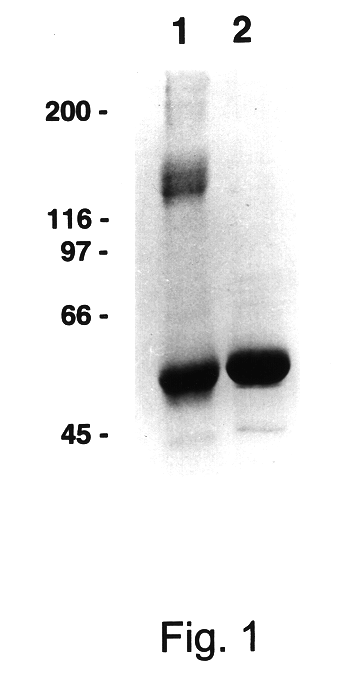
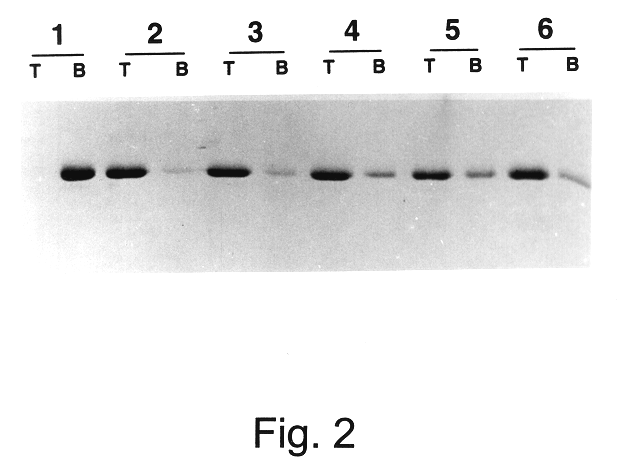
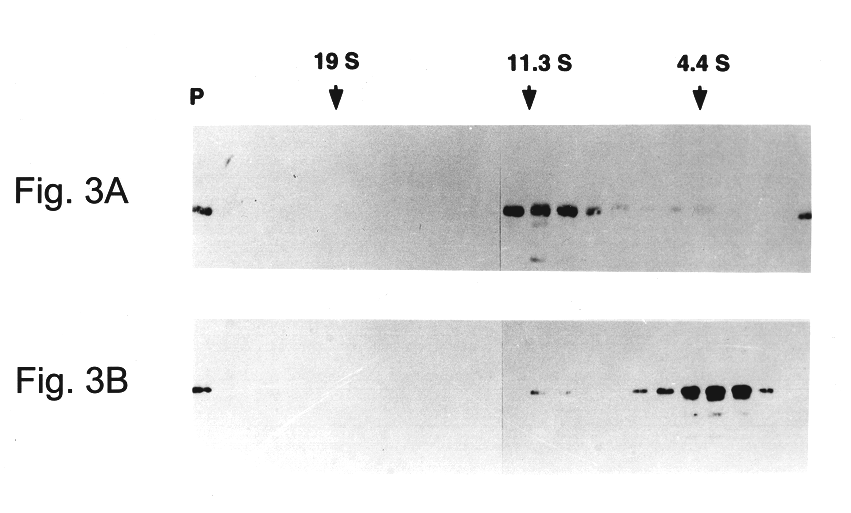
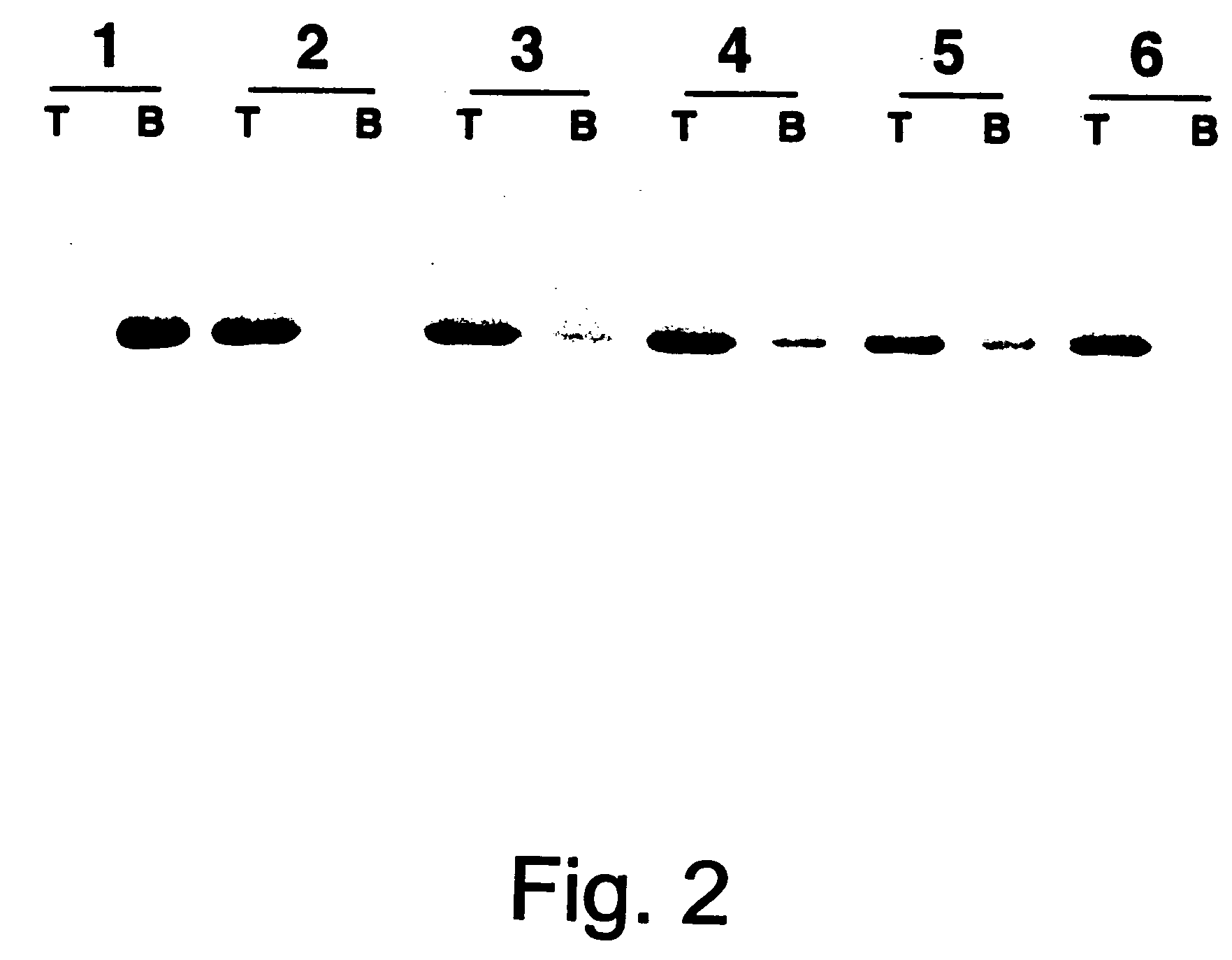
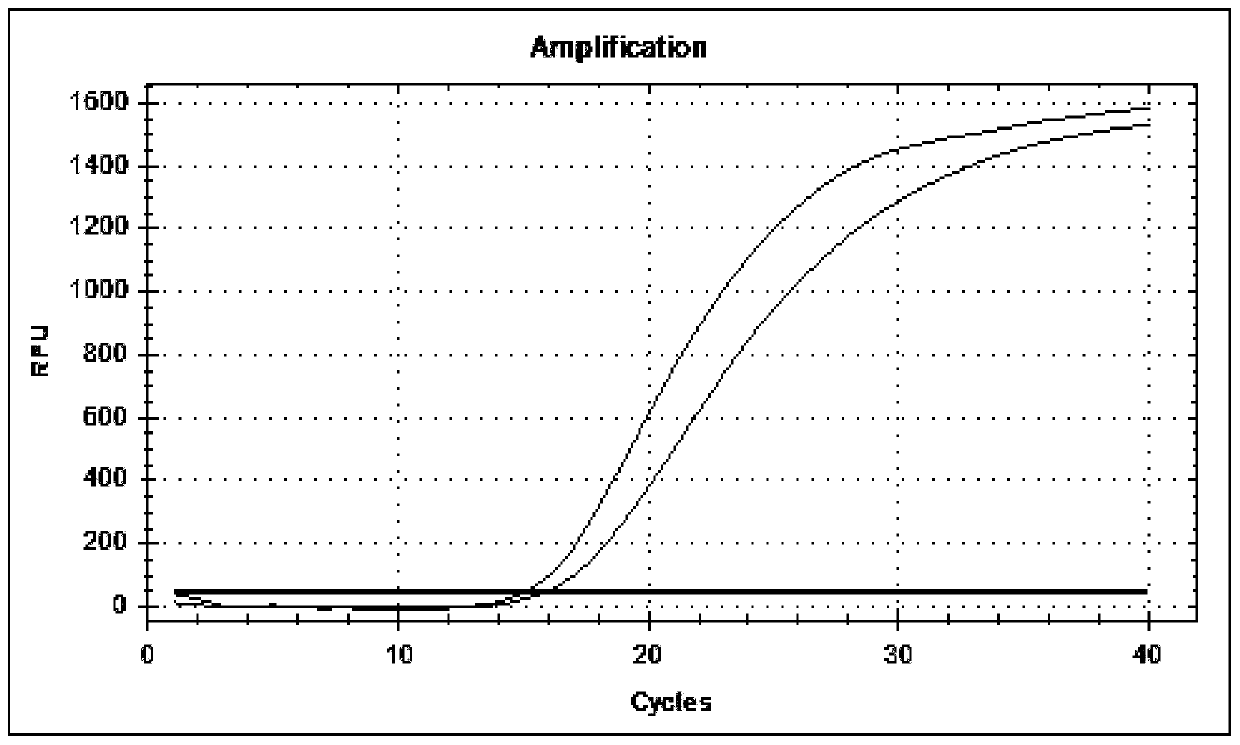
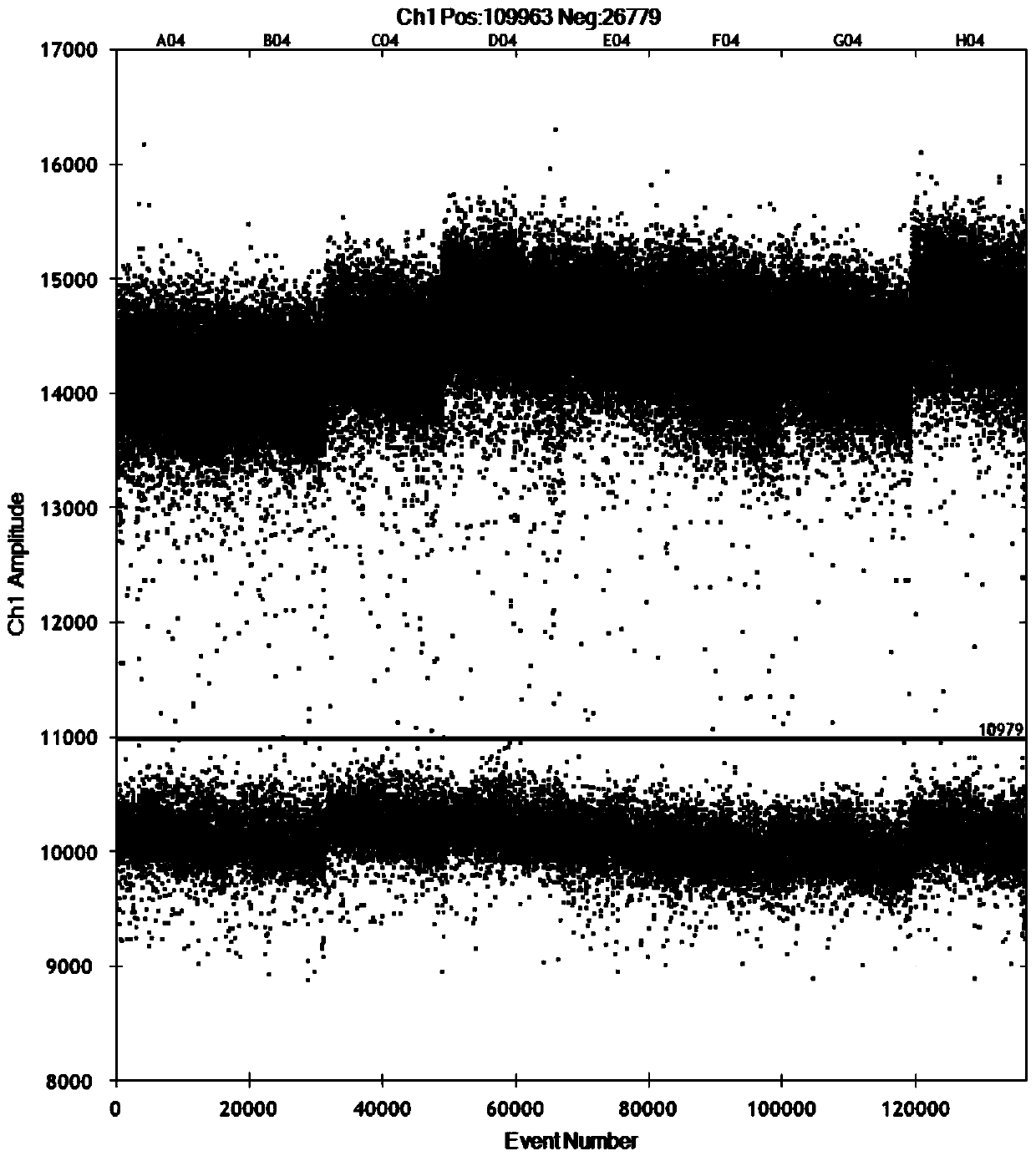
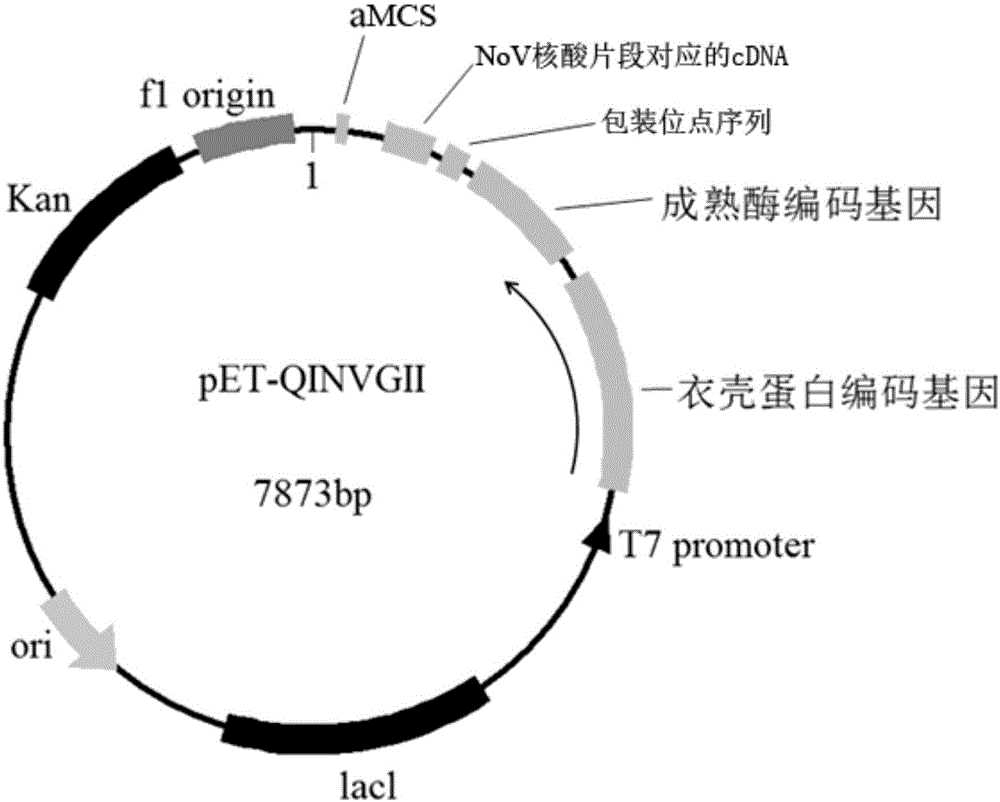
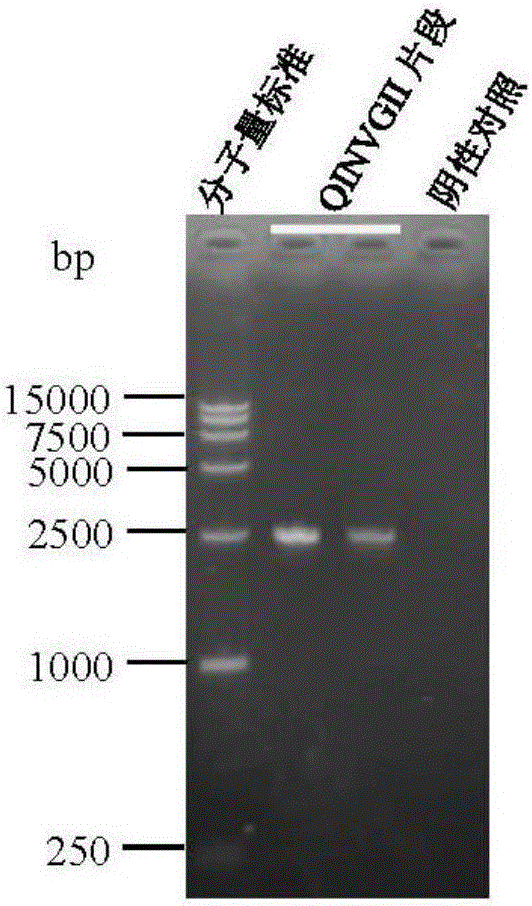
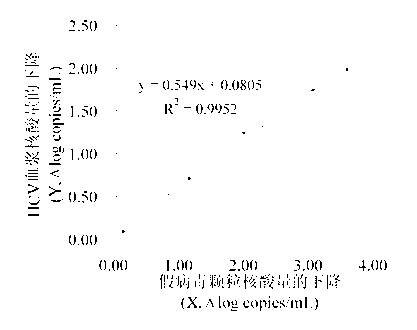Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Pseudovirion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pseudovirions are synthetic viruses used to inject genetic material, including DNA and RNA, with specific and desired traits into bacterial and eukaryotic cells. Pseudoviruses are closely related to viruses in structure and behavior but lack many characterists exhibited by true viruses, including the capability to replicate.
Vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPS)
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or "marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
In vitro method for disassmbly/reassembly of papillomavirus virus-like particles (VLPs). Homogeneous VLP and cavsomere compositions produced by said methods: use thereof as vehicle for improved purification, and delivery of active agents
InactiveUS7351533B2Increase ionic strengthStabilize VLPsMicroencapsulation basedMicrobiological testing/measurementEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavinis VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or marker” DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
In vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPs)
InactiveUS6261765B1Stabilize VLPsHigh strength conditionNanotechMicroencapsulation basedEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or "marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Momlv-based pseudovirion packaging cell line
The present invention discloses Moloney murine leukemia virus (MoMLV)-based viral packaging cell line for the production of anti-viral vaccines. The invention also includes methods of making, administering and formulating pseudovirions and replicon deficient viral particles of the invention and methods of inducing immunity.
Owner:BIOPROTECTION SYST
In vitro method for disassmbly/reassembly of papillomavirus virus-like particles (VLPs). Homogeneous VLP and cavsomere compositions produced by said methods: use thereof as vehicle for improved purification, and delivery of active agents
InactiveUS20040152181A1Stabilize VLPsIncrease ionic strengthMicroencapsulation basedMicrobiological testing/measurementEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavinis VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Pseudovirion vector and preparation method and application thereof
ActiveCN102559731AReduce cumbersome operationsQuality improvementMicrobiological testing/measurementMicroorganism based processesProtein containing complexNucleotide
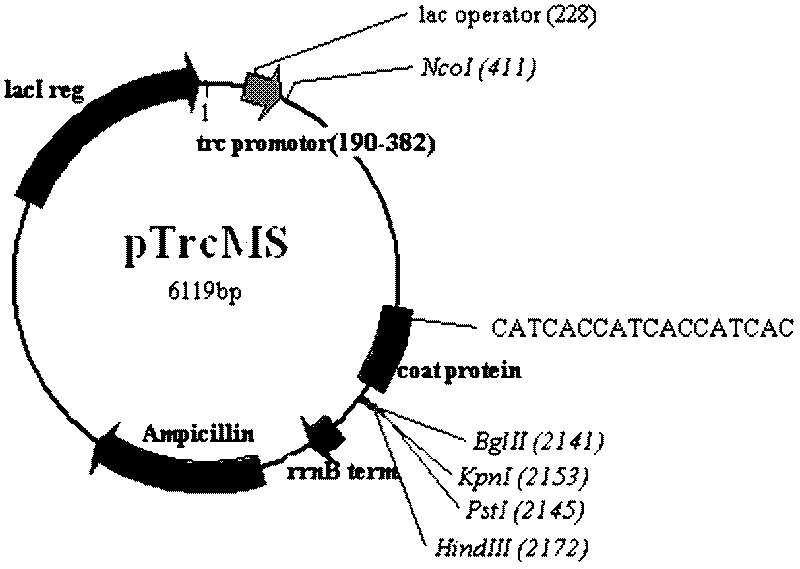
The invention discloses an animal pathogen microbial pseudovirion vector and a preparation technology thereof. The gene coding sequences of a maturase protein and a coat protein of an MS2 phage and a cDNA (complementary Deoxyribose Nucleic Acid) sequence which corresponds to a 5' non-coded sequence of a gene regulating element sequence included by a genome part are connected to the downstream of a pTrcHis2A vector promoter, so that a pseudovirion vector is constructed. A 6His purification tag is added between the fifteenth and sixteenth amine acids of the coat protein in the MS2 phage througha gene insertion method, a pseudovirion particle comprising an exogenous gene is expressed into an RNA (Ribonucleic Acid)-protein complex, and the pseudovirion particle is captured with a protein purifying method, so that the complex operating process for preparing the pseudovirion particle can be simplified, and the purification quality of the pseudovirion particle is enhanced simultaneously. The pseudovirion vector is pTrcMS of which the nucleotide sequence is shown as SEQ ID No.1.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Pseudovirions and preparation method and application thereof
InactiveCN105200018ANon-infectiousImprove stabilityMicrobiological testing/measurementInactivation/attenuationNucleic acid detectionRibonuclease
The invention discloses pseudovirions; a capsid protein of MS2 bacteriophage is wrapped with a DNA-protein complex of a detection sequence formed by series connection of three base fragments of influenza A virus, influenza B virus and beta-actin successively. The invention also provides a preparation method of the pseudovirions and an application of the pseudovirions as quality control products for detecting the influenza A virus and the hepatitis B virus. The preparation method of the pseudovirions is simple and is easy to operate; the obtained pseudovirus is high in purity, can be used as the quality control products in an influenza A virus and influenza B virus nucleic acid detection kit, and has the characteristics of no infectivity, good stability, ribonuclease resistance and the like.
Owner:GUANGDONG HECIN SCI INC
Pseudovirion containing hepatitis c virus RNA (Ribonucleic Acid) fragment and preparation method thereof
InactiveCN104845993ANo dangerImprove the simulation effectMicrobiological testing/measurementInactivation/attenuationEscherichia coliPHA granule
The invention provides a pseudovirion containing hepatitis c virus (HCV) RNA (Ribonucleic Acid) fragment and preparation method thereof which are applied in the field of biomedical clinical vitro diagnostic. The pseudovirion is a RNA-protein complexes that MS2 bacteriophage capsid protein wraps the hepatitis c virus, and the complexes is icosahedral; the method comprises the following steps of designing and synthesizing primer to obtain target gene MS2 by overlapping and splicing PCR (Polymerase Chain Reaction) method; connecting the target gene MS2 to plasmid pET-32a (+) to obtain recombinant plasmid pET-MS2; connecting the recombinant plasmid pET-MS2 and the HCV fragment to obtain pET-MS2-HCV recombinant plasmid; the obtained pET-MS2-HCV recombinant plasmid is introduced into escherichia coli for prokaryotic expression; releasing virus-like particles by adopting ultrasonication and the obtained virus-like particles are the pseudovirion containing the hepatitis c virus RNA fragment. The pseudovirion preparation method provided by the invention has the advantages that the preparation method is simple, the operation is easy, the obtained pseudovirus is high in purity, and the pseudovirion can be used as standard and quality control material of detection of RT-PCR (Reverse Transcription-Polymerase Chain Reaction), is good in stability, has no infectivity, has RNase-resistant, and the like.
Owner:宝瑞源生物技术(北京)有限公司
In vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPS), homogeneous vlp and capsomere compositions produced by said methods; use thereof as vehicle for improved purification, and delivery of active agents
InactiveUS6962777B1Stabilize VLPsIncrease ionic strengthMicrobiological testing/measurementVirus peptidesEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or “marker” DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Pseudoviral particle containing hepatitis e virus RNA (Ribose Nucleic Acid) fragment and preparation method thereof
InactiveCN103923887AImprove versatilityEasy to storeInactivation/attenuationMicroorganism based processesEscherichia coliPolyethylene glycol
The invention provides a pseudoviral particle containing a hepatitis e virus (HEV) RNA (Ribose Nucleic Acid) fragment and a preparation method thereof. The pseudoviral particle is an RNA-protein complex formed by coating hepatitis e virus RNA by an MS2 bacteriophage coat protein, and the RNA-protein complex is spherical. The preparation method comprises the steps of designing and artificially synthesizing a primer to obtain a target gene MS2 through a PCR (Polymerase Chain Reaction) method, connecting the target gene MS2 to a plasmid pET (polyethylene glycol terephthalate)-28b(+) to obtain a recombinant plasmid pET-28b / MS2 / HEV, guiding the recombinant plasmid into escherichia coli for prokaryotic expression, and settling a virus-like particle by adopting a polyethylene glycol method, wherein the virus-like particle is the pseudoviral particle containing the hepatitis e virus RNA fragment. The pseudoviral particle provided by the invention can be used as a standard substance and a quality control product of general HEV gene I-IV type RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection, and has the characteristics no infectivity, safety, reliability, high stability and resistance to ribonuclease.
