respiratory syncytial virus detection method is low in sensitivity and that serological test has high probability of false positive and high cost. The kit comprises an Mighty Amp enzyme, a 2xBuffer Mix buffer liquid, gold nanoparticle sol, sterile double distilled water, a pair of specific primers and bovine respiratory syncytial virus positive reference plasmid; the pair of specific primers include P1(5'-TATGCTATGTCCCGATTGG-3') and P2(5'-ACTGATTTGGCTAGTACACCC-3'). The sequence of the bovine respiratory syncytial virus positive reference plasmid is as shown in SEQ ID NO.2. The kit is high in sensitivity, simple in operation and low in cost.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Porcine parvovirus (PPV) virus-like particles (VLPs) antibody detection kit as well as preparation method and application thereof
InactiveCN108776225AHigh sensitivityGood specificity and reproducibilityVirus peptidesBiological material analysisSystem developmentParvovirus
The invention discloses a porcine parvovirus (PPV) virus-like particles (VLPs) antibody detection kit as well as a preparation method and application thereof. The detection kit comprises an enzyme label plate pre-enveloped with PPV VLPs, a blocking solution, sample diluent, an enzyme conjugate, a concentrated washing solution, an enzyme substrate solution and a stop buffer. According to the preparation method, the kit for detecting PPV is established by using the PPV VLPs as an envelope antigen for the first time; the kit also can be used for quickly detecting the antibody level of the PPV inserum; the kit provided by the invention is high in detection sensitivity, good in specificity and reproducibility, and stable in results. Furthermore, the used VLPs are safe and harmless to an operator and the environment, and are free from infectivity. In addition, the kit provided by the invention is applied to the development of an escherichia coli expression system, thus having the advantagesof being economical and inexpensive.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Influenza virus carried HCV nucleic acid test quality control product and preparation method thereof
ActiveCN105132583ALow costGood for mass manufacturingMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionTranscriptional expression
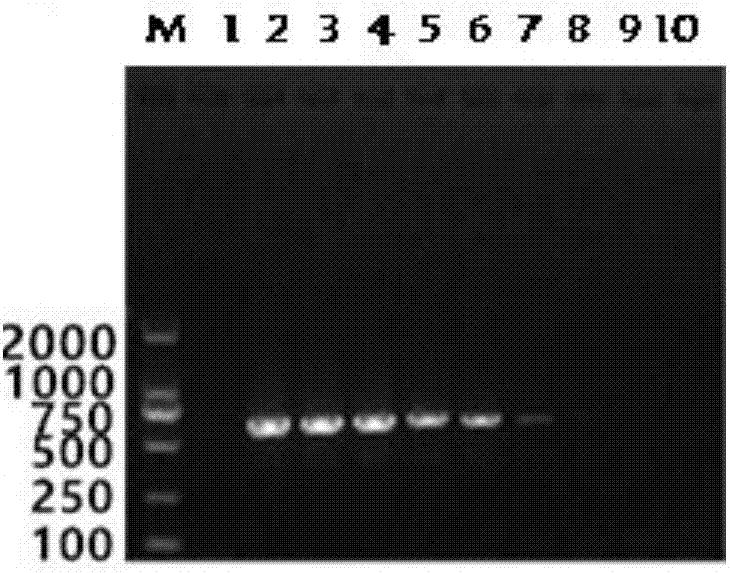
The invention relates to an influenza virus carried HCV nucleic acid test quality control product and a preparation method thereof; the quality control product is prepared from HCV conserved gene carried recombinant influenza virus through enlarged culture, inactivation and purification; the preparation method comprises the following steps: (1) constructing a plasmid which is used for rescuing influenza virus and is interpolated with HCV conserved gene in PR8 virus NS gene; (2) co-transfecting 293T cell to the constructed plasmid with plasmids which are respectively used for transcriptional expression of PR8 viruses PB2, PB1, PA, HA, NP, NA and M, so as to rescue and obtain HCV 5' UTR carried recombinant influenza virus; and (3) enlarged-culturing, inactivating and purifying the recombinant influenza virus so as to obtain the quality control product. The quality control product disclosed by the invention can be used for really simulating HCV pathogens and achieving all-around monitoring on HCV detection; and the quality control product is easy in large-scale preparation, low in cost, good in stability, easy in storage and transportation, and good in application prospect.
