Methods and compositions for inhibiting HIV transmission
A composition and animal technology, applied in the field of medicine, can solve the problem of not protecting the infection of the African green monkey homologous virus and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1: Preparation of hyperimmune colostrum comprising polyclonal anti-Env antibodies
[0184] Step 1 - Vaccine Preparation for Dairy Cows
[0185] The methods for preparing antigens reported in Publication No. WO / 2004 / 078209, International Application No. PCT / AU2004 / 000277 (the contents of which are incorporated herein by reference) were used.
[0186] Step 2 - used the method reported in Publication No. WO / 2004 / 078209, International Application No. PCT / AU2004 / 000277 (the contents of which are incorporated herein by reference) for the preparation of antibodies from vaccinated cows Methods.
Embodiment 2
[0187] Example 2: Preparation of Polyclonal Antibody Binding to HIV Env and Confirmation of Neutralization
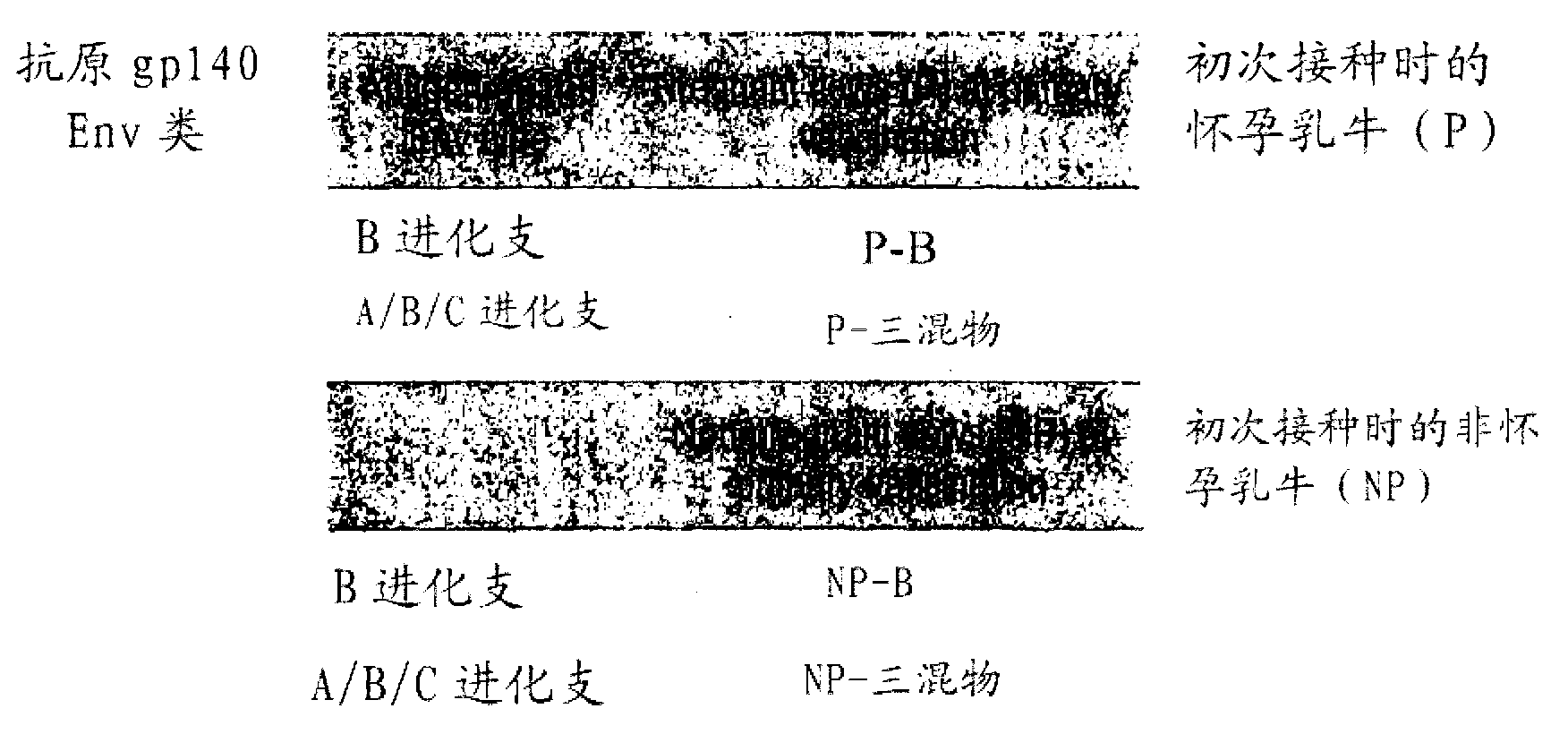
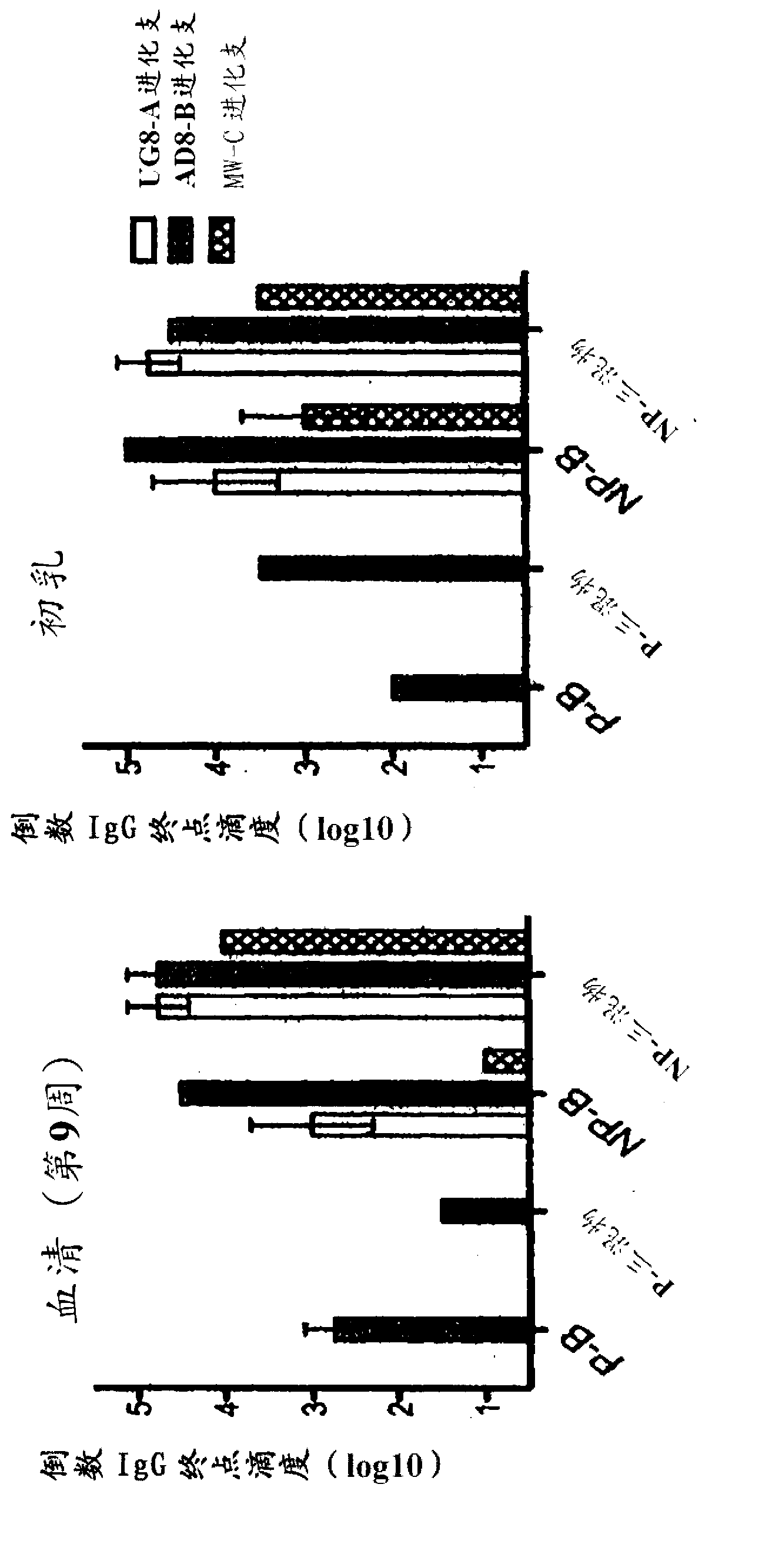
[0188] Soluble Env gp140 oligomers have been prepared from Clade A, B and C HIV strains from Hela and / or 293T cells and purified by lentil agglutinin affinity chromatography and gel filtration chromatography.