Owner:JINZHOU MEDICAL UNIV
Preparation method and applications of H5N1 subtype bird flu pseudovirion
InactiveCN102191223ANo diseaseWill not cause diseaseMicrobiological testing/measurementMicroorganism based processesBird fluBiology
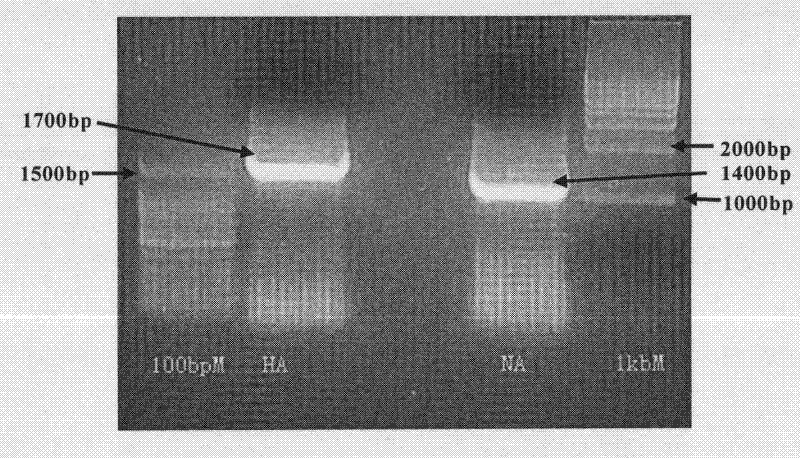
The invention relates to a preparation method and applications of an H5N1 subtype bird flu pseudovirion. Specifically, the application relates to a bird flu pseudovirion with packaging plasmid and the HA gene and NA gene of the highly virulent form of the bird flu virus and a preparation method and applications thereof; and the application also relates to a system for preparing the pseudovirion.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
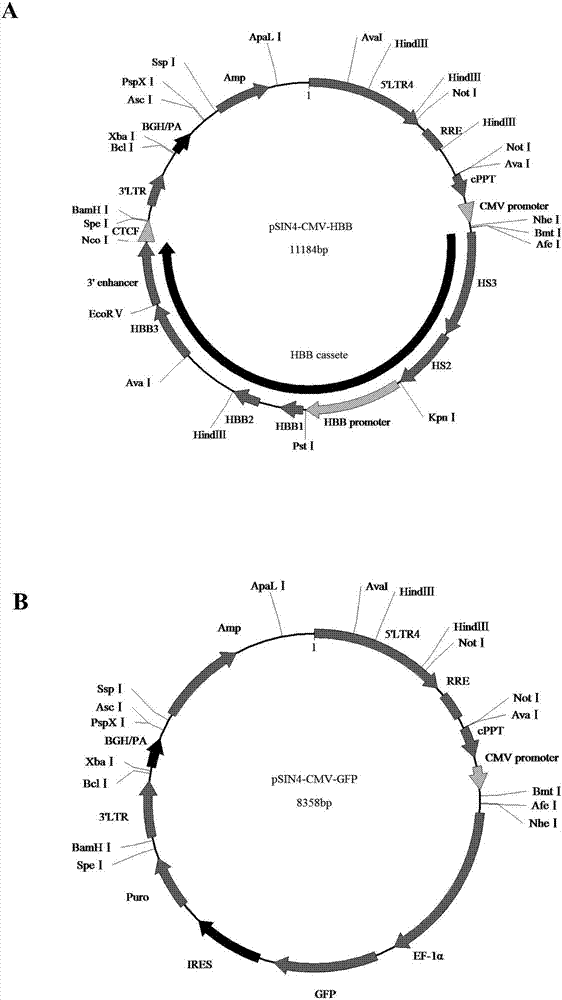
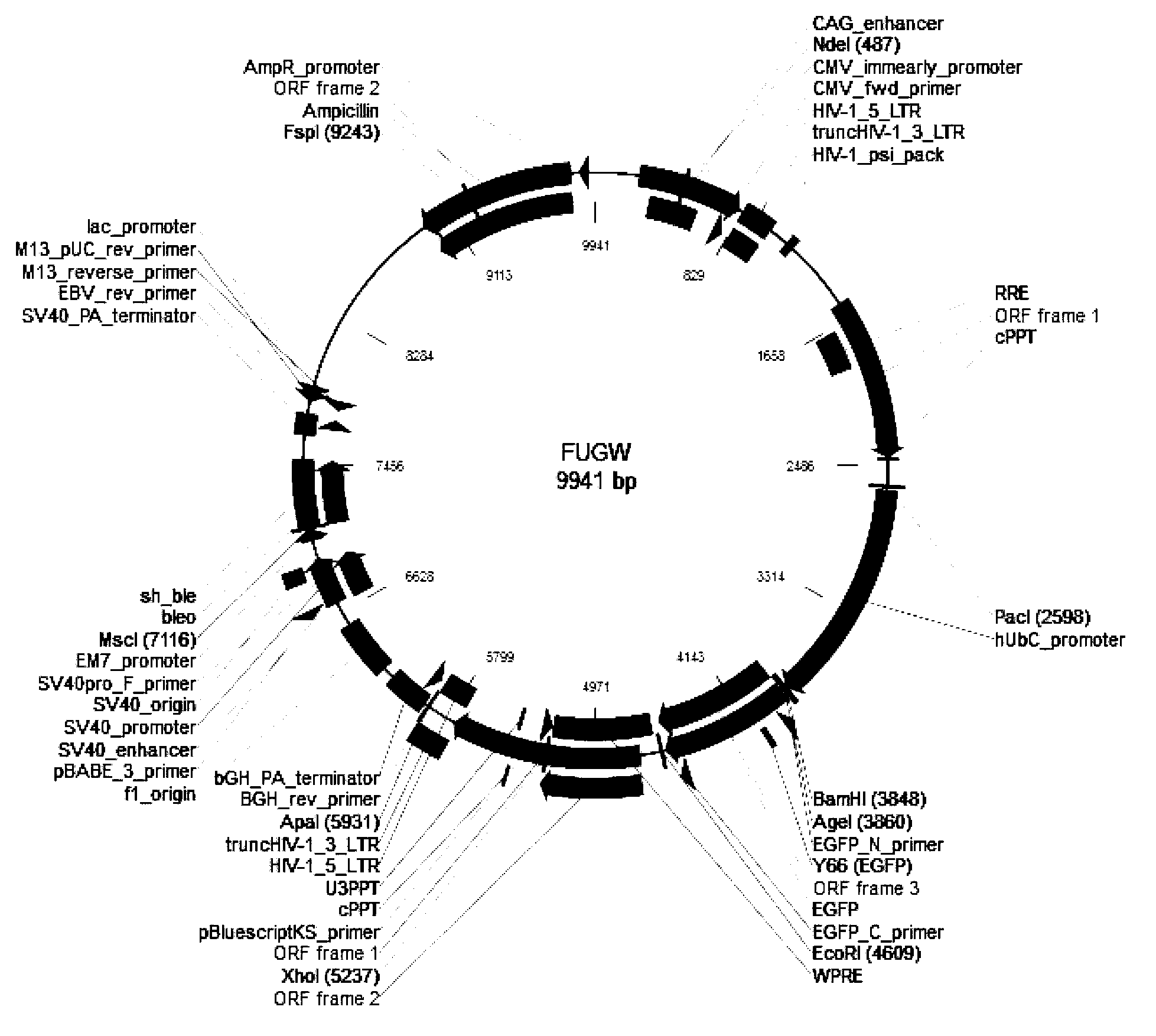
Beta-globin recombinant lentiviral vector and application thereof
Owner:济南赛尔生物科技股份有限公司
Kit for real-time fluorescence quantification RT-qPCR detection of hepatis c viral nucleic acid by means of one-step method
ActiveCN106119415AShorten the lengthReduce the chance of being degraded by enzymesMicrobiological testing/measurementFluorescenceReverse transcriptase
The invention discloses a kit for real-time fluorescence quantification RT-qPCR detection of hepatis c viral nucleic acid by means of a one-step method. The kit comprises a viral nucleic acid extraction reagent, a probe containing detection target polynucleotide and an internal control, RT-qPCR reaction buffer used for amplification of a target polynucleotide primer, an RT-PCR enzyme mixture containing warm start DNA polymerase, Revert AidTM M-MLV reverse transcriptase and RiboLockTM ribonuclease inhibitor, and a quantitative criterion reference containing virus-like particles. By the adoption of the novel hepatitis c virus target sequence probe and primer, a secondary structure and an incision enzyme site which cause degradation easily in a hepatis c virus 5'-UTR area are avoided innovatively, original viral load can still be reflected to the maximum degree even if viral nucleic acid is unstable, all six genotypes of hepatis c viral nucleic acid RNA in samples subjected to improper acquisition, transportation, storage and treatment can be tested, and accurate basis is provided for auxiliary diagnosis of hepatis c virus infection and monitoring of drug therapy of infectors.
Owner:山东探克生物科技股份有限公司
Preparation method of artificial dual false virus particle comprising HCV (hepatitis C virus) and HIV (human immunodeficiency virus) nucleic acid fragments
InactiveCN103789277AHigh clone purityImprove stabilityInactivation/attenuationFermentationHuman immunodeficiencyCompanion animal
Owner:东北制药集团辽宁生物医药有限公司
Novel coronavirus nucleic acid pseudovirus standard substance for detection and preparation method thereof
ActiveCN112359022AImprove securityImprove stabilitySsRNA viruses positive-senseMicrobiological testing/measurementRNA extractionFreeze-drying
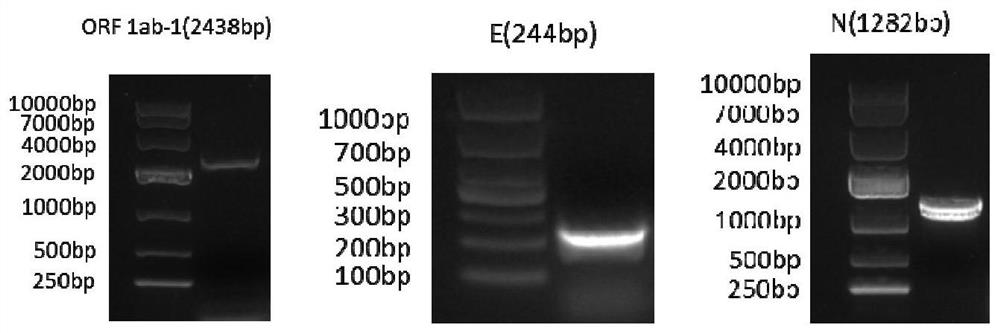
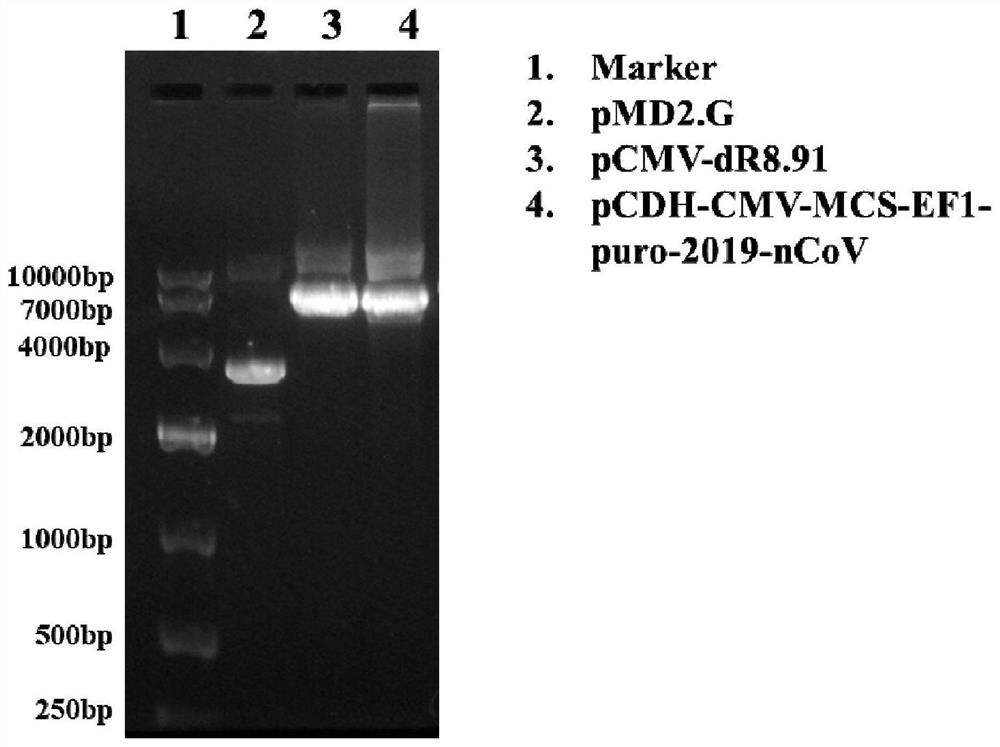
The invention relates to a pseudovirus standard substance for nucleic acid detection of a novel coronavirus (2019-nCoV or called SARS-CoV-2) and a preparation method of the pseudovirus standard substance. Novel coronavirus nucleic acid is integrated into a lentiviral expression plasmid vector, then constructed lentiviral expression plasmids and packaging plasmids are jointly transfected into cells, and a large number of pseudoviral particles packaged with novel coronavirus RNA are obtained after cell expression. After plasmid DNA is removed through DNase I digestion and sucrose density gradient ultracentrifugation, freeze drying is performed to obtain a pseudovirus particle freeze-dried product. The pseudovirus standard substance has the partial RNA sequence packaged with the virus and also has a pseudovirus standard substance packaged with all the sequences, can completely simulate the whole process of virus RNA extraction and nucleic acid detection, and can be used for the verification evaluation and the laboratory quality control of the novel coronavirus nucleic acid qualitative and quantitative detection method. The obtained standard substance has the characteristics of high purity, good safety, accurate quantity value and the like, is good in stability, can be transported at normal temperature, and provides guarantee for quantity value traceability and transmission of thestandard substance.