Owner:上海市临床检验中心
Rapid quantitative test paper for type-O foot-and-mouth disease virus
InactiveCN109799340ARapid Quantitative DetectionImproving immunogenicityMaterial analysisRare earthControl line
The invention discloses rapid quantitative test paper for a type-O foot-and-mouth disease virus. The test paper comprises a bottom plate, a sample pad, a conjugate pad, an analysis membrane and a water absorption pad. The bottom plate is made of a waterproof material, and the sample pad, the conjugate pad, the analysis membrane formed by a nitrocellulose membrane and the water absorption pad madeof a water absorbing material are sequentially fixed to the bottom plate made of the waterproof material. A rare earth luminescent material and staphylococcus aureus A protein coupled conjugate is added to the conjugate pad, and the nitrocellulose membrane is provided with a detection line made of type-O foot-and-mouth disease virus-like particles and a control line made of rabbit IgG. By adoptionof the type-O foot-and-mouth disease virus-like particles as capture antigens, rapid quantitative testing of the type-O foot-and-mouth disease virus antibody level in serum can be realized, high immunogenicity is achieved while threats of virus shedding are avoided, and safety and harmlessness are guaranteed for operators and the environment.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for efficiently expressing PCV2 Cap and PCV3 Cap fusion proteins
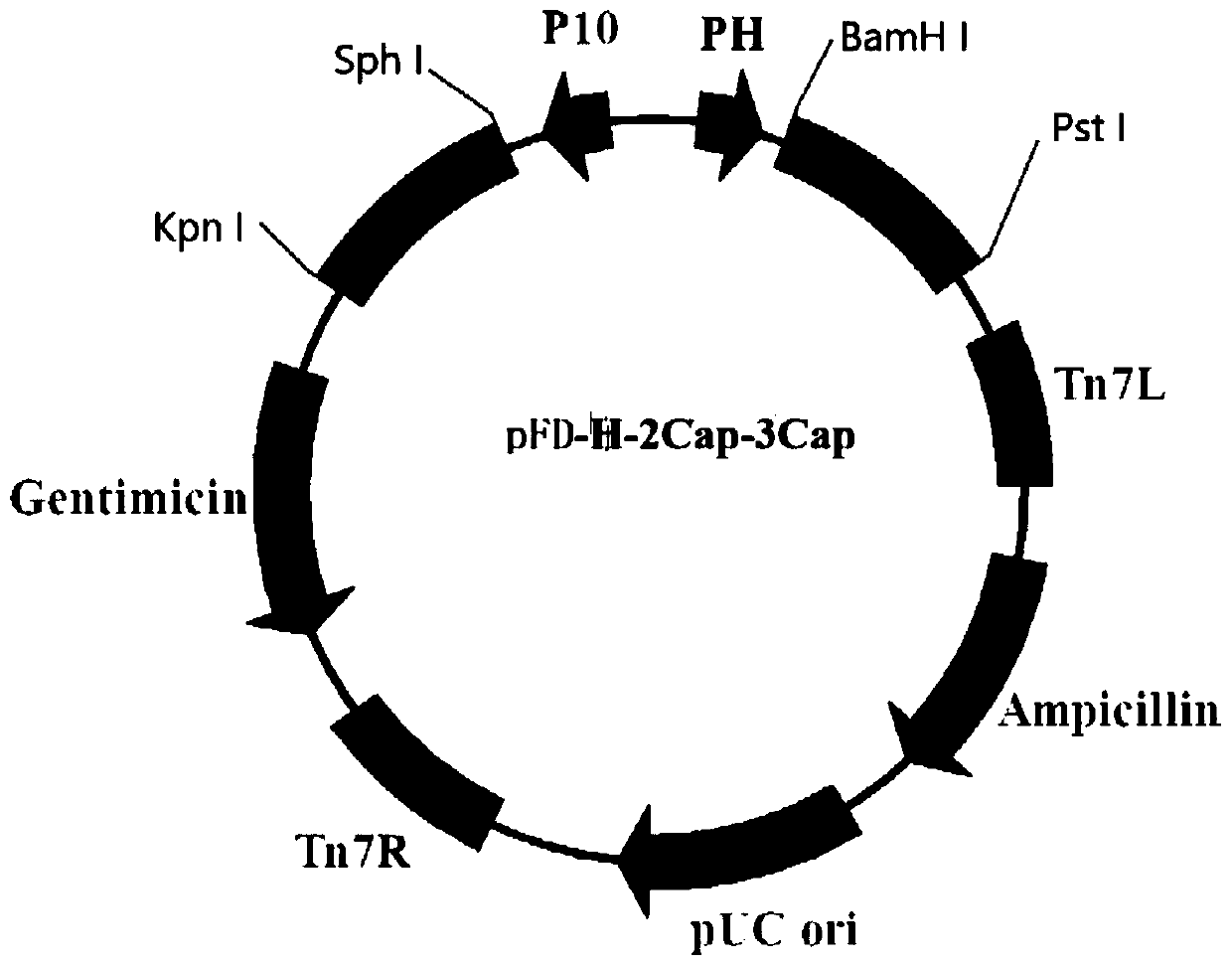
ActiveCN111187353AEasy to purifySimple identification stepsAntibody mimetics/scaffoldsVirus peptidesNucleotideVertebrate Animals
The invention provides a method for efficiently expressing PCV2 Cap and PCV3 Cap fusion proteins. The method comprises the following steps: firstly, constructing a recombinant baculovirus for efficiently expressing PCV2Cap protein and PCV3Cap protein; connecting the nucleotide sequence of the PCV2Cap protein of which the nuclear localization signal is cut off with the nucleotide sequence of the PCV3Cap protein; connecting the middle by using a hydrophobic flexible protein linker sequence; and adding a bee element signal peptide sequence to the N ends of the PCV2Cap protein sequence and the PCV3Cap protein sequence to promote secretory expression. Proteins expressed by recombinant positive baculovirus infected sf9 insect cells are subjected to various post-translational modifications, and the modified proteins are close to natural virus coded proteins and have high biological activity. Moreover, the baculovirus has high species specificity, only infects the insect cells, has no infectivity to vertebrate cells, and expression products of baculovirus are safe and reliable and can be used for subsequent tests after simple treatment. Therefore, the method for expressing the PCV2 Cap andPCV3 Cap fusion proteins is safer and more effective than a traditional mode.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Steam driving urination boosting system based on magnetic resonance coupling
InactiveCN106361480ACause infectionSimple structureNon-surgical orthopedic devicesCapacitanceHigh frequency power
The invention provides a steam driving urination boosting system based on magnetic resonance coupling. The system comprises a high-frequency power supply, a first compensation capacitor, a second compensation capacitor, resonance coils comprising a transmitting coil and a receiving coil, a bed frame which is fixed on the pubis, a urethra valve which is fixed on the bed frame and a driving sac of which the upper end is fixed on the bed frame, wherein a working medium is stored in the closed driving sac; the transmitting coil is held in a hand and presses the anterior abdominal wall; the receiving coil is fixed in the closed driving sac; the first compensation capacitor is connected in series with the transmitting coil and the high-frequency power supply; a heating wire is soaked in the liquid-state working medium, and is connected in series with the receiving coil and the second compensation capacitor; the bed frame and the urethra valve are fixed on the periphery of a covering film; the urethra valve is an elastic element coating urethra, and is fixed on the bed frame. The system has a simple and light structure, realizes high-efficiency non-contact electric transmission, has stable driving capacity, and does not cause infection, injury, urine leakage or backflow.
