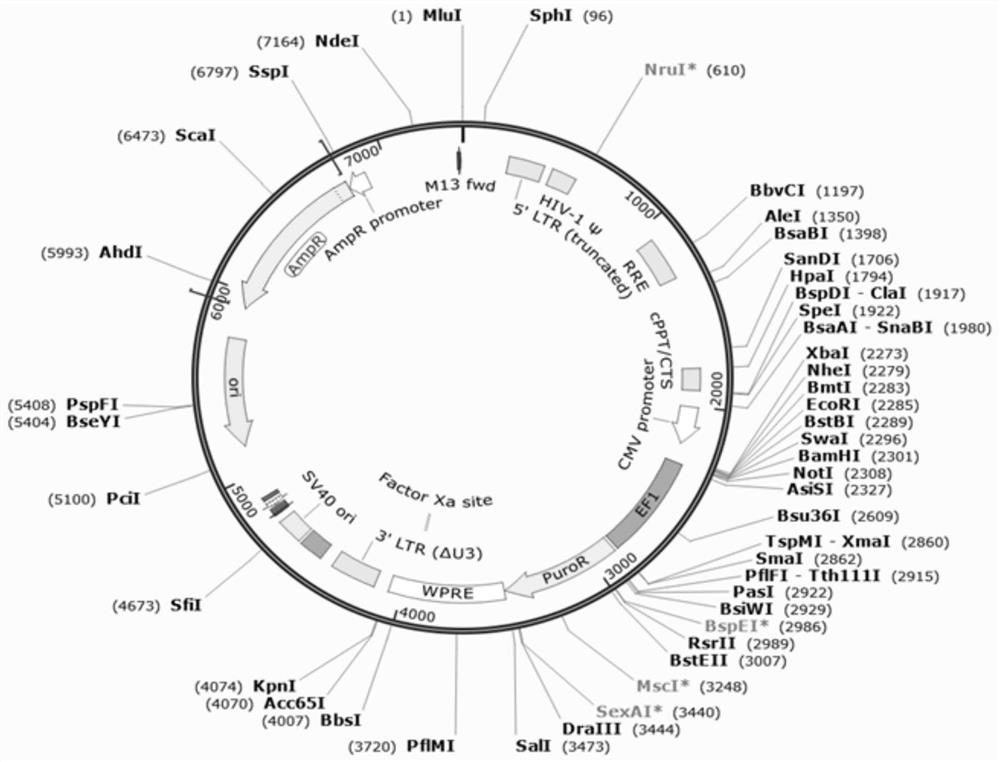
Pseudovirus particle capable of being used for evaluating activity of new coronavirus neutralizing antibody and serving as nucleic acid detection standard substance and preparation method of pseudovirus particle
A technology of fake virus particles and viruses, applied in the field of standardization, can solve problems such as biological safety hazards, virus replication safety, hidden dangers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The characteristics of the pseudovirion particles prepared by the present invention
[0075] Step S011: Inoculate 293T cells in each well of a six-well culture plate and grow to a monolayer density of 70%;
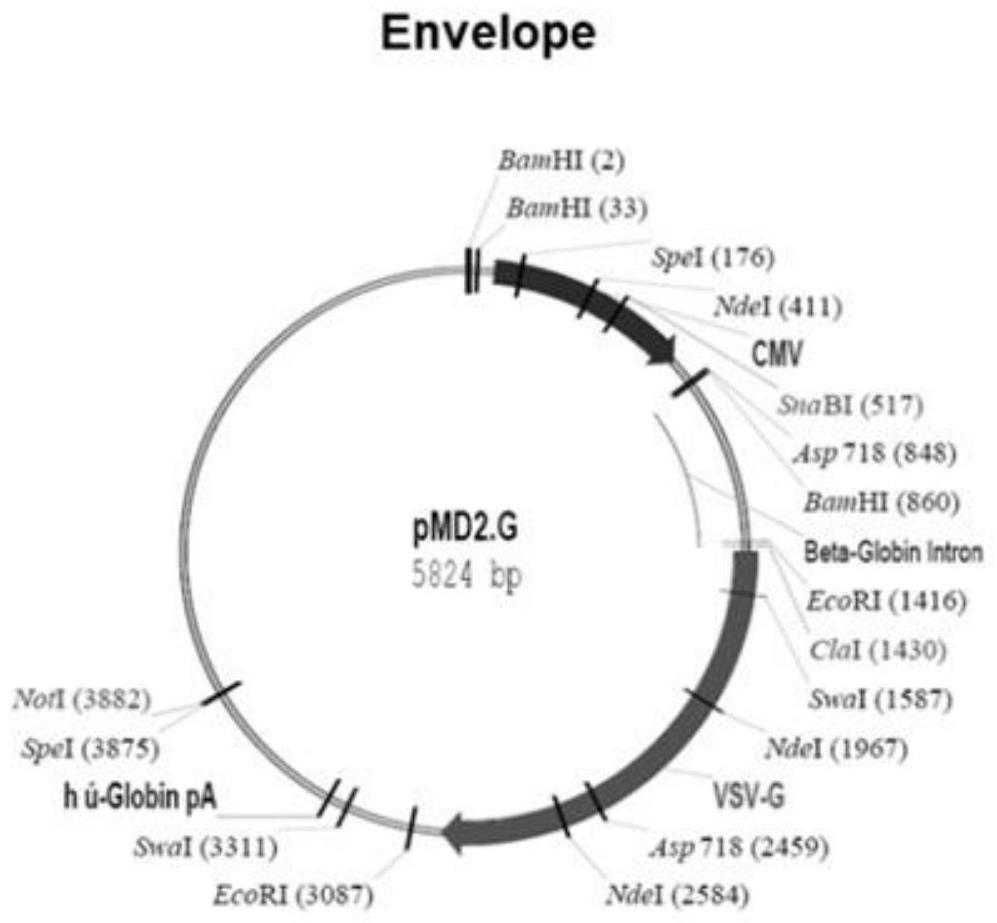
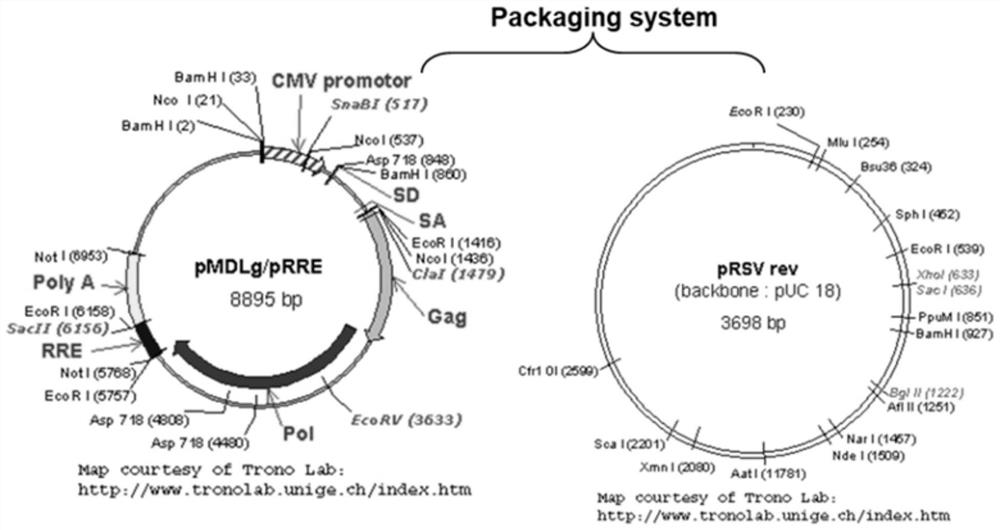
[0076] Step S012: mix the VSV-G-RBM envelope protein expression plasmid of the present invention, the new coronavirus 7-segment core expression plasmid, and the pMDLg / pRRE and pRSV rev plasmids with liposomes, and transfect 293T cells;
[0077] Step S013: 48 hours after transfection, collect the cell supernatant;
[0078] Step S014: Prepare a six-well cell culture plate, inoculate 293T cells, and grow to a monolayer density of 80%;
[0079] Step S015: inoculate 2 ml of the supernatant harvested in step S013 into each well of the cells in step S014, and continue culturing;
[0080] Step S016: After 48 hours, observe the proportion of fluorescent cells under a fluorescence microscope;
[0081] Step S017: It can be seen that about 60% of the cells can emit green flu...
Embodiment 2
[0088] Embodiment 2 anti-RBM antibody is to the blocking effect of pseudovirion infection Vero cell