Owner:NAT INST OF METROLOGY CHINA
Japanese encephalitis virus JEV replicon vector and application thereof
InactiveCN101712965AFree from virus attacksViral antigen ingredientsGenetic material ingredientsForeign proteinTerra firma
The invention provides a replicon vector taking Japanese encephalitis virus genome as a framework, as well as a cell line for packaging the same and a packaging system. The JEV replicon vector has the capability of efficiently expressing foreign protein. Mice are immunized with the replicon vector, and the titer of an anti-JEV antibody reaches 1:1280 after three immunizations so as to protect 75 percent of suckling mice from virus attack. The cell line provided can produce 1.6*105 U / ml of pseudovirus particles after packaging JEV replicon, and the titer of the anti-JEV antibody reaches 1:2560 after two immunizations so as to protect 73 percent of suckling mice from virus attack. The invention establishes a technical platform for a JEV replicon vector system for the first time, and explores the feasibility of researching replicon vaccines and pseudovirus vaccines through the JEV replicon vector system, thereby laying a solid foundation for developing and researching a plurality of novel vaccines used to prevent and treat tumors and viral diseases in future.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
In vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPs), homogeneous VLP and capsomere compositions produced by said methods; use thereof as vehicle for improved purification, and delivery of active agents
InactiveUS20030096259A1Stabilize VLPsHigh strength conditionMicrobiological testing/measurementVirus peptidesEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or "marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
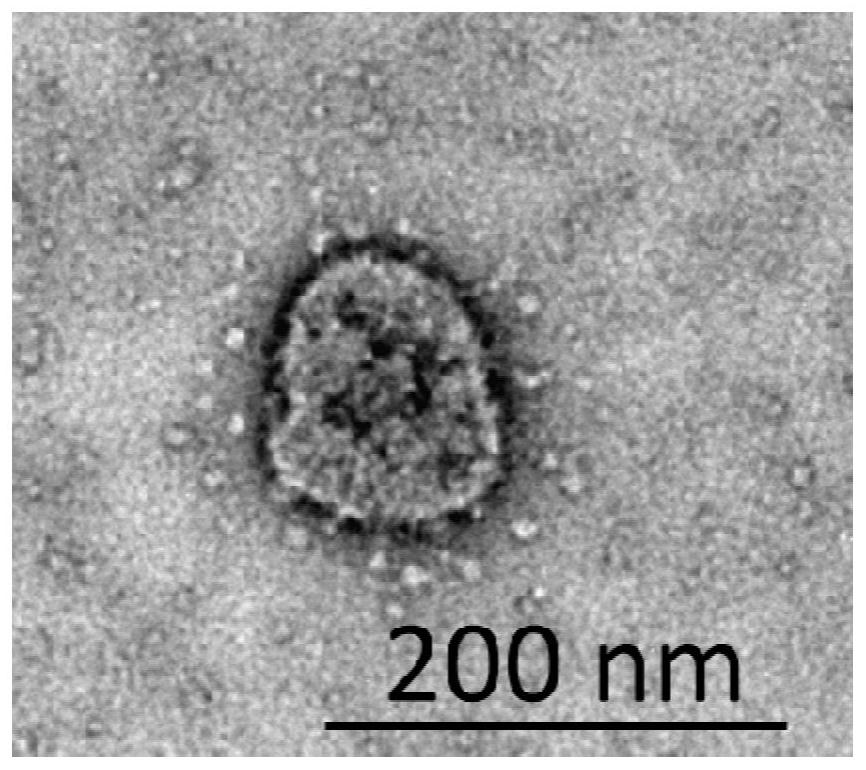
Novel coronavirus pseudovirus, and preparation method and application thereof
InactiveCN111575245AThe method is simple and effectiveImprove efficiencySsRNA viruses positive-senseMicrobiological testing/measurementPcr methodBioinformatics
The invention belongs to the technical field of biology, and particularly discloses a novel coronavirus pseudovirus, and a preparation method and application thereof. The prepared pseudovirus has no replication nor infection activity, and the biological safety is reliable. The suspension can reach about 10<6> copies / microliter, the indexes of uniformity and stability have no significant difference, and the requirements of positive reference substances can be met. Pseudovirus particles are used as a positive reference substance, and a triple fluorescent RT-PCR method is developed. The linear fitting coefficients between the nucleic acid content and the amplification Ct values of three target genes are all 0.99 or above, and the positive reference substance is calibrated at 1.2*10<4> copies / microliter. An RT-RPA constant-temperature isothermal amplification method is developed, and the detection limit can reach 10 copies / microliter.
