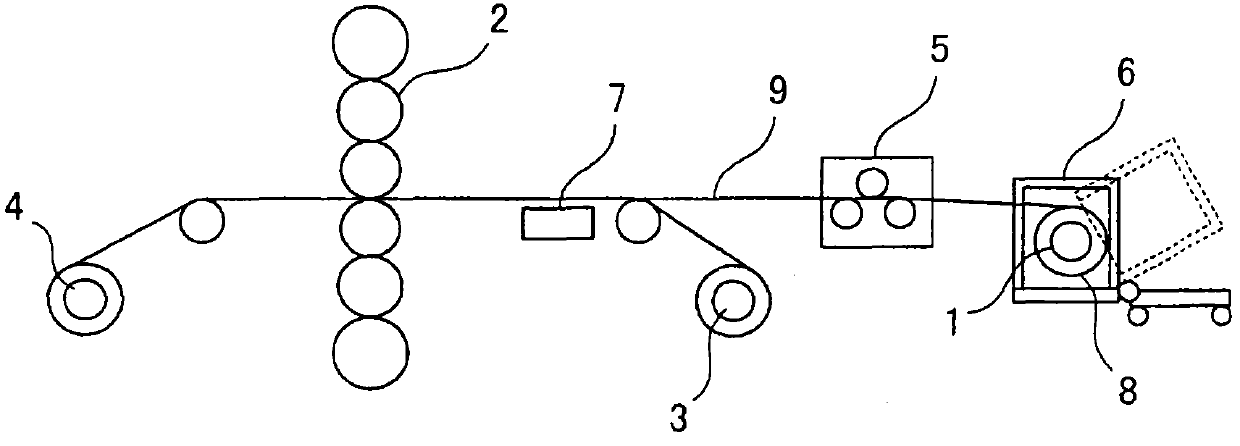
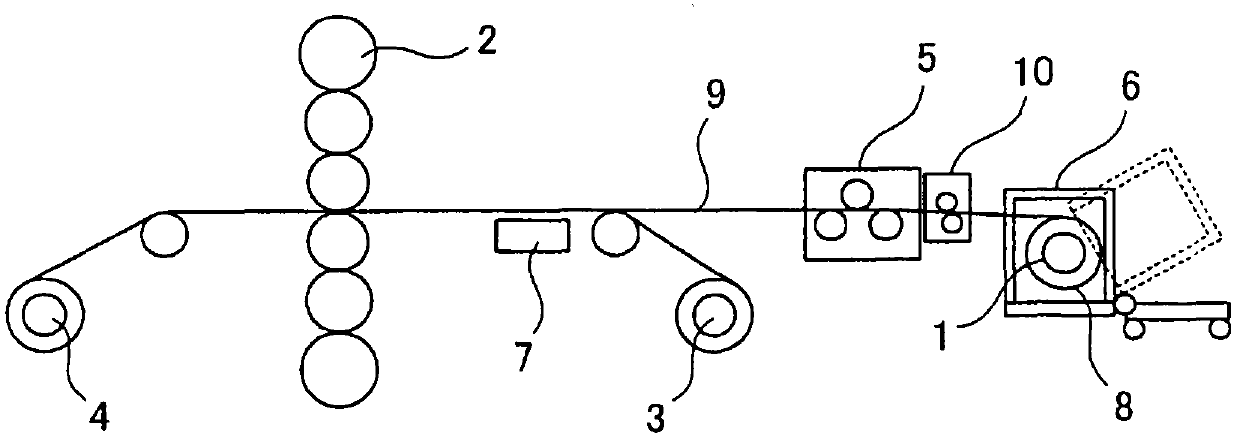
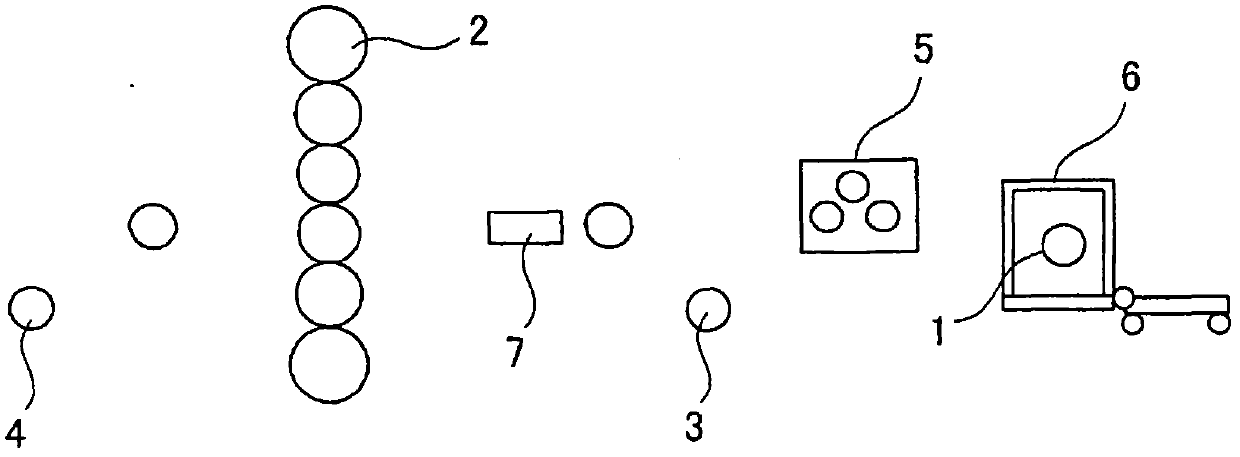
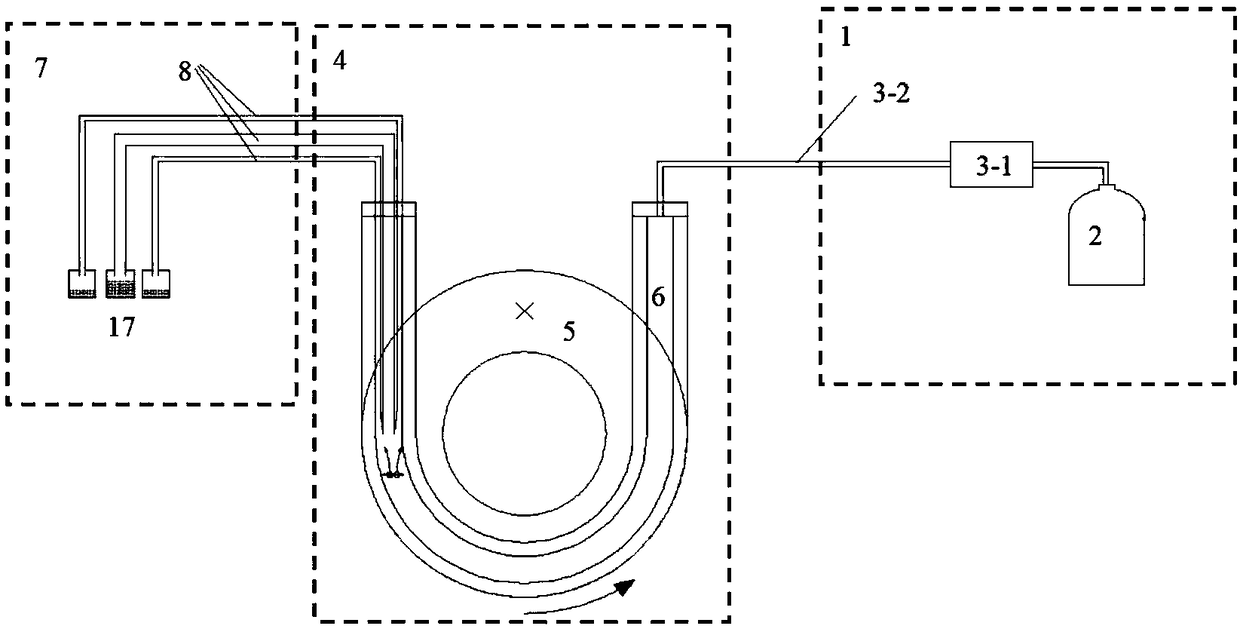
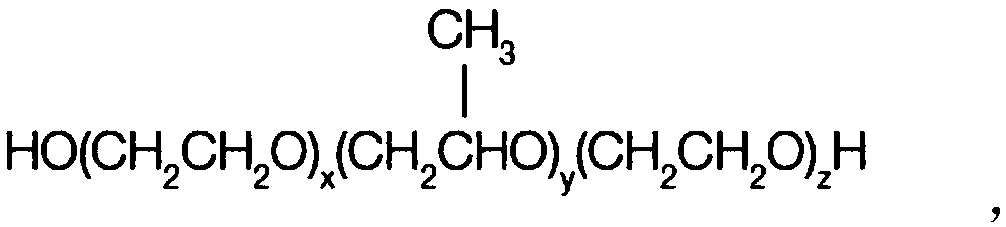
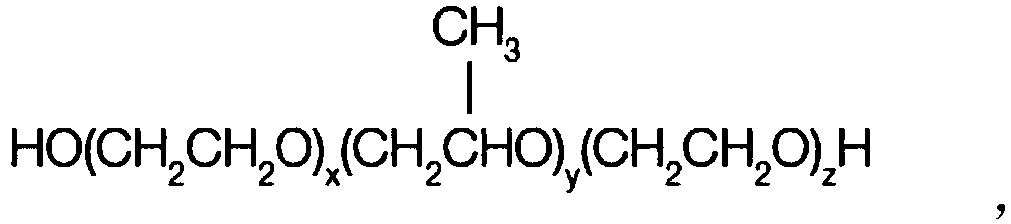Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67results about How to "No corrosion problems" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of open pore foamed aluminum capable of easily removing pore-forming agent
The invention relates to a preparation method of an open pore foamed aluminum capable of easily removing a pore-forming agent. The preparation method of the open pore foamed aluminum comprises the following steps: firstly weighing ammonium bicarbonate and aluminite powder according to a volume ratio of (1-4) to 1, then uniformly mixing, subsequently adding absolute ethyl alcohol so as to improve the uniformity of mixed powder so as to obtain mixed powder sample, then pressing the mixed powder sample to obtain a cylindrical or strip-shaped pressed blank sample; putting the pressed blank sample in water bath for dipping to remove a spherical ammonium bicarbonate pore-forming agent, then drying the pressed blank sample in a drying oven; finally, sintering the pressed blank sample in vacuum, then cooling the pressed blank sample to the room temperature so as to obtain open pore foamed aluminum which is uniform in pore structure and controllable in porosity. The preparation method of the open pore foamed aluminum has the advantages that compared with the irregularly-shaped pore-forming agent, the foamed aluminum prepared by the spherical ammonium bicarbonate is capable of avoiding crack initiation to a great extent under the action of stress; the mechanical property of the foamed aluminum is improved; the pore-forming agent can be thoroughly removed; the corrosion of the residual pore-forming agent to an aluminum substrate can be avoided; the process period is shortened; the economic benefit is increased.