Owner:GUANGDONG UNIV OF TECH
A kind of porcine epidemic diarrhea recombinant baculovirus genetically engineered subunit vaccine and its preparation method and application
InactiveCN103585625BImprove abilitiesPracticalMicroorganism based processesAntiviralsElisa kitEngineered genetic
The invention belongs to the technical field of biological vaccine preparation, in particular to a porcine epidemic diarrhea recombinant baculovirus genetically engineered subunit vaccine and its preparation method and application. The present invention selects the S1 gene and M gene of the current new epidemic strain of PEDV as the reference sequence, uses the baculovirus expression system to express the S1 protein or part of the S1 protein and M protein, and prepares the obtained recombinant protein into a subunit vaccine for effective Control the occurrence of porcine epidemic diarrhea. The porcine epidemic diarrhea genetically engineered subunit vaccine prepared by the method of the present invention solves the defects of the current porcine epidemic diarrhea virus traditional vaccine, can be used to prevent and treat porcine epidemic diarrhea virus infection and related diseases caused by it, and can also It is also suitable for preparing the coating antigen of the ELISA kit for detecting porcine epidemic diarrhea virus antibody.
Owner:SOUTH CHINA AGRI UNIV
CTLA-4 gene armored RNA standard substance and applications thereof
InactiveCN104531740AGood protectionStrong stabilityMicrobiological testing/measurementVector-based foreign material introductionBiologyExpression vector
The invention provides a CTLA-4 gene armored RNA standard substance and applications thereof. The RNA standard substance is prepared through the following steps: firstly, obtaining a C-based gene sequence for CTLA-4 nucleic acid detection, and then obtaining an expression vector containing MS2 phage mature zymoprotein genes and capsid protein genes, namely, a PET32a-CP vector; then, obtaining a prokaryotic expression vector PET32a-CP-CTLA-4 of CTLA-4 C-genes and CP genes; transferring the prokaryotic expression vector into a prokaryotic expression strain, carrying out inducible expression, carrying out frozen low-temperature freeze thawing, centrifuging, and purifying, so that an expression product particle is obtained; and carrying out DNase I digestion and purification, and then diluting the obtained product, so that the CTLA-4 gene armored RNA standard substance is obtained. The substance disclosed by the invention has the characteristics of stability, no biological infectivity, ribonuclease resistance, and the like, and is used as a standard substance in the nucleic acid detection of immune suppression factors CTLA-4.
Owner:ETEMUS BIOMEDICINE XIAMEN
Preparation method of tomato spotted wilt virus nucleic acid standard substance
PendingCN112553220ALittle source informationWith precisionSsRNA viruses negative-senseVirus peptidesTomato spotted wilt virusGenus Dependovirus
The invention discloses a preparation method of a tomato spotted wilt virus nucleic acid standard substance. The virus nucleic acid standard substance is prepared by the following steps of selecting agene conserved region as an amplification target region; designing primers and synthesizing; carrying out sequence amplification; constructing an expression vector; carrying out in-vitro transcription; measuring the concentration and diluting; performing split charging; and carrying out detection quantification. The standard substance prepared by the method has the characteristics of stability, no biological infectivity, wide application range and the like, and can be used for detecting the tomato spotted wilt virus by utilizing reverse transcription PCR and fluorescent quantitative PCR.