Owner:武汉海关技术中心
Novel application of neferine to inhibition of SARS-CoV and SARS-CoV-2 infection
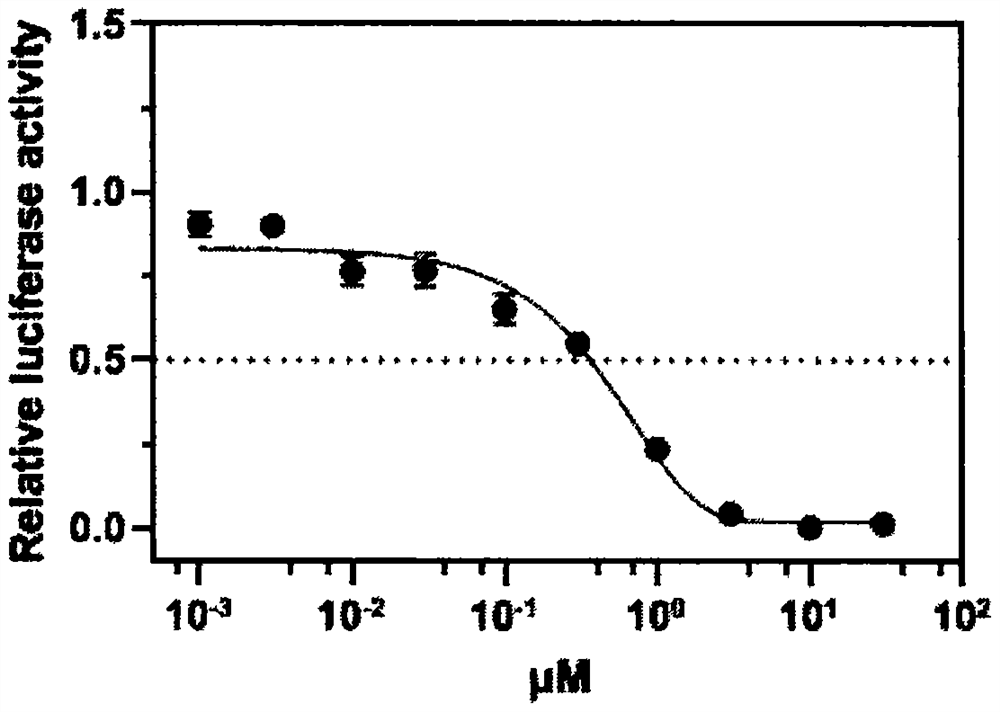
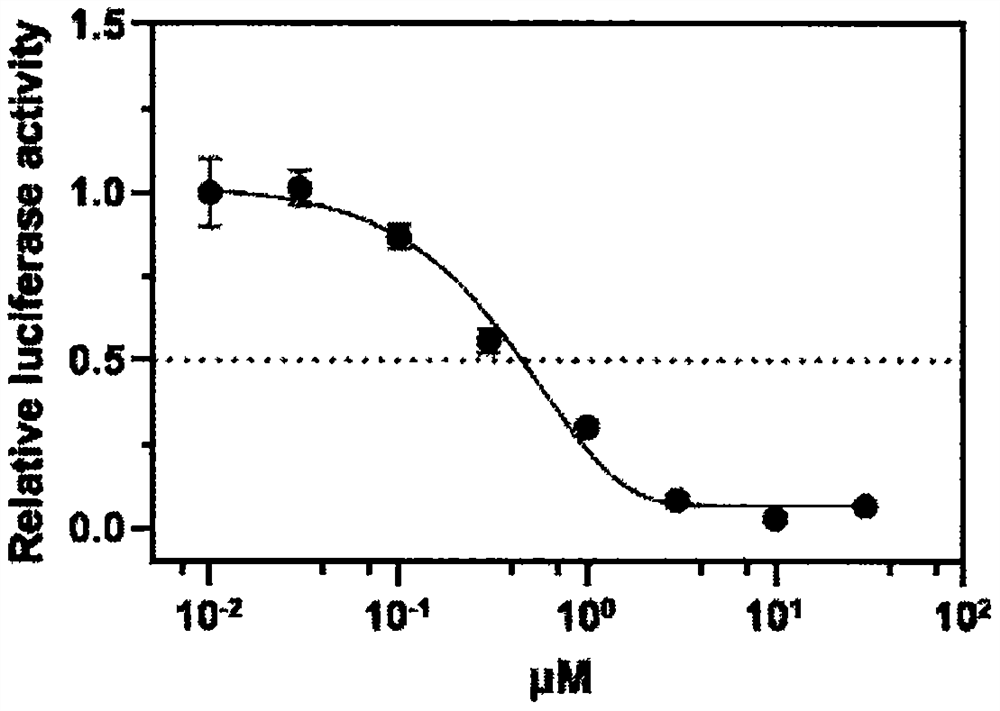
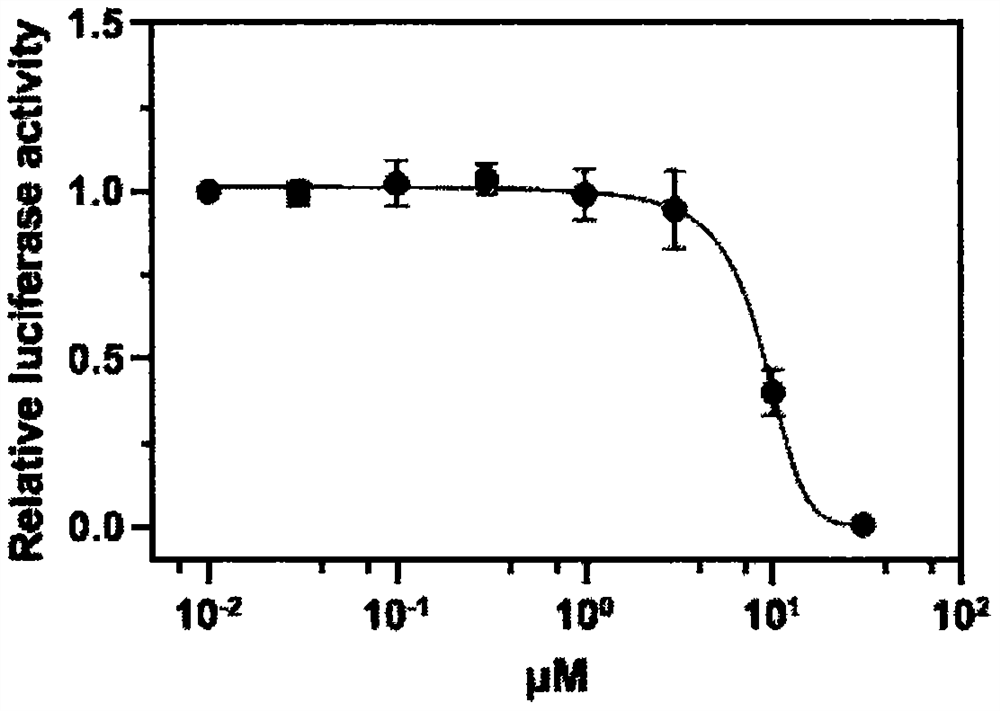
The invention discloses an application of neferine to inhibition of SARS-CoV and SARS-CoV-2 infection, and belongs to the technical field of natural medicines. Experimental results show that the neferine (Nef) can be used for safely and effectively inhibiting infection of SARS-CoV and SARS-CoV-2 pseudovirus particles on host cells. Along with the increase of the concentration of the neferine, the infection rate of pseudovirus particles to cells is gradually reduced. The median inhibitory concentration (IC50) of the neferine acting on the SARS-CoV pseudovirus is 0.43[mu]M, and the IC50 of the neferine acting on the SARS-CoV-2 pseudovirus is 0.36 [mu]M. In addition, the CCK8 result shows that the median toxicity concentration (CC50) of the neferine is 29.17 [mu]M, and the calculated selectivity indexes (SI=CC50 / IC50) of the neferine are 67.84 and 81.03, respectively. Through administration at different time points and a VSV-G pseudovirus elimination experiment, the infection stage of the neferine acting on the SARS-CoV and the SARS-CoV-2 is confirmed. The research proves that the neferine can be used as a candidate inhibitor for SARS-CoV and SARS-CoV-2 infection, and the new application of the neferine as a clinical treatment medicine and preparation is shown.
Owner:ACADEMY OF MILITARY MEDICAL SCI
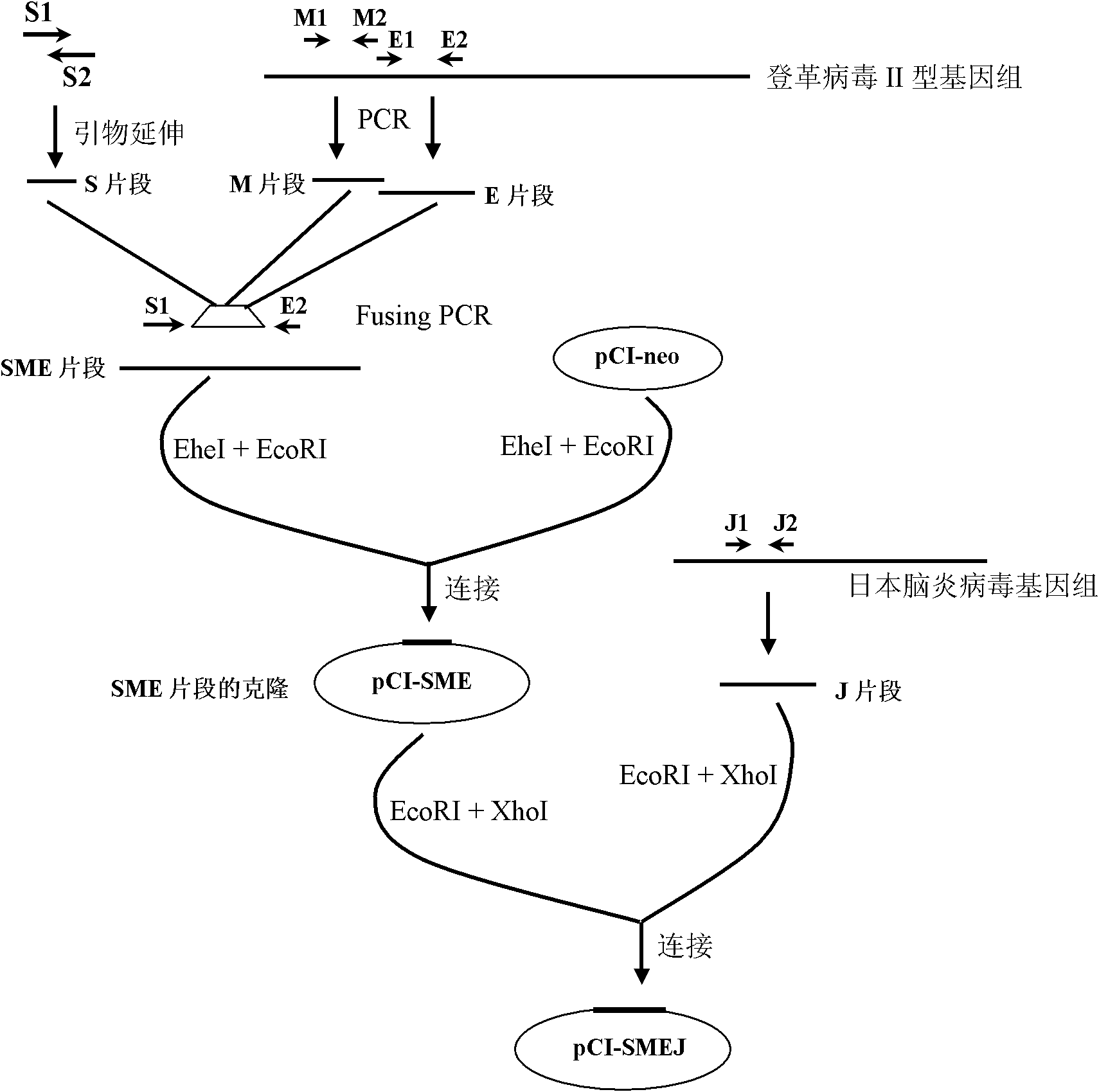
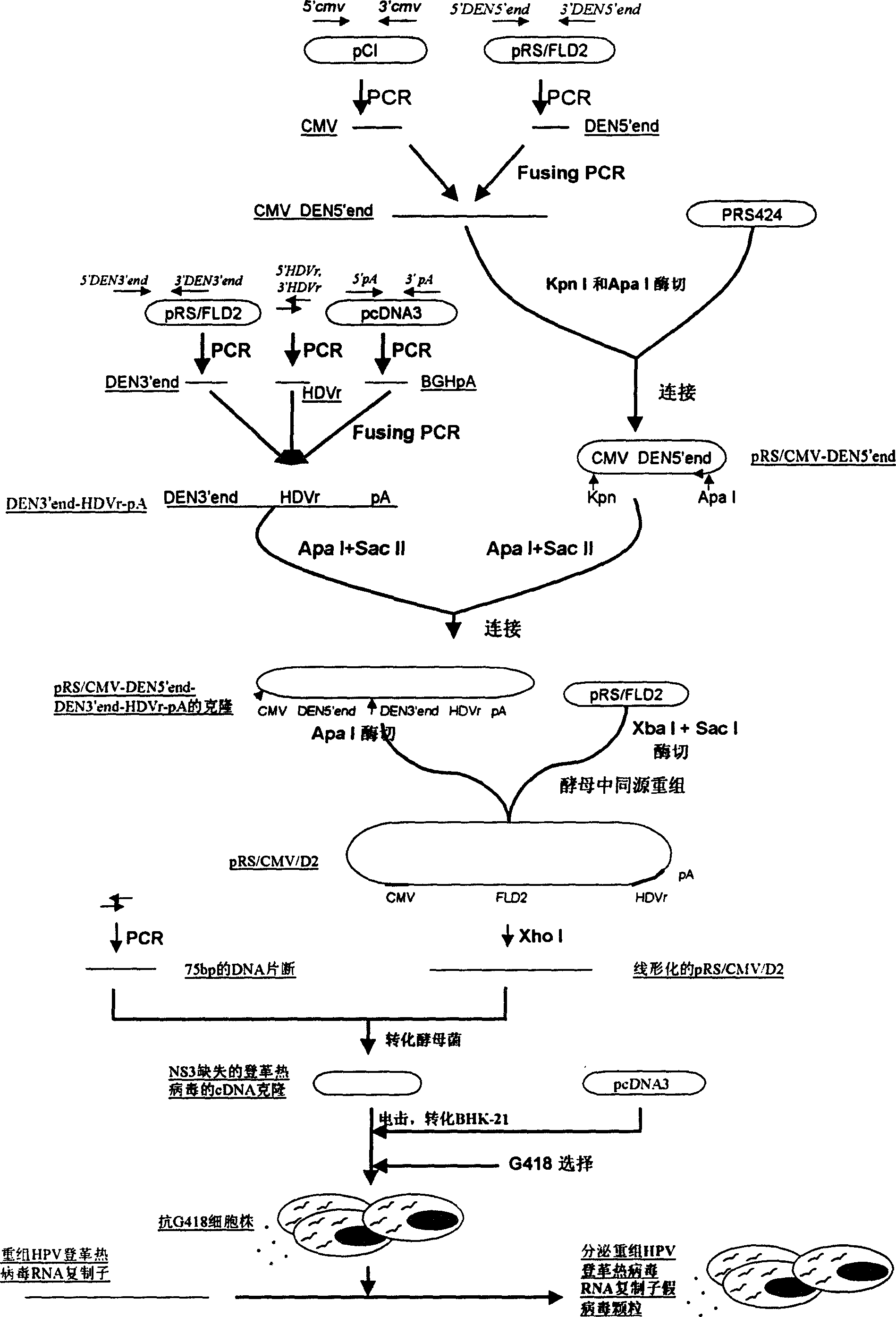
Dengue virus and Japanese encephalitis virus embedded pseudo virus particle vaccine and preparation method thereof
ActiveCN101899466AImprove effectivenessStimulate immune responseNervous disorderInactivation/attenuationStructural proteinViral Vaccine
The invention relates to a dengue virus and Japanese encephalitis virus embedded pseudo virus particle vaccine and a preparation method thereof. The embedded pseudo virus particles are formed by transfectting and expressing recombinant expression plasmids of a cloned dengue virus and Japanese encephalitis virus structural gene in target cells. The preparation method of the embedded pseudo virus comprises the following steps of: (1) constructing the recombinant expression plasmids containing a dengue virus-Japanese encephalitis virus structural protein coding sequence; (2) introducing the recombinant plasmid into the target cells and sieving stably-transfectted cells for cloning; (3) culturing the stably-transfectted cells so as to generate dengue virus-Japanese encephalitis virus embedded pseudo virus particles; and (4) extracting and purifying the embedded pseudo virus particles from cell culture supernatant fluid. The vaccine embedded with the dengue virus-Japanese encephalitis virus antigens can stimulate the organism to generate immune response, further prevents dengue virus and / or Japanese encephalitis virus infectious diseases, and has the advantages of good immune effect, safety and industrial production suitability.
Owner:ARMY MEDICAL UNIV
Novel microbicide anhydride modified anti-SEVI (semen-derived enhancer of viral infection) polyclonal antibody for preventing HIV (human immunodeficiency virus) sexual transmission
InactiveCN103736100ABlocking enhancementHighly effective anti-HIV activitySuppositories deliveryAntiviralsAnti virusHuman immunodeficiency
The invention provides a novel microbicide anhydride modified anti-SEVI (semen-derived enhancer of viral infection) polyclonal antibody for preventing HIV (human immunodeficiency virus) sexual transmission. The novel microbicide anhydride modified anti-SEVI polyclonal antibody comprises an anti-SEVI polyclonal antibody, wherein the anti-SEVI polyclonal antibody can be used for immunizing rabbit or other tested animals by a conventional method by synthesizing a polypeptide fragment PAP248-286 of SEVI, antiserum of anti-SEVI is collected, and IgG (immunoglobulin G) of the antibody is purified to obtain the polyclonal antibody. The anhydride modified anti-SEVI polyclonal antibody is obtained by performing anhydride modification on the antibody. The anhydride modified anti-SEVI polyclonal antibody has excellent anti-virus activity to HIV-1 pseudovirion strain, zoo virus strain and anti-HIV medicinal HIV-1 drug-resistance strain, and the anhydride modified anti-SEVI polyclonal antibody can specifically bind SEVI, so that the reinforcing effect of the SEVI in seminal fluid to HIV infection can be blocked. The antibody has the beneficial effects that a dual-functional HIV sexual transmission preventing candidate microbicide can be used for resisting the strengthening capability of SEVI to HIV infection and has high-effective HIV activity.