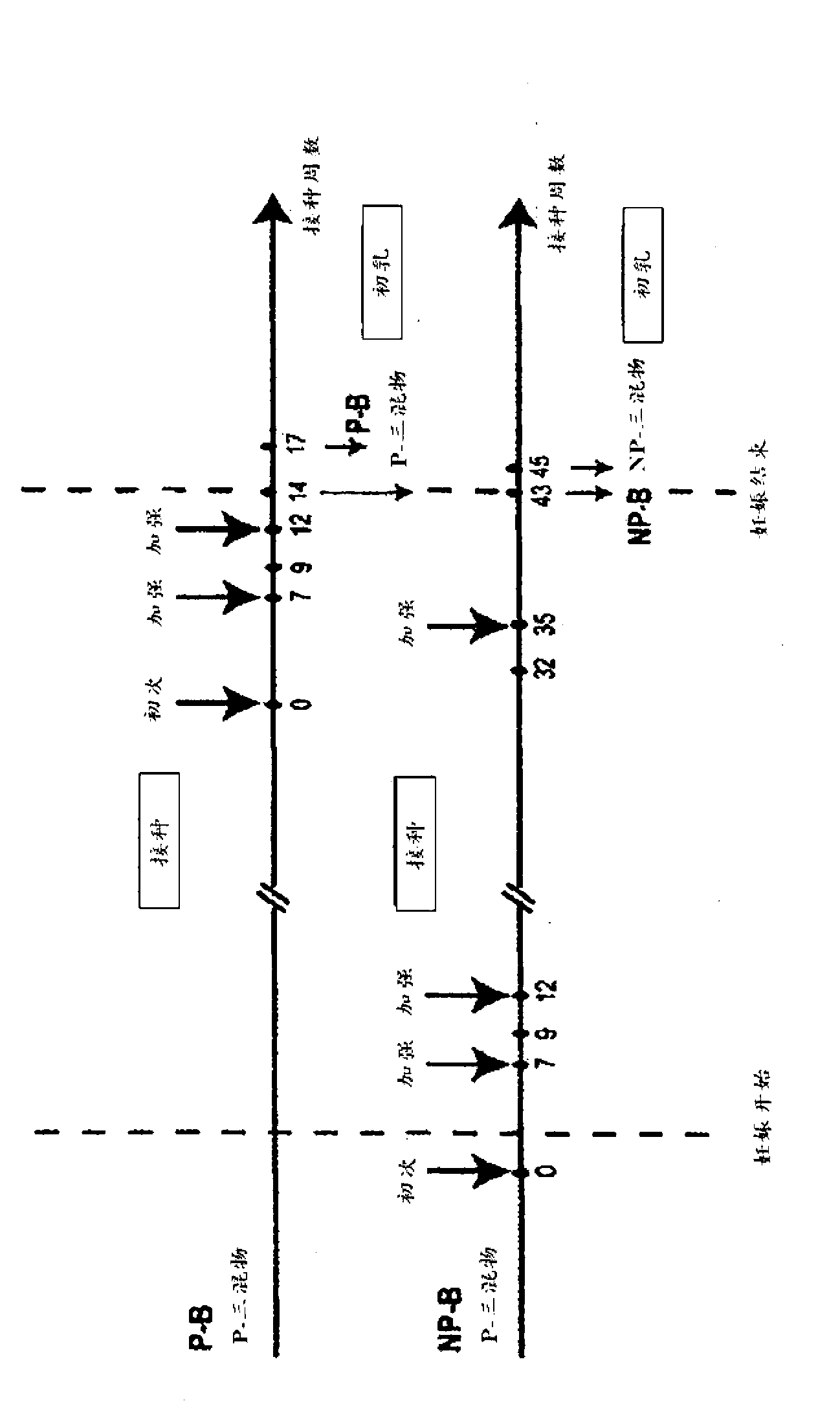
[0189] Four dairy cows (two in the second semester and two initially non-pregnant) were vaccinated with 100 μg of purified HIV-1 Env gp140 oligomer in Montanide adjuvant. Two groups of two each were inoculated with clade B only (AD8) or with equal amounts (33.33 μg) of clade A, B and C Env gp140 (UG8, AD8 and MW) (termed "triple mix") Dairy cows (one pregnant and one non-pregnant). All four cows received at least 3 vaccinations, with the last vaccination given 4 weeks before farrowing. All four cows seroconverted within 9 weeks. The reciprocal endpoint serum IgG titer for pregnant dairy cows was up to 1×10 as determined by a newly established anti-bovine IgG H...
Embodiment 3
[0199] Example 3: Polyclonal neutralizing antibodies against HIV-1 Env from bovine colostrum
[0200] Twelve BSE-free pregnant dairy cows housed on an authorized isolation farm in Victoria were vaccinated with 100 μg of an equimolar mixture of four HIV-1 Env gp140 oligomers:
[0201] 1) SC35 B clade pre-seroconversion strain Env gp140, which adopts an open structure and mainly exhibits important neutralizing epitopes;
[0202] 2) ADA primary R5 tropism B clade Env gp140;
[0203] 3) 966 A / E clade Env gp140; and
[0204] 4) MW C clade Env gp140.
[0205] These Envs were formulated with an adjuvant (Montanide) and administered by a registered veterinarian twice before conception and at least twice at 3-week intervals during the second trimester.
[0206] Env-specific IgG levels in routine blood samples taken during the vaccination and pregnancy were monitored using the Env ELISA test and Western blot, and vaccination was continued until high titers of IgG were detected. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com