Owner:TONGJI UNIV
Water-based dust depressor and preparation method thereof
InactiveCN104693339AImprove securityAvoid the decrease of wind erosion resistanceOther chemical processesWater basedAqueous solution
The invention provides a water-based dust depressor and a preparation method thereof. The dust depressor is prepared through water solution copolymerization, the dust depressor comprises 6-25 parts of unsaturated acid; 2-10 parts of unsaturated esters; 0.1-0.5 part of cross-linking agent; 0.1-2.0 parts of initiating agent and 100 parts of water; the raw materials further comprise antalkali, and the quantity of the antalkali adjusts the pH value of a reaction system to 6.0-9.0. The dust depressor is prepared by the unsaturated acid and the unsaturated esters through the water solution copolymerization technology, a shell layer can be formed on the surface of a dust particle, a good moisturizing function and a dust depressing function are achieved, and an obtained product contains neither volatile low-molecular compounds nor corrosive high valence metal ions. The prepared chemical dust depressor can be used by being diluted with water and sprayed, the comprehensive performance is good, and the safety and environmental protection are achieved.
Owner:HEBEI UNIV OF TECH
Boiler-turbine coupled flue gas waste heat utilization system capable of preheating air based on condensed water
InactiveCN103486567ANo corrosion problemsReduce the temperatureCombustion processIndirect carbon-dioxide mitigationAir preheaterDeaerator
The invention discloses a boiler-turbine coupled flue gas waste heat utilization system capable of preheating air based on condensed water, which belongs to the field of energy conservation of electric stations. The boiler-turbine coupled flue gas waste heat utilization system mainly comprises a main economizer, a main flue, a bypass flue, a confluence flue, a main air preheater, smoke water heat exchangers, a high-pressure heater, a low-pressure heater, a deaerator, a flue gas-air heater and a condensed water-air heater. Cold air is preheated by extracting low-temperature condensed water in some of thermodynamic systems and low-temperature flue gas of a boiler through utilizing the condensed water-air heater; a primary smoke water heat exchanger and a secondary smoke water heat exchanger are used for heating the feeding water and the condensed water so as to replace extraction steam, with higher pressure, of a steam turbine. According to the flue gas waste heat utilization system, waste heat is effectively utilized, and the air is preheated by utilizing the low-level condensed water and the low-temperature flue gas of the boiler, so that the high-pressure extraction steam used for heat return is saved, thus work of a machine set is remarkably increased, and deep energy conservation and consumption reduction of a coal-fired generator set can be realized; furthermore, a problem of low-temperature corrosion of a waste heat utilization heating surface is relieved, and remarkable economic benefit is obtained.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