Owner:昆明海关技术中心
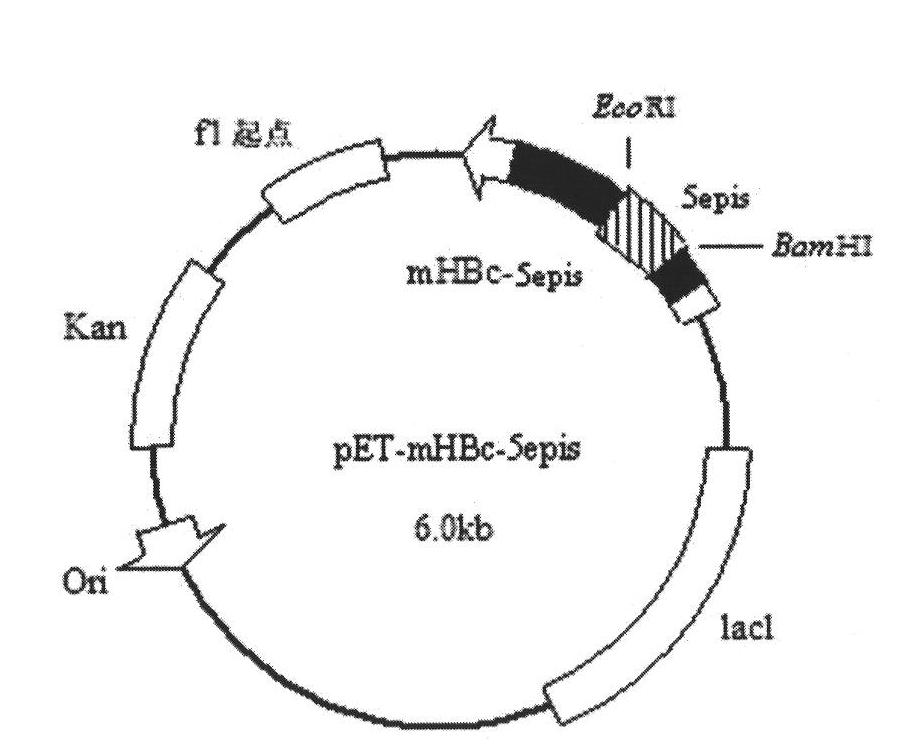
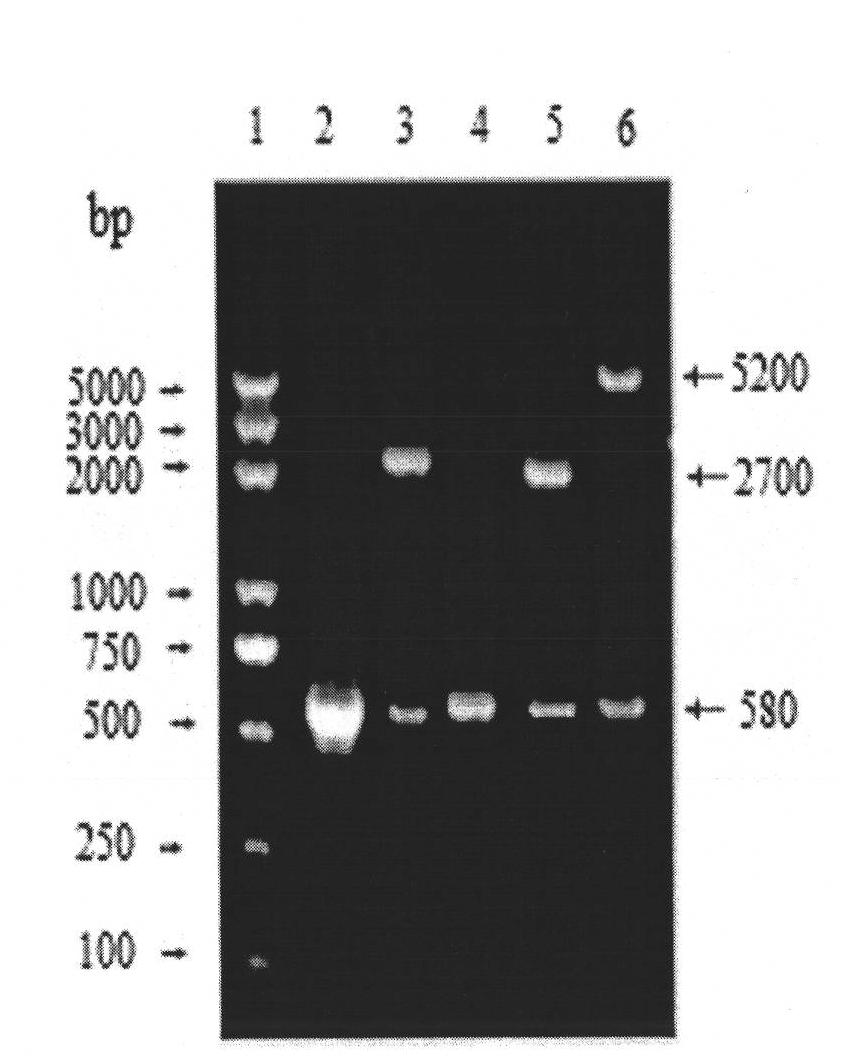
Molecular design of IBDV (Infectious Bursal Disease Virus) antigen epitope and HBcAg (Hepatitis B Core Antigen) chimeric gene
InactiveCN101818162AImprove stabilityHigh expressionViral antigen ingredientsMicroorganism based processesGenetic engineeringPolymerase chain reaction
The invention relates to a molecular design of an IBDV (Infectious Bursal Disease Virus) antigen epitope and HBcAg (Hepatitis B Core Antigen) chimeric gene, belonging to the field of biological pharmacy. The molecular design comprises the following steps of: establishing an expression plasmid pET-mHBc of an HBcAg-modified gene by applying an overlap extension PCR (Polymerase Chain Reaction) technology; directionally inserting an infectious bursal disease virus gene 5epis into the expression plasmid mHBC gene, acquiring an expression plasmid pEt-mHBc-5epis, and expressing the pEt-mHBc-5epis in colibacillus, wherein the molecular weight of recombinant chimeric protein is about 29kDa, and the expression level accounts for about 47.6 percent of total bacterial protein, the diameters of virus-like particles are about 60 to 80nm, and the antigen valence reaches 2.0*102. The 5epis and HBcAg chimeric gene which is successfully established by adopting the molecular design efficiently expresses the recombinant chimeric protein with the IBDV antigen reactivity in the colibacillus, forms the virus-like particles, and has the application potential of becoming a granulated epitope-based IBD (Infectious Bursal Disease) genetic engineering vaccine.
Owner:中国人民解放军南京军区军事医学研究所 +1
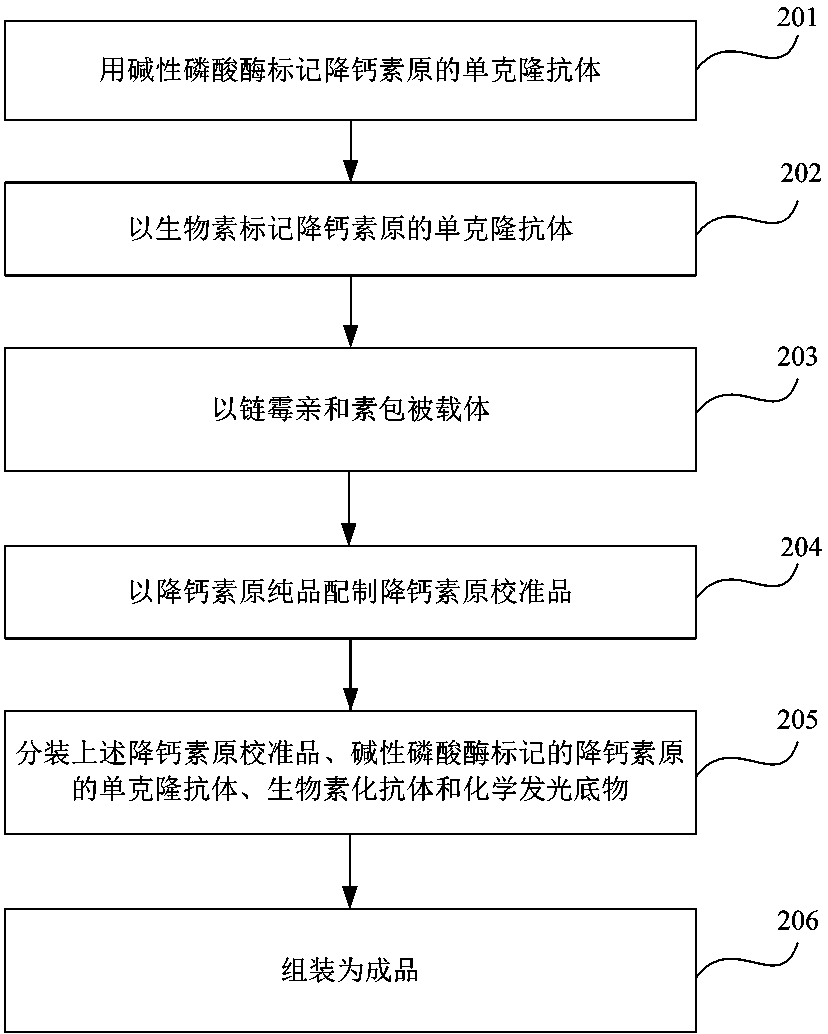
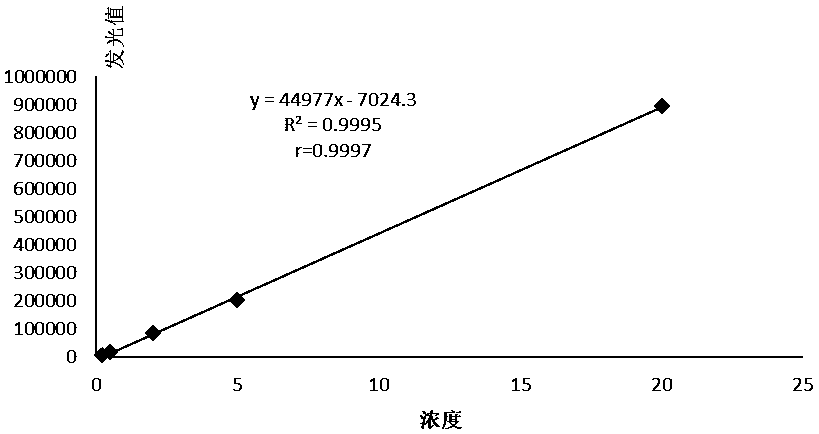
Kit for quantitatively detecting procalcitonin and preparation method thereof
InactiveCN108169489AStrong specificityHigh purityMaterial analysisBiotin-streptavidin complexMonoclonal antibody