[0089]Step S021: 6 BALB / c mice, 3 of which were controls, and the other 3 were intraperitoneally immunized with the new coronavirus S protein, 200ug / mouse / time, a total of 4 times, with an interval of one week between each time. Serum was collected one week after the fourth immunization for use.
[0090] Step S022: Inoculate Vero cells on a 96-well cell culture plate and grow to a monolayer density of 80%;
[0091] Step S023: Add 10ul of mouse immune serum to each well of A1 and A2; add 10ul of normal mouse serum to each well of B1 and B2; add 10ul of normal mouse serum to each well of C1 and C2;
[0092] Step S024: A1, A2, B1, B2, C1, C2, add 20ul of the pseudovirus particles harvested in Step S013 of Example 1 to each well, and continue to cultivate;
[0093] Step S025: observe the number of fluorescent cells in each well under a fluorescence microscope after 48 hours;
...
Embodiment 3
[0095] qPCR and ddPCR quantification of embodiment 3 pseudovirus particles
[0096] Step S031: Extract RNA from viral particles in the supernatant according to the novel coronavirus nucleic acid detection process;
[0097] Step S032: using Oligo dT(16t) as a primer to perform reverse transcription to obtain cDNA;
[0098] Step S032: The pseudovirus particle cDNA containing 861 base RNA of the new coronavirus is subjected to 2-fold serial dilution and quantified by real-time fluorescent quantitative PCR (qPCR) and digital droplet PCR (ddPCR). During the quantification process, SYBER GREEN was used as a fluorescent indicator, and specific primers for different fragments were used to perform real-time fluorescence quantitative PCR (qPCR) amplification and digital droplet PCR (ddPCR) amplification and quantification in parallel.
[0099] The results showed that it was difficult for qPCR to distinguish the 2-fold dilution of the new coronavirus gene template, the lack of regularit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com