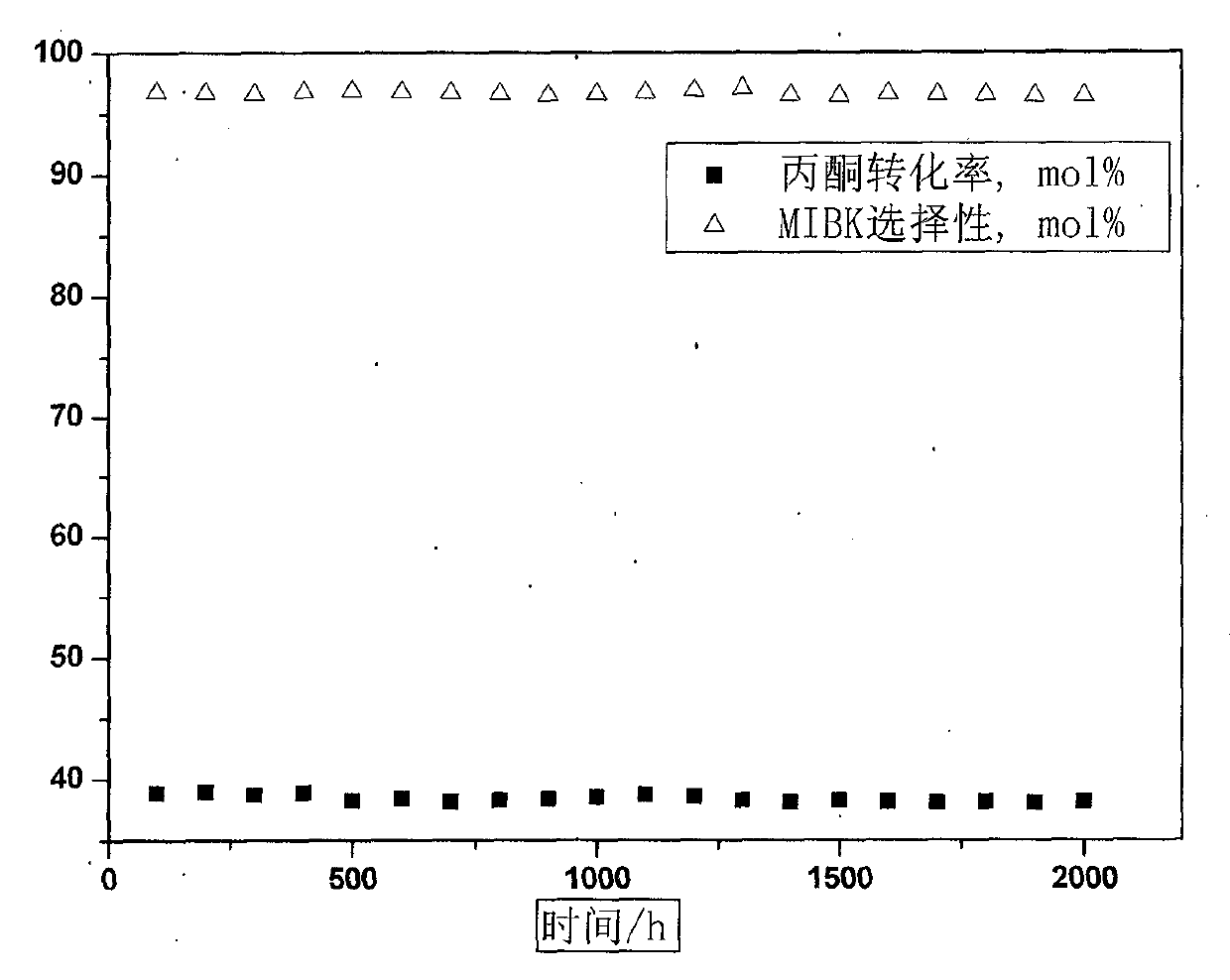
Preparation method of catalyst for hexone synthesis by acetone hydrogenation and application
InactiveCN102698761AReduce manufacturing costShort processOrganic compound preparationCarbonyl compound preparationCatalytic functionAcetone
The invention belongs to the technical field of catalysis, and relates to a preparation method of a catalyst for hexone synthesis by acetone hydrogenation and an application; the catalyst is composed of active component nickel loaded on an alumina carrier modified by transition metal oxides. The preparation method of the catalyst comprises two procedures: the first one is that transition metal oxides and alumina are mixed, molded, dried, roasted, and subject to hydro-thermal treatment, and the active component Ni is immersed to obtain the catalyst; the second one is that an alumina carrier is prepared firstly, then transition metal oxides and Ni are introduced by an immersion method, and finally hydro-thermal treatment is performed to obtain the catalyst. The catalyst of the invention adopts non-noble metal as the main active component, is simple in preparation, low in cost, strong in raw material adaptability, long in service life, and high in activity and selectivity, and combines three catalytic functions of acetone condensation, dehydration, and hydrogenation as a whole, and the reaction process flow is simplified greatly.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Novel FRP (fiber reinforced plastic)-steel tube-epoxy resin-sea sand concrete compression member
The invention belongs to the field of engineering structures, and particularly relates to a novel FRP (fiber reinforced plastic)-steel tube-epoxy resin-sea sand concrete compression member. The member is characterized in that an epoxy resin layer is coated on the inner surface of a steel tube, and the steel tube is internally filled with sea sand concrete; an FRP material layer is overwrapped on the outer surface of the steel tube; because the FRP material layer is overwrapped outside the steel tube, and the steel tube is prevented from being in direct contact with the external environment, so that the durability of the steel tube is improved obviously; meanwhile the epoxy resin layer is coated on the inner surface of the steel tube, so that the sea sand concrete and the steel tube are isolated, and the corrosion effect of chloride ions in sea sand on the steel tube is avoided; and the potential safety hazards of the sea sand concrete are reduced, and the application of the sea sand concrete is promoted. The member is applied to high-corrosion coastal and offshore areas and the like, bears the load mainly focused on axle load, and has favorable economical and environmental benefits.