The invention provides a kit for quantitatively detecting procalcitonin and a preparation method thereof. The kit comprises an alkaline phosphatase labeled monoclonal antibody of procalcitonin, a biotin labeled monoclonal antibody of procalcitonin, a carrier with coated streptavidin, a procalcitonin calibration product, and a chemiluminescent substrate for alkaline phosphatase. At the same time, the kit preparation method comprises following steps: labeling a procalcitonin monoclonal antibody by alkaline phosphatase; labeling a procalcitonin monoclonal antibody by biotin; coating a carrier bya procalcitonin monoclonal antibody; preparing a procalcitonin calibration product by using pure procalcitonin; split-charging the calibration product, the alkaline phosphatase labeled procalcitonin monoclonal antibody, the biotinylated antibody, and a chemiluminescent substrate; and carrying out assembly to obtain a finished product. The provided kit has the advantages of high sensitivity, good specificity, high accuracy of detection results of quantitative detection, low using cost, and easiness for popularization and application.
Owner:浙江艾明德生物科技有限公司
Magnetic targeting carrier capable of carrying gene and drug, preparation method and application thereof
InactiveCN101797387BFree from degradationNot easy to degradeGenetic material ingredientsInorganic non-active ingredientsMesoporous materialSuperparamagnetism
The invention discloses a magnetic targeting carrier capable of carrying genes and drugs, a preparation method and an application thereof. The invention is a carrier which has stability, safety and targeting and has controlled release behavior for non-viral magnetic gene therapy and drug therapy. The carrier material of the invention is characterized in that the carrier material is a bunchy silica mesoporous material with magnetism; the length-diameter ratio is not less than 3; the loading capability is big; the material has a protective effect on loaded genes and carriers and superparamagnetism, is not easy to agglomerate, and can control release speed of genes and drugs in vitro; and the surface thereof is easy to modify various functional groups, thus having wide adaptability. The invention also provides a preparation method of the carrier. When in use, therapeutic short chain DNA, siRNA or drugs enter in holes or are combined with surface modified functional genes by a soaking mode, then reach a targeted tissue by guidance of an applied magnetic field, and release the short chain DNA, siRNA or drugs carried thereby under the action of an alternating magnetic field, thus achieving the purpose of magnetic targeting controlled therapy.
