Dengue virus and Japanese encephalitis virus embedded pseudo virus particle vaccine and preparation method thereof
A technology of dengue virus and encephalitis virus, applied in the field of dengue virus-Japanese encephalitis virus chimeric pseudovirus as a vaccine research field, can solve the problems of poor effect and lack of particle structure of chimeric protein, so as to improve production efficiency , the effect of reducing risk and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
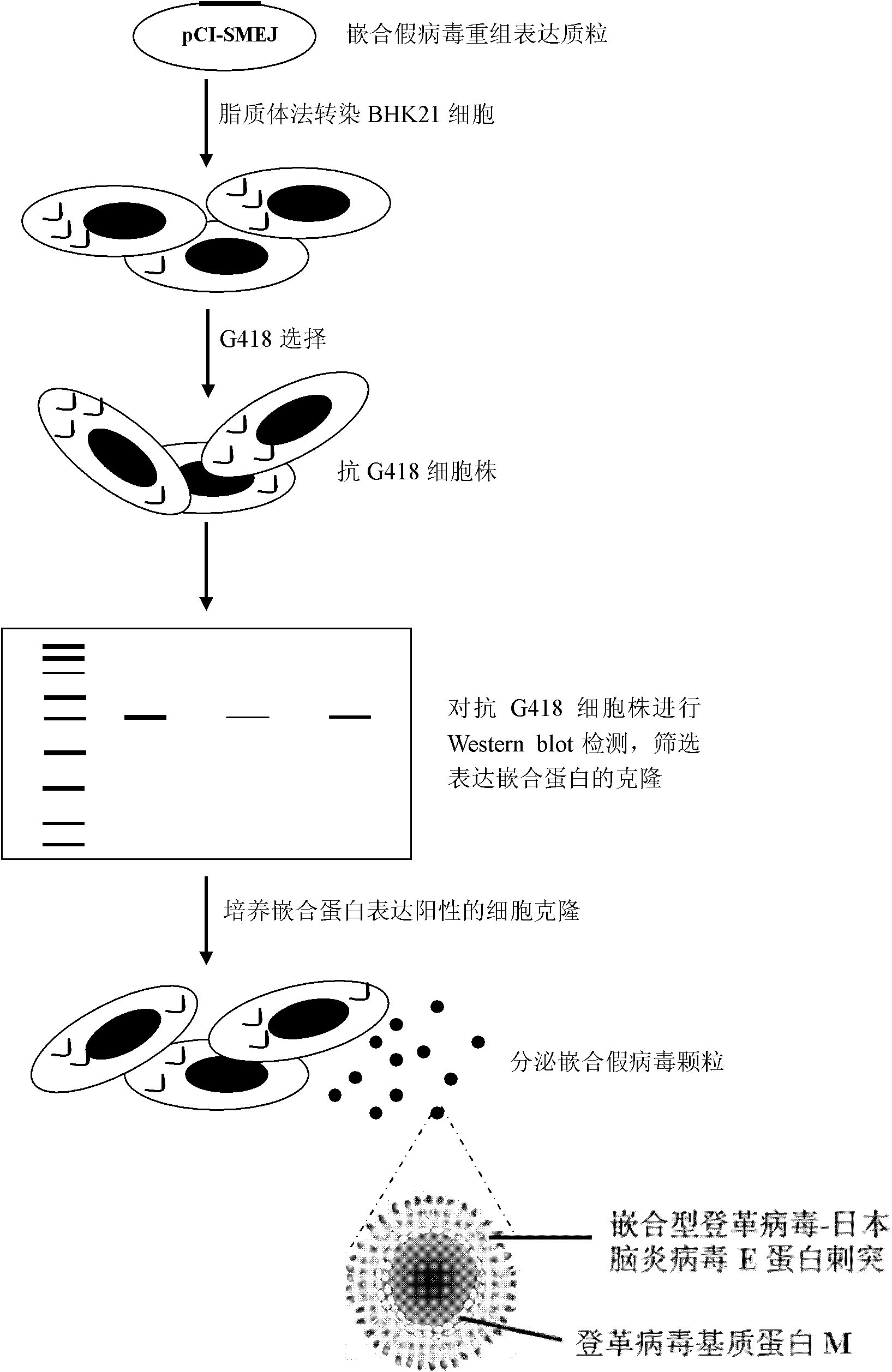
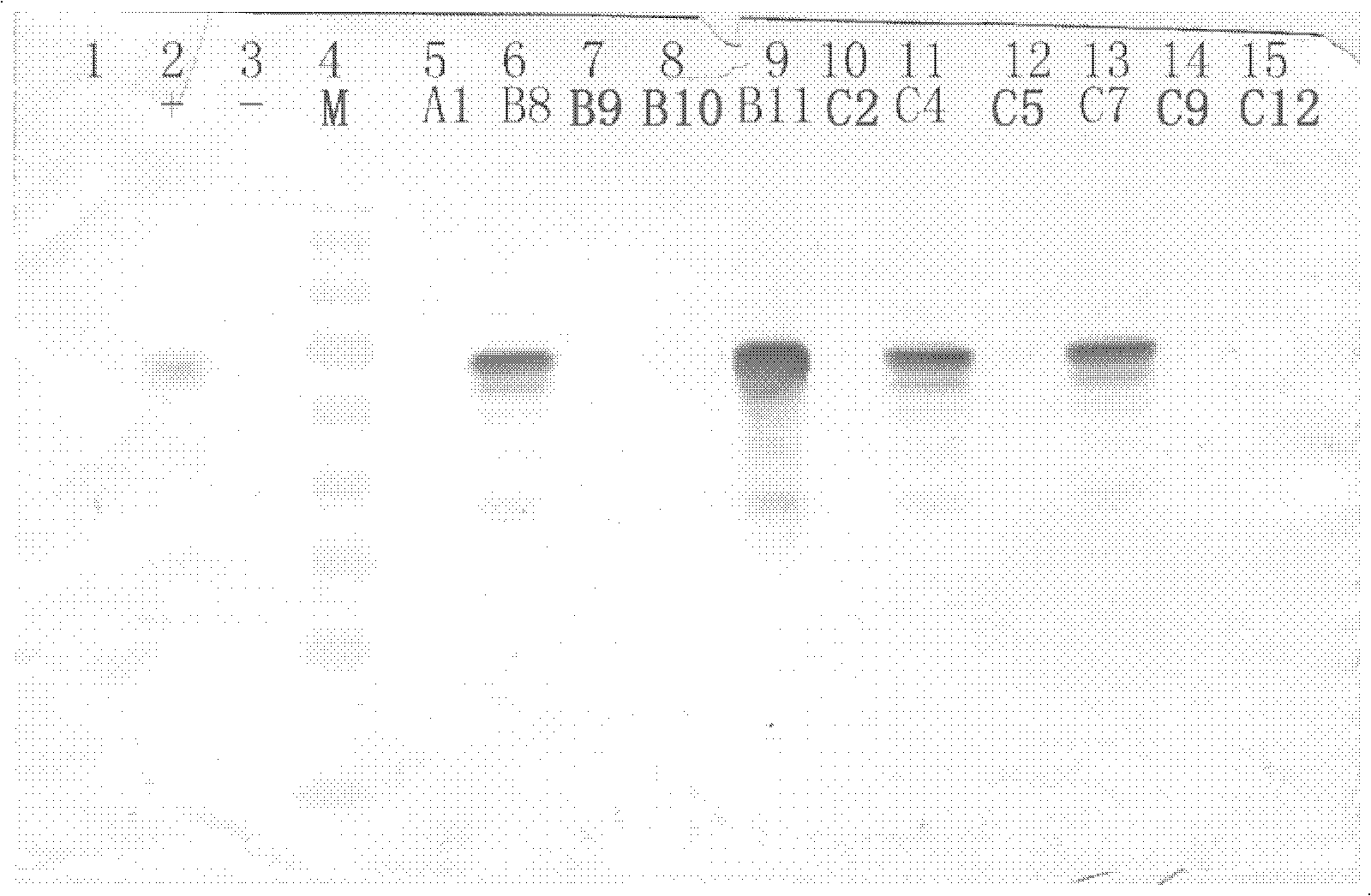
[0068] After obtaining the recombinant expression plasmid, a conventional method is to introduce it into target cells, cultivate the transfected cells, and collect the culture supernatant after a period of time (such as 48 hours), and obtain the chimeric pseudovirus by ultracentrifugation. Another preferred method is to use the antibiotic (such as G418) corresponding to the resistance gene carried by the plasmid to screen the cells transfected with the plasmid to obtain a cell clone with stable resistance, and then cultivate the clone for a period of time (such as 48 hours). Afterwards, the culture supernatant was collected and centrifuged to obtain chimeric pseudovirus particles.