Owner:SOUTHERN MEDICAL UNIVERSITY
Preparation method of H7N9 pseudoviruses and applications of H7N9 pseudoviruses
ActiveCN105177043ASimple methodMicrobiological testing/measurementImmunoglobulins against virusesAntibodyAvian influenza virus
The invention discloses a preparation method of H7N9 pseudoviruses and applications of H7N9 pseudoviruses, which belong to the field of bio-medicine techniques. The preparation method comprises the following steps: cloning HA and NA gene sequences of H7N9 avian influenza viruses to mammalian expression vectors pVAX-1; and then, co-transfecting 293T cells by using pVAX-HA and retrovirus vectors pNL-4.3. Luc. E-R-isoplasmids, so that H7N9 pseudoviruses are generated. Cytostatics and neuraminidase inhibitors for inhibiting the entry of H7N9 avian influenza viruses are screened by using H7N9 pseudoviruses and a pseudovirus cell model, studies on influenza virus neutralizing antibodies are performed by using H7N9 pseudoviruses, and influenza virus infections are prevented and treated by using H7N9 pseudoviruses as anti-influenza immunological preparations.
Owner:杭州庆正鸿科技有限公司
Method for forming nerve cells by induction and composition
The invention discloses a method for forming nerve cells by induction and a composition. The invention provides a method for forming nerve cells by specially inducing colloid cells to differentiate, and specifically the method comprises the following steps: firstly, constructing a NeuroD1 gene on a virus vector, and packaging to obtain virus-like particles with NeuroD1; and after the NeuroD1 virus-like particles are infected with the colloid cells, cultivating for a certain time to differentiate the colloid cells into the nerve cells. The obtained never cells and the composition thereof can have potential value in treating neurodegenerative diseases such as Parkinsonism.
Owner:SHANGHAI GENECHEM
Pseudovirion particle and preparation method and application thereof
The invention discloses a pseudovirion particle and a preparation method and application thereof. The pseudovirion particle comprises RNA fragments of a monkey type D retrovirus encapsulated by MS2 phage capsid protein. The preparation method of the pseudovirion particle is simple, operation is easy, fineness of the obtained pseudovirion particle is high, and the pseudovirion particlecan be used as an external standard quality control product of a nuclein detecting method of monkey type D retrovirus detection. The method has the characteristics of high stability and nuclease resistance, a target template sequence exists only in the form of RNA, infectivity is avoided, and security is high.
Owner:海南出入境检验检疫局检验检疫技术中心
Pseudovirus particle containing human immunodeficiency virus RNA segment and preparation method thereof
ActiveCN106755043AImprove packaging efficiencyHigh purityInactivation/attenuationVector-based foreign material introductionConserved sequenceHuman immunodeficiency
The invention discloses a preparation method for a pseudovirus particle containing a human immunodeficiency virus RNA segment. The preparation method comprises the following steps: 1) preparing a clone plasmid containing a MS2 gene sequence; 2) screening an HIV gene conserved sequence and preparing an HIV clone plasmid; 3) performing double-plasmid cotransfection to induce the expression of the pseudovirus particle. The pseudovirus particle prepared according to the preparation method is obviously increased, is high in stability, can be stored for a long time, is high in purity, contains high-purity RNA, contains no DNS and is suitable for large-scale production and application.