Owner:CENT SOUTH UNIV
Methods and compositions for inhibiting HIV transmission
InactiveCN103167881ANeutralizing potencyNon-infectiousMilk immunoglobulinsAntiviralsImmunodeficiency virusHiv transmission
The present invention provides methods and compositions useful in the field of medicine, and particularly in the treatment of viral infections. More particularly, the invention relates to the use of methods and compositions for the inhibition of human immunodeficiency virus (HIV) transmission.
Owner:REEF PHARMA PTY LTD
PD-1 gene armored RNA standard substance and applications thereof
InactiveCN104531857AAvoid degradationImprove protectionMicrobiological testing/measurementFermentationFreeze thawingNucleic acid detection
The invention provides a PD-1 gene armored RNA standard substance and applications thereof. The RNA standard substance is prepared through the following steps: firstly, obtaining a W-based gene sequence for PD-1 nucleic acid detection, and then obtaining an expression vector containing MS2 phage mature zymoprotein genes and capsid protein genes, namely, a PET32a-CP vector; then, obtaining a prokaryotic expression vector PET32a-CP-PD-1 of W genes and CP genes; transferring the prokaryotic expression vector into a prokaryotic expression strain, carrying out inducible expression, carrying out frozen low-temperature freeze thawing, centrifuging, and purifying, so that an expression product particle is obtained; and carrying out DNase I digestion and purification, and then diluting the obtained product, so that the PD-1 gene armored RNA standard substance is obtained. The substance disclosed by the invention has the characteristics of stability, no biological infectivity, ribonuclease resistance, and the like, and is used as a standard substance in the nucleic acid detection of immune suppression factors PD-1.