Owner:TSINGHUA UNIV
Electromagnetic pump for conveying liquid nonferrous metal
ActiveCN105591521ANo corrosion problemsEasy flow controlDynamo-electric machinesEngineeringBody movement
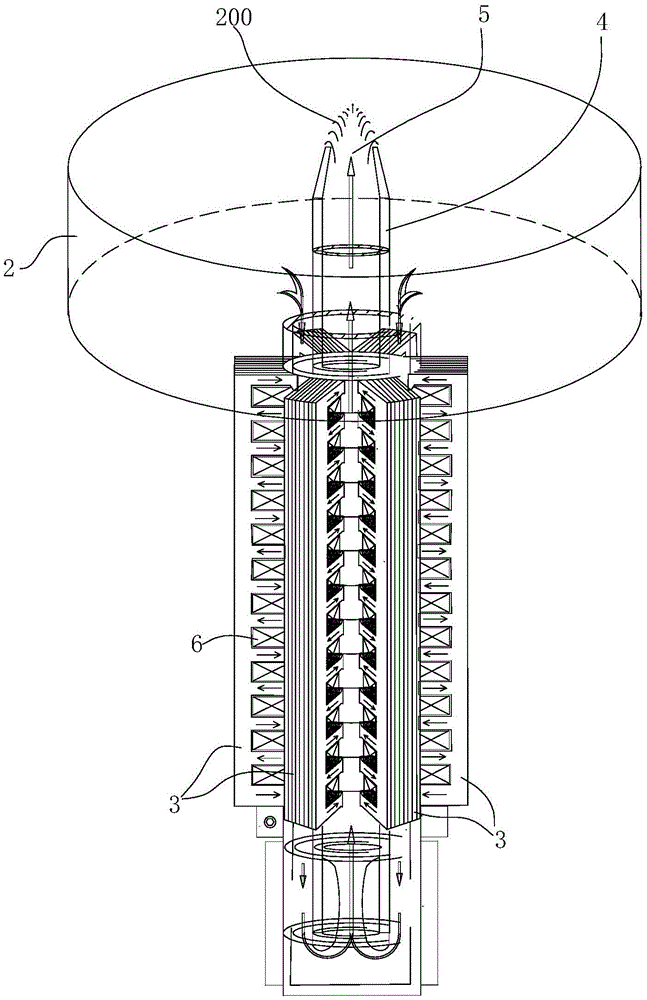
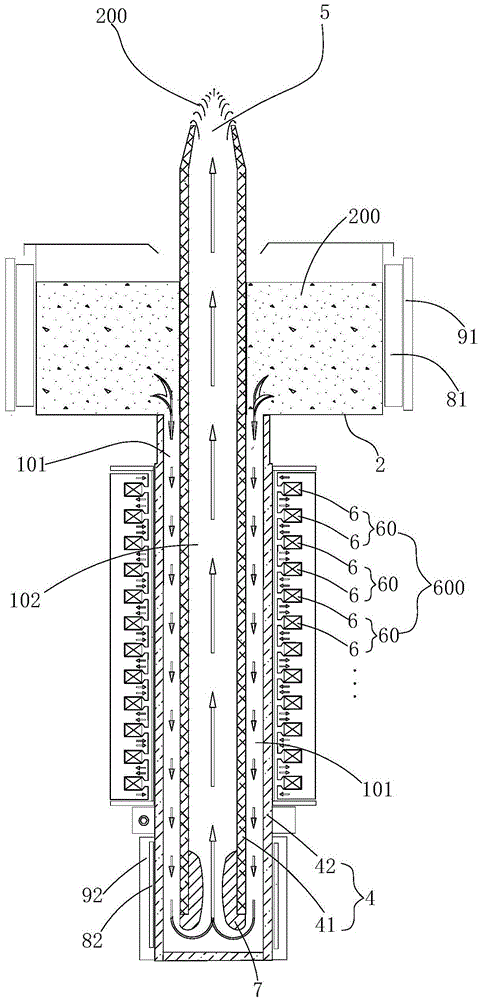
The invention is suitable for the technical field of liquid nonferrous metal conveying equipment, and provides an electromagnetic pump for conveying liquid nonferrous metal. The electromagnetic pump comprises a power supply, a pulse generator, a pot body, an iron core lamination stack, a guide pipe and a spout. A plurality of electromagnetic windings which equidistantly arranged in the axial direction of the guide pipe are embedded into the iron core lamination stack. The iron core lamination stack and the adjacent electromagnetic winding on the iron core lamination stack form a pushing force unit. Furthermore a same-phase driving pulse is input. After energization, instantaneous magnetic fields with opposite polarities are generated among polar teeth of the iron core lamination stack. A plurality of pushing force units form a pushing force set. Driving pulses with different time sequences are respectively input into the pushing force units in the pushing force set. The pushing force units form a movable magnetic field which changes according to the time sequence from an inlet to an outlet. The electromagnetic pump utilizes an electromagnetic pushing force for pushing the liquid nonferrous metal to move. No movable component such as mechanical rotation component exists, and a problem of pump body movement assembly corrosion is prevented. Furthermore high flowing stability of the molten metal is realized, and speed and flow of the molten metal can be controlled.