Owner:GUANGZHOU SUPBIO BIO TECH & SCI
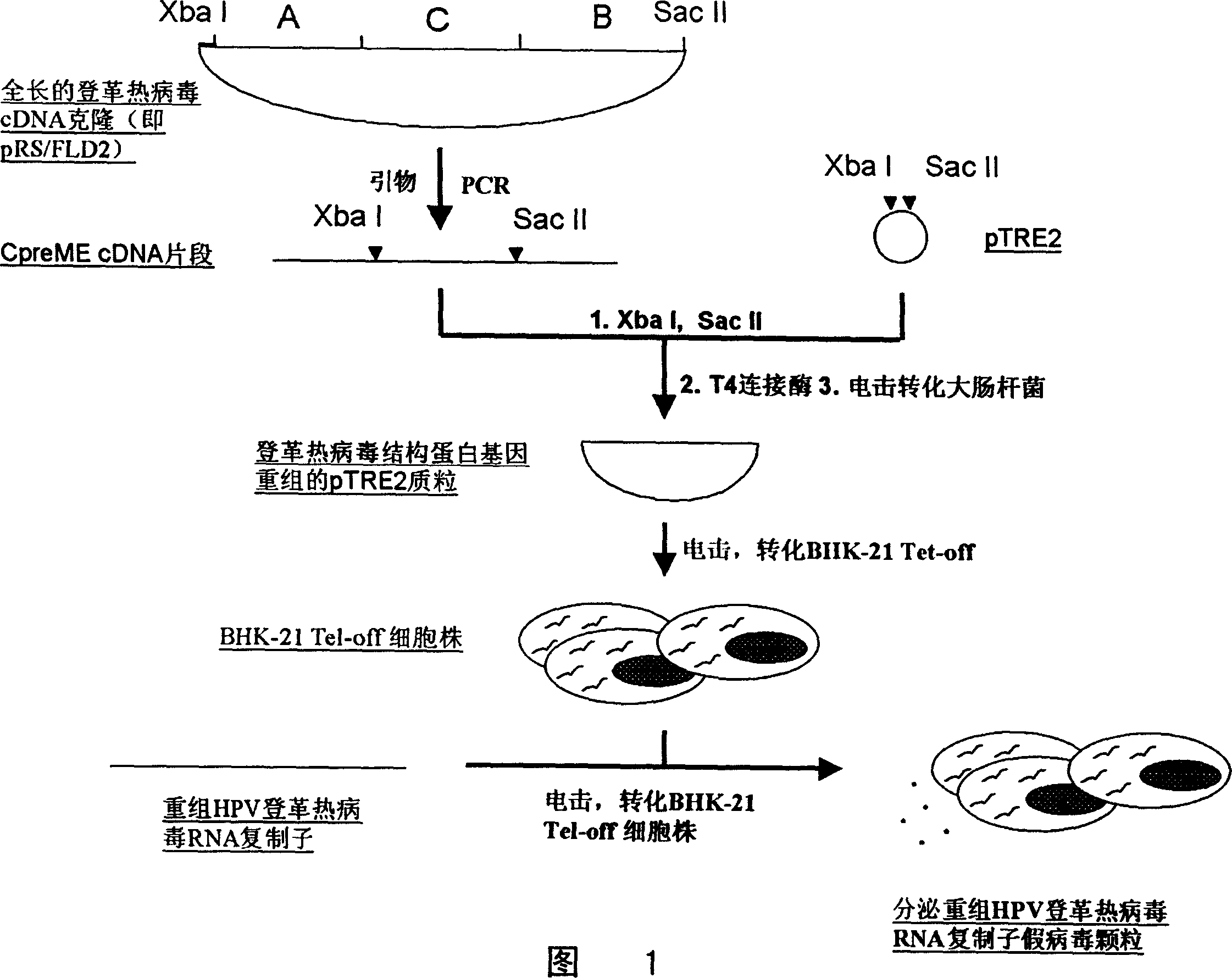
False virosome vaccine with recombinant replicon of dengue fever virus as carrier
InactiveCN1524952AImprove effectivenessEffective immunityViral antigen ingredientsViral/bacteriophage medical ingredientsDendritic cellDengue virus
The invention provides a false virus granule vaccine with dengue fever virus recombination replicon as a core and method for preparation, wherein the vaccine can express antigens with high efficiency in the affected cells, the antigen can be extracted effectively with good immunity effect. The vaccine can be used for preventing and treating tumor and virosis.
Owner:上海天甲生物医药有限公司 +2
Method for preparing lentiviral particle packaged EBOV RNA as positive reference substance for EBOV nucleic acid detection
InactiveCN110669793AHigh copy numberImprove stabilitySsRNA viruses negative-senseMicrobiological testing/measurementViral nucleic acidT cell
The invention discloses a method for preparing lentiviral particle packaged EBOV RNA as a positive reference substance for EBOV nucleic acid detection. The preparation method comprises the following steps: connecting part of segments of three genes NP, GP and L of EBOV in series and then integrating the segments into an expression vector of lentivirus, separately amplifying the constructed lentiviral expression plasmid and packaging plasmid in an engineering escherichia coli strain to obtain a large number of plasmids, then jointly transfecting the constructed lentiviral expression plasmid andpackaging plasmid into HEK293T cells, and carrying out cell expression to obtain a large number of lentiviral particles packaged with RNA genes of EBOV. The invention relates to the technical field of gene engineering. According to the method for preparing lentiviral particle packaged EBOV RNA as a positive reference substance for EBOV nucleic acid detection. The obtained particles are used as apositive reference substance for EBOV nucleic acid detection and have the advantage of well simulating the whole process from virus nucleic acid extraction to nucleic acid detection in an actual sample, and freeze-dried powder obtained by freeze-drying pseudovirus particles of EBOV through a freeze-drying protective agent is very stable.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
High-stability pseudoviral particles as well as plasmid vector and method for preparing pseudoviral particles
ActiveCN105950565ALow priceEasy to insertSsRNA viruses positive-senseMicroorganism based processesPlasmid VectorDNA fragmentation
The invention provides high-stability pseudoviral particles as well as a plasmid vector and a method for preparing the pseudoviral particles. The pseudoviral particles contain a nucleic acid fragment of norovirus, wherein the nucleic acid fragment can serve as a target for detecting the norovirus. The plasmid vector contains a maturase coding gene of a Qbeta phage, a capsid protein coding gene, a packaging site sequence and a DNA segment including a cDNA sequence in correspondence with the nucleic acid fragment. The method comprises the following steps: constructing the plasmid vector, then transcribing and / or translating the plasmid vector in escherichia coli cells, and conducting separating and purifying, so that the pseudoviral particles are obtained. With the application of the method disclosed by the invention, the pseudoviral particles, which contain the norovirus nucleic acid fragment functioning as the detection target, can be prepared; and the pseudoviral particles have the advantages of being free from infectivity, high in copy number, good in stability and the like.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
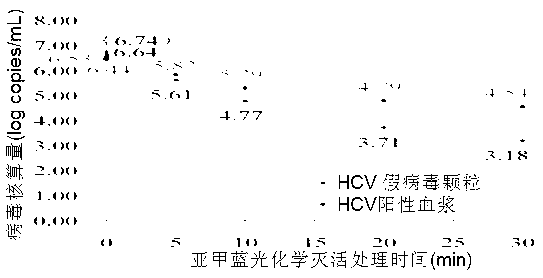
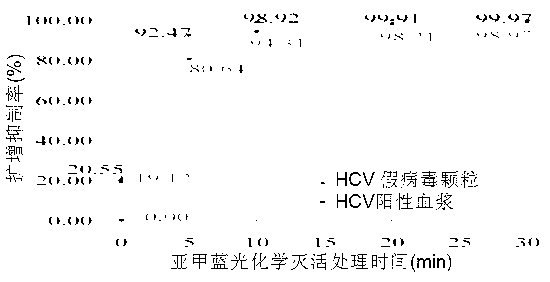
Detection method of photochemical inactivation effect of methylene blue on HCV pathogen
InactiveCN102965450AEasy to operateImprove accuracyMicrobiological testing/measurementMicroorganism based processesWhole blood productVirus inactivation
The invention provides a method for detection and evaluation of photochemical virus inactivation effect of methylene blue, which adopts HCV pseudovirion constructed by enveloped RNA technology as a quality control, and performs methylene blue photochemical virus inactivation of the quality control and the blood or blood products needing to be subjected to virus inactivation under a same conition. The method has no virus infection risk to human body, and can reflect the disappearance condition of HCV infection by the damage degree of pseudovirion nucleic acid.
Owner:SHANGHAI BLOOD CENT
Medicine composition for treating neuron degeneration disease
The invention discloses a medicine composition for treating neuron degeneration disease. The invention provides a method for forming neurons by specifically inducing spongiocyte differentiation. The method disclosed by the invention specifically comprises the following steps: firstly establishing a Myt1L (Myelin transcription factor 1-like) gene on a virus vector and packaging to obtain pseudovirus particles with Myt1L; and infecting spongiocyte by the pseudovirus particles with the Myt1L, and culturing for a period of time, thus differentiating the spongiocyte into neuron cells. The neuron cells and the composition thereof have a potential value in treatment of neuron degeneration diseases, such as Parkinson disease.
Owner:SHANGHAI GENECHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com