Owner:ETEMUS BIOMEDICINE XIAMEN
Bovine-para-influenza virus 3 nano-PCR (polymerase chain reaction) detection kit and preparation method thereof
InactiveCN106906307ASpecific and accurateAccurate specificityMicrobiological testing/measurementMicroorganism based processesBovine parainfluenza virusPositive control
The invention discloses a bovine-para-influenza virus 3 nano-PCR (polymerase chain reaction) detection kit and a preparation method thereof, relates to the field of bovine para-influenza virus detection, and solves the problems that an existing bovine-para-influenza virus 3 detection method is low in sensitivity, serological detection is high in false positive and foreign importing reagents are expensive. The kit comprises a MightyAmp enzyme, 2xBuffer Mix solution, gold nanoparticle sol, aseptic double-distilled water, a pair of specific primers P1 (5'-GCTCTTCTCTTTTTGTCCCATTCTT-3') and P2 (5'-AACCCCTTCCTCAATCCTGATATAC-3') and a bovine-para-influenza virus 3 positive control plasmid. The sequence of the bovine-para-influenza virus 3 positive control plasmid is as shown in SEQ ID NO: 2. The kit is high in sensitivity, simple, convenient, rapid, efficient and low in cost.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
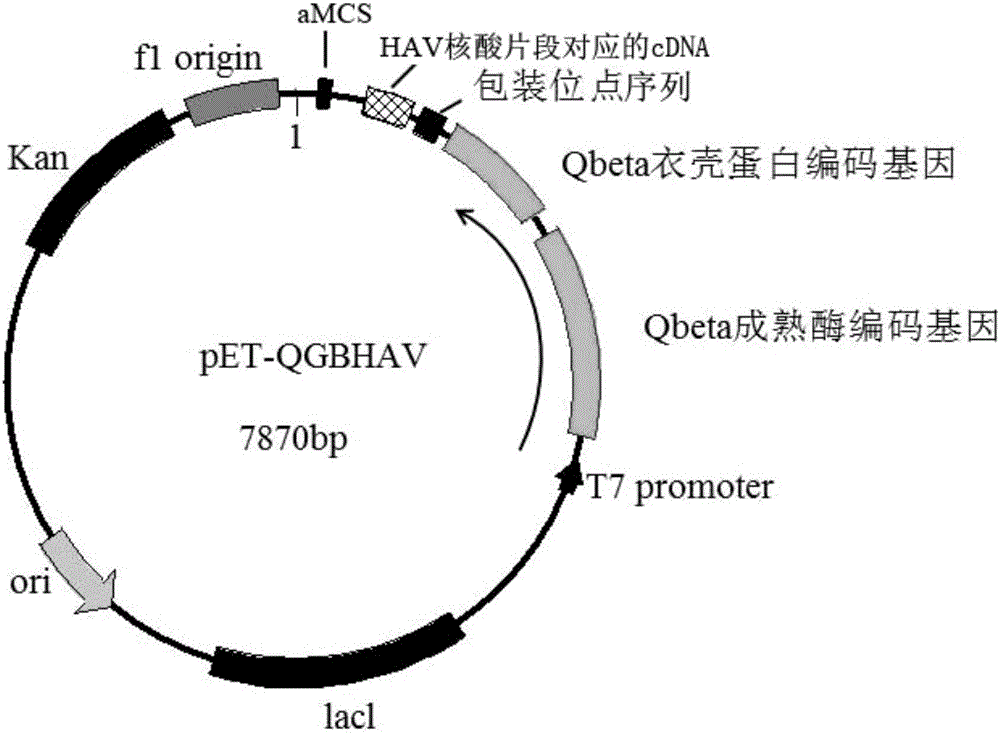
Pseudovirus particle comprising hepatitis-A-virus nucleic acid fragment and preparation method of pseudovirus particle
InactiveCN105907726ALow costReduce excess spaceSsRNA viruses positive-senseMicroorganism based processesHigh concentrationHepatitis A viruses
The invention provides a pseudovirus particle comprising a hepatitis-A-virus nucleic acid fragment and a preparation method of the pseudovirus particle. The pseudovirus particle comprises the hepatitis-A-virus nucleic acid fragment which can be used as a target for detecting hepatitis A virus. The preparation method includes creating a plasmid vector comprising a maturase coding gene, a capsid protein coding gene and a packaging site sequence of a coding Qbeta phage as well as a cDNA (complementary deoxyribonucleic acid) sequence corresponding to the hepatitis-A-virus nucleic acid fragment, performing transcription and / or translational expression on the plasmid vector in an escherichia coli cell, and performing separation and purification to obtain the pseudovirus particle. The pseudovirus particle can serve as a standard positive sample for detecting the hepatitis A virus, and is applied to hospitals, food sanitation testing organizations, quality testing organizations, scientific research institutions and other testing organizations. By the preparation method, the non-infectious pseudovirus particle with high concentration and stability can be prepared.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Pseudovirus particle capable of being used for evaluating activity of new coronavirus neutralizing antibody and serving as nucleic acid detection standard substance and preparation method of pseudovirus particle
PendingCN114075553ASolve biological safety hazardsNon-infectiousSsRNA viruses negative-senseSsRNA viruses positive-senseVirus ProteinBiological potential
The invention provides a pseudovirus particle which can be used for evaluating the activity of a new coronavirus neutralizing antibody and used as a nucleic acid detection standard substance and a preparation method of the pseudovirus particle. The problems of biological potential safety hazards caused by the adoption of new crown live viruses for neutralizing antibody effect evaluation in current new crown antiviral research and potential safety hazards such as virus replication possibly existing in preparation of a pseudovirus nucleic acid standard substance for new crown nucleic acid detection are solved. The pseudovirus particle takes lentivirus as a framework; the outer membrane protein is the outer membrane protein fused by a VSV-G protein and a new coronavirus S protein receptor binding motif; the core gene of the gene is an SARS-CoV-2 virus gene segment; the SARS-CoV-2 virus gene segment is a concatemer of seven RNA (Ribonucleic Acid) segments of the virus; the seven RNA fragments are respectively a basic group at the site 13340 to the site 13462, a basic group at the site 15430 to the site 15530, a basic group at the site 22721 to the site 22861, a basic group at the site 26269 to the site 26382, a basic group at the site 28706 to the site 28834, a basic group at the site 28880 to the site 28981 and a basic group at the site 29741 to the site 29890 of the virus gene.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Inoculation method for infecting hibiscus cannabinus with multan cotton leaf curl virus