Embodiment 1
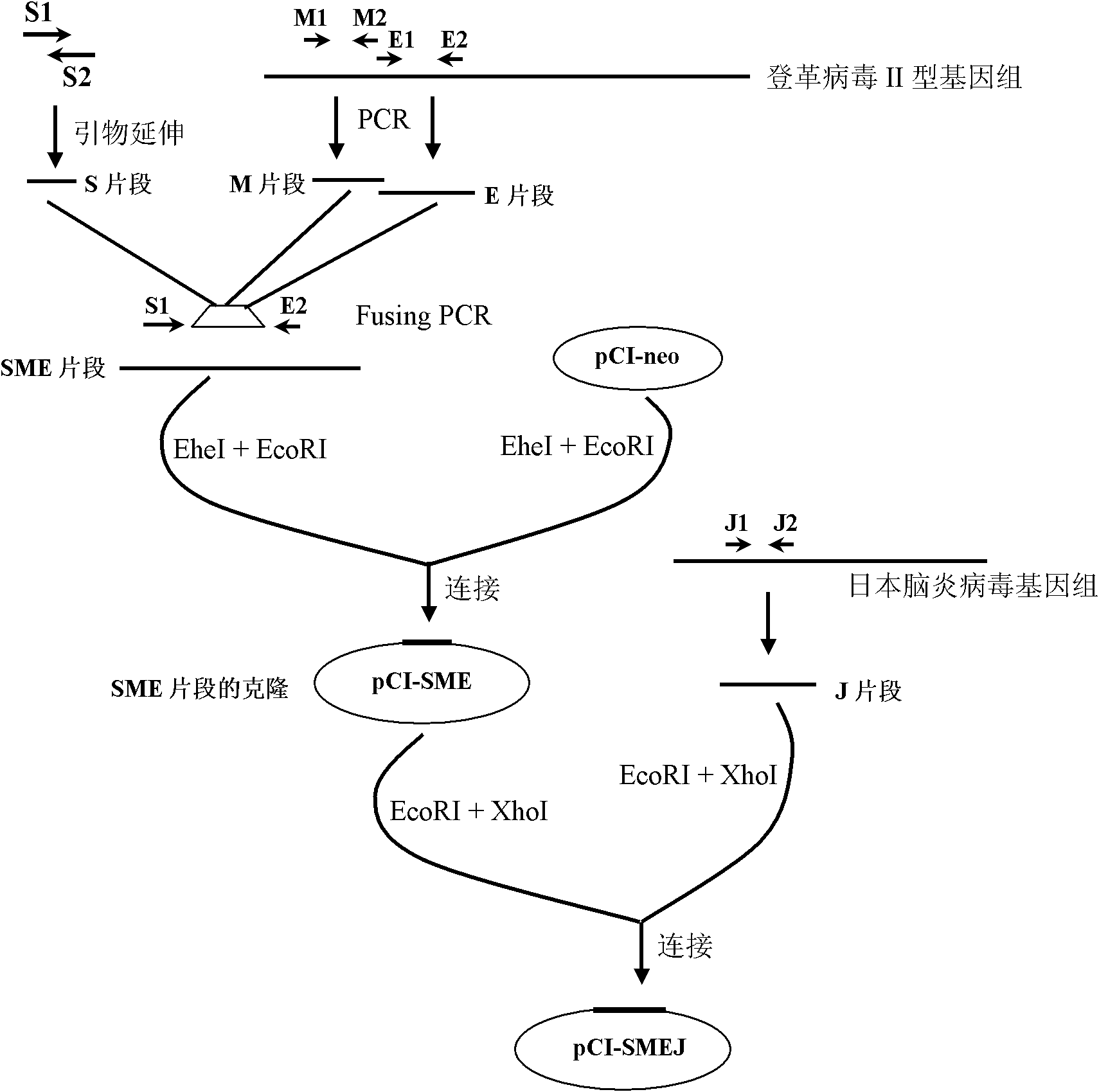
[0069] Example 1 Construction of chimeric pseudovirus recombinant expression plasmid
[0070] 1. Preparation of Flavivirus Signal Peptide Coding Sequence (S Fragment)
[0071] (1) Design a pair of oligonucleotide fragments according to the Japanese encephalitis virus signal peptide sequence shown in SEQ NO (Davis BS etal, J Virol 2001, 75 (9): 4040-4047): S1: ATAGGCTAGCGCCATGGGCAAGAGATCGGCGGGCTCAATCATGTGGCTCGCAAGCTTGG (SEQ ID No: 5), S2: GCGTGTGGTTAAATGGAATGCTCCTGCGTAAGCTATGACAACTGCCAAGCTTGCGAGC (SEQ ID No: 6), synthesized by the DNA Synthesis Department of Dalian TakaRa Company;
[0072] (2) Synthesis of S fragment by primer extension method (the 5' end of S1 chain has an EheI restriction site): because the 3' end of S1 and S2 oligonucleotide fragments is designed with a 14bp complementary sequence, the primer extension method can be used The S fragment is generated, and the amino acid sequence encoded by the fragment is shown in SEQ ID No:2. The specific operation is: prep...
Embodiment 3
[0138] The preparation process of embodiment 3 chimeric pseudovirion vaccine
[0139] 1. recover the stable transfected clone obtained in Example 2, use the DMEM medium containing 10% calf serum to expand the culture, after reaching the required cell amount, use the DMEM medium culture of 2% calf serum instead, Collect the supernatant once every 48 hours, centrifuge at 6000 g for 30 minutes (Sigma 3T3 centrifuge), and save the supernatant.
[0140] 2. Centrifugal purification of chimeric pseudovirus particles: add PEG8000 (Shanghai Shenggong, final concentration is 7.1%) and NaCl (Shanghai Shenggong, final concentration is 2.7%) in culture supernatant, shake overnight at 4 ℃ (16 -20 hours), centrifuge at 50000g at 4°C for 1 hour, and resuspend the pellet with 1 / 100 volume of TNE buffer (10mM Tris, 1mM EDTA, 100mM NaCl, pH 8.0) to make a concentrated virus solution.
[0141] The preparation of TNE buffer is as follows: Weigh 1.21g Tris (Shanghai Sangong), 0.37g EDTA (Shanghai ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com