Owner:SUNEAST ELECTRONICS TECH SHENZHEN
Continuous casting mold flux for automobile sheet
The invention discloses continuous casting mold flux for an automobile sheet. The continuous casting mold flux is formed by mixing a base body and carbonaceous materials with the weight which is 1 percent to 2 percent of the total weight of the base body. The base body comprises, by weight, not more than 0.25 percent of Na2O, 0.5 percent to 4.5 percent of MgO, 0.3 percent to 1.0 percent of Fe2O3, 5 percent to 25 percent of Al2O3, 0.5 percent to 6.5 percent of Li2O, 0 percent to 16 percent of BaO, 5 percent to 9 percent of F, 20 percent to 40 percent of SiO2 and not more than 2 percent of impurities. The weight ratio of CaO to SiO2 ranges from 0.9 to 1.4. The base body meets the requirements that the proportion of a pre-melting portion is not less than 70 percent, the melting point of the base body ranges from 1100 DEG C to 1200 DEG C, the viscosity ranges from 0.25 Pa.s to 0.45 Pa.s at the temperature of 1300 DEG C, and the surface tension ranges from 0.43 N / m to 0.53 N / m at the temperature of 1400 DEG C. The continuous casting mold flux has the higher viscosity and the higher surface tension, and the slag inclusion occurrence rate of a continuous casting sheet billet of the automobile sheet is better reduced.
Owner:BAOSHAN IRON & STEEL CO LTD
Rechargeable battery
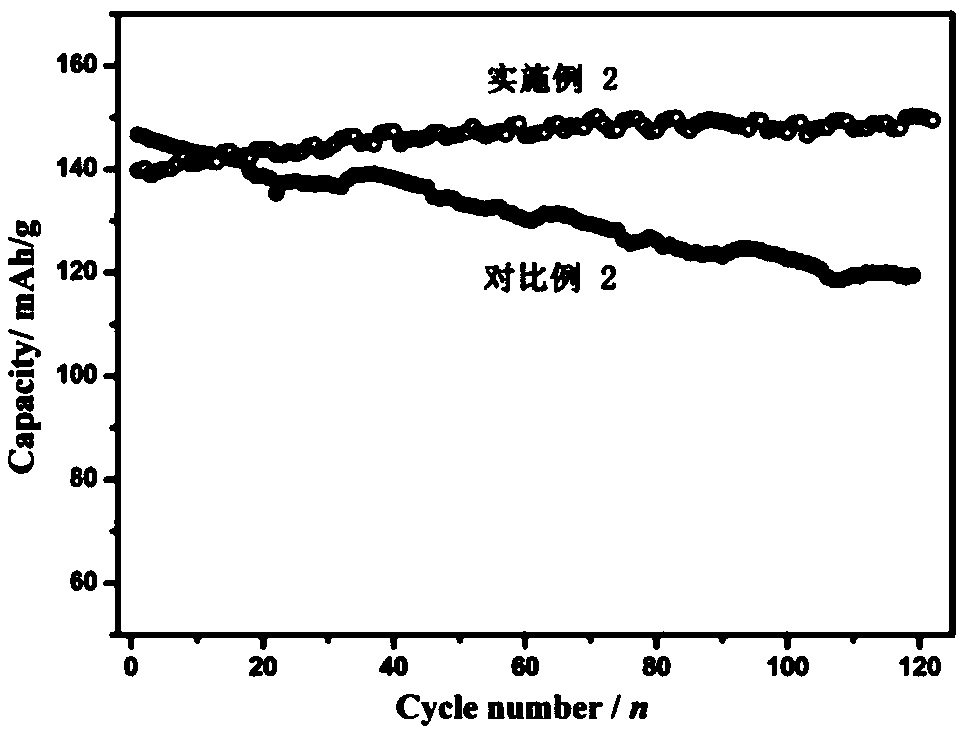
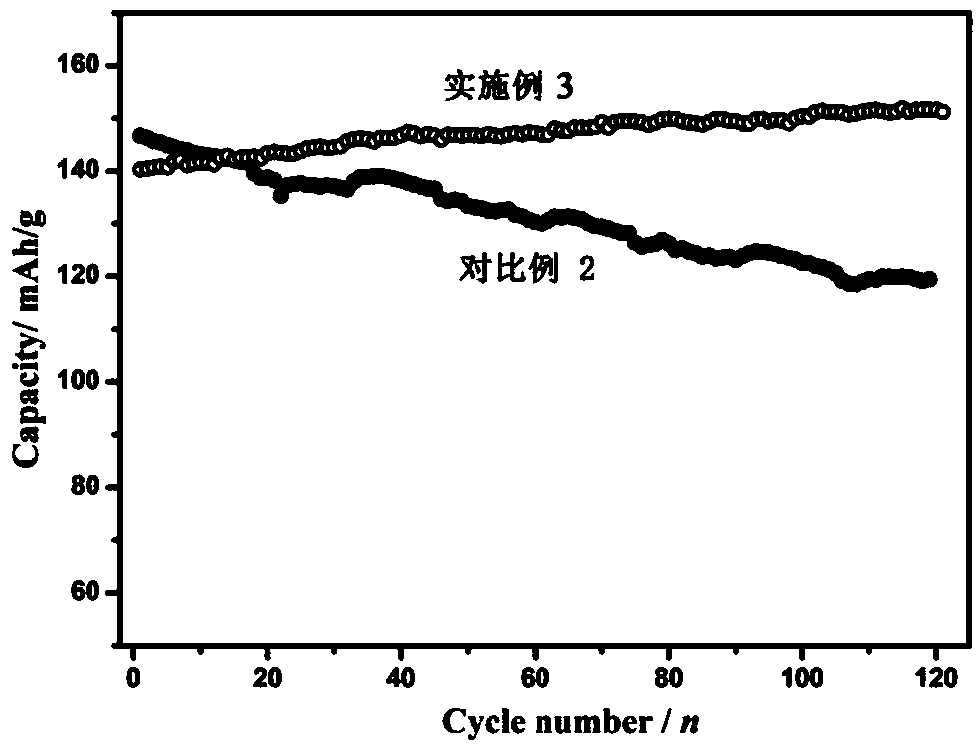
ActiveCN109037794AImprove cycle lifeIncrease energy densityFinal product manufactureNegative electrodesHigh energyManganese oxide
A rechargeable battery includes an electrolyte, a positive electrode, a negative electrode, and an isolation film disposed between the positive electrode and the negative electrode, The active material of the positive electrode comprises at least one of manganese oxide and manganese oxyhydroxide, the active material of the negative electrode comprises zinc element, and the electrolyte salt in theelectrolyte solution comprises at least one of zinc alkyl sulfonate, zinc aryl sulfonate, zinc fluoroborate, zinc alkyl sulfonate hydrate, zinc aryl sulfonate hydrate and zinc fluoroborate hydrate. The rechargeable battery not only can effectively avoid irreversible sulfation of the positive electrode, the cycle life of rechargeable batteries is improved by increasing the reversibility of the cathode materials, and the rechargeable batteries have higher energy density. Moreover, the corrosion of chloride ions and the reduction of nitrate ions are avoided. Compared with the lithium batteries onthe market, the raw materials used are cheaper, so the rechargeable battery has better economic benefits.
Owner:刘小林
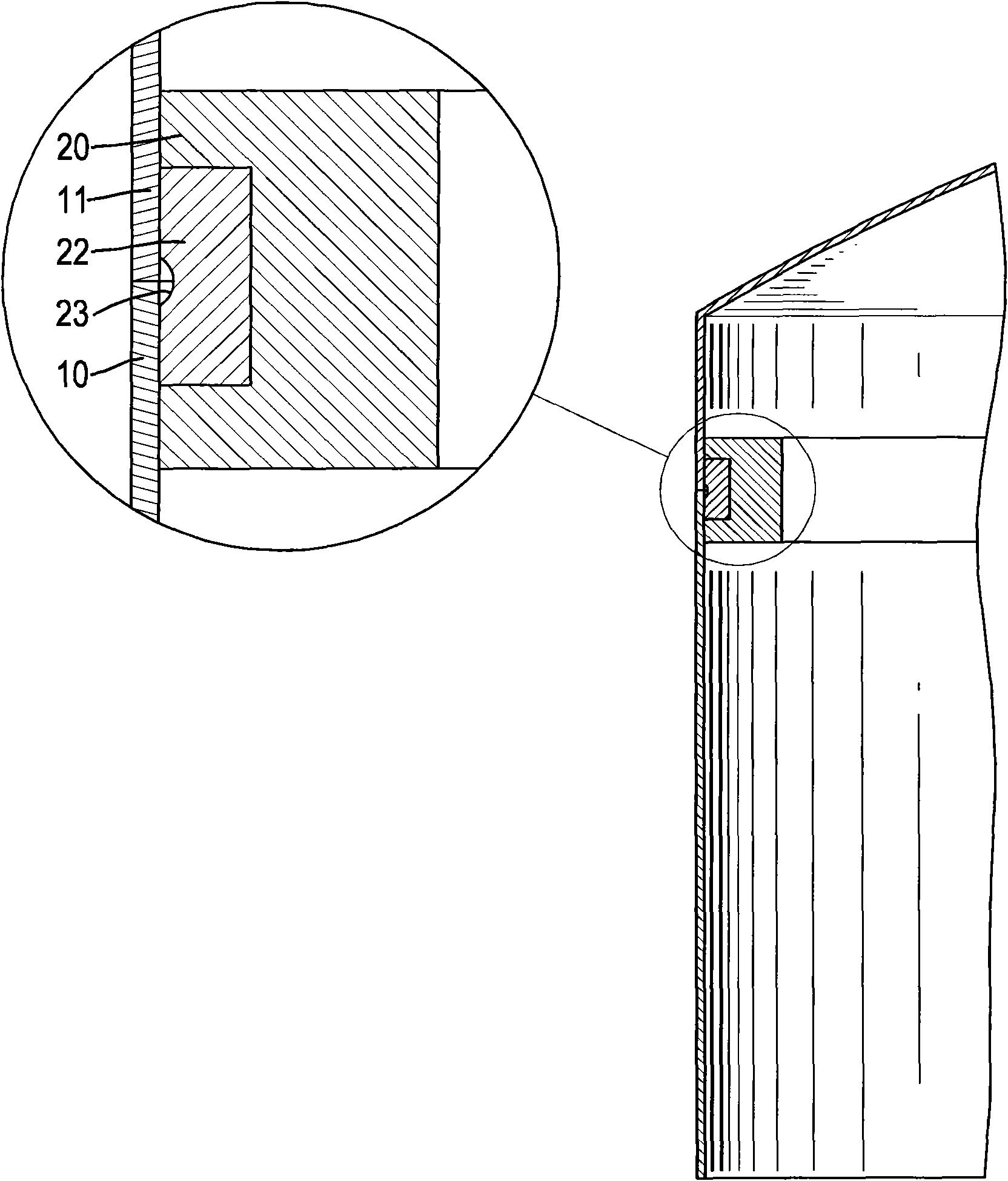
Screwed pipe joint and method for the production thereof
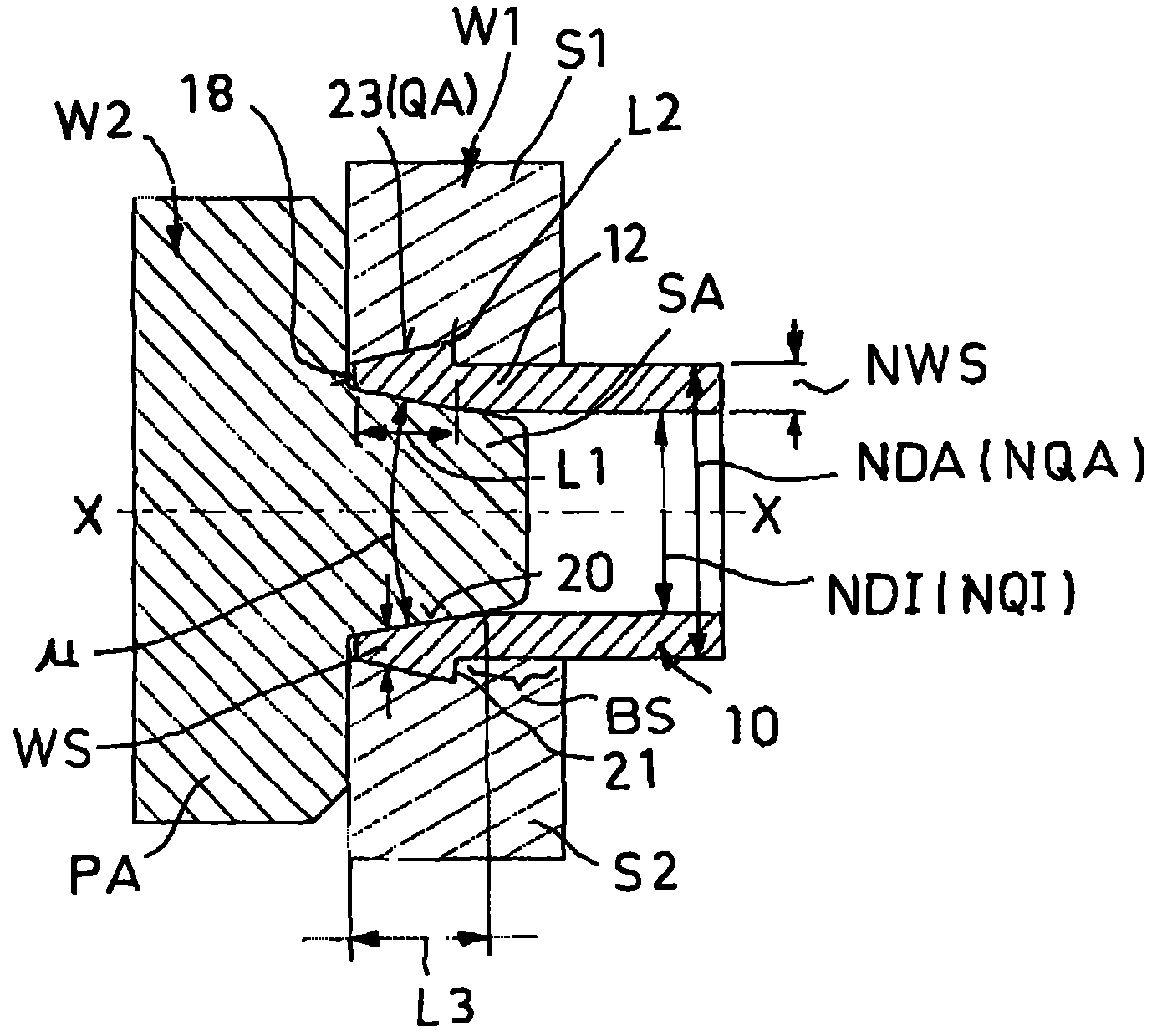