InactiveCN105219801ANon-infectiousInfection achievedGenetic engineeringFermentationVascular bundleHibiscus
The invention belongs to the field of plant protection, and particularly relates to an inoculation method for infecting hibiscus cannabinus with multan cotton leaf curl virus. The method comprises the steps that a manual injection method is adopted for conducting infectivity clone inoculation on mesophyll tissue and stem vascular bundle tissue of hibiscus cannabinus plants in the 4-5 leaf stage through multan cotton leaf curl virus mediated by agrobacterium tumefaciens, and then hibiscus cannabinus is infected by the multan cotton leaf curl virus. Compared with a gene gun blasting method frequently adopted by the pathogenicity research of the multan cotton leaf curl virus at present, the method has the advantages of being free of special instruments, easy to implement, accurate in result, good in repeatability, low in cost and the like, and is simple, convenient to implement, economical and effective.
Owner:PLANT PROTECTION RES INST OF GUANGDONG ACADEMY OF AGRI SCI
Harmless brucella detecting method
InactiveCN108743995ADetection securityReduce Personal Safety RisksMicrobiological testing/measurementMicroorganism based processesFungicideSecretion
The invention relates to a harmless brucella detecting method. The method includes that genital secretions, eye secretions, feces or tissues and organs of diseased animals are obtained by utilizing the bacteria killing effect of a fungicide, possible brucella is killed with the fungicide, but possible brucella DNA still remains, the purified brucella DNA can be obtained by laboratory cleaning, andharmless detection of the brucella is carried out by the steps of conventional PCR, electrophoresis and the like. The method has the greatest advantage of harmlessness. On the basis of the advantagesof high detection accuracy and detection speed, the detection of the brucella is safer and non-toxic, and personal safety risks of breeders, veterinarians, samplers, laboratory personnel, epidemic prevention personnel, butchers and citizens are reduced.
Owner:董武 +1
A method for efficiently expressing pcv2cap and pcv3cap fusion proteins
ActiveCN111187353BImprove expression efficiencySpeed up expressionAntibody mimetics/scaffoldsVirus peptidesSecretion expressionNucleic acid sequencing
The invention provides a method for efficiently expressing PCV2 Cap and PCV3 Cap fusion proteins. First, construct a recombinant baculovirus that efficiently expresses PCV2Cap and PCV3Cap proteins: the nucleic acid sequence of the PCV2Cap protein with the nuclear localization signal cut off and the nucleic acid sequence of the PCV3Cap protein Connection, a hydrophobic flexible protein linker is used in the middle, and a beeline signal peptide sequence is added to the N-terminus of the PCV2Cap and PCV3Cap sequences to promote their secretion and expression. The protein expressed by the recombinant positive baculovirus infected sf9 insect cells undergoes various post-translational modifications, which is close to the natural virus-encoded protein and has high biological activity. Moreover, the baculovirus is highly species-specific and only infects Insect cells are non-infectious to vertebrate cells, and their expression products are safe and reliable, and can be used in follow-up experiments after simple treatment, which is safer and more effective than traditional methods.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Anti-cancer drug composition and preparation method thereof
InactiveCN102698257AGood attackImprove anti-cancer effectPowder deliveryPeptide/protein ingredientsSide effectCancer cell
The invention relates to an anti-cancer drug composition. The anti-cancer drug composition comprises active ingredients: NDV (Newcastle Disease Virus) particles and NA (neuraminidase), and the activity of NDV is 10<6>-10<7> PFU / ml, and the activity of NA is 0.01-0.02u / ml. The anti-cancer drug composition has the advantages of wide anti-tumor spectrum, and obvious therapeutic effect, no obvious toxic or side effect, immunization, and direct killing effect on cancer cells. The invention further provides a preparation method of the anti-cancer drug composition.
Owner:张炳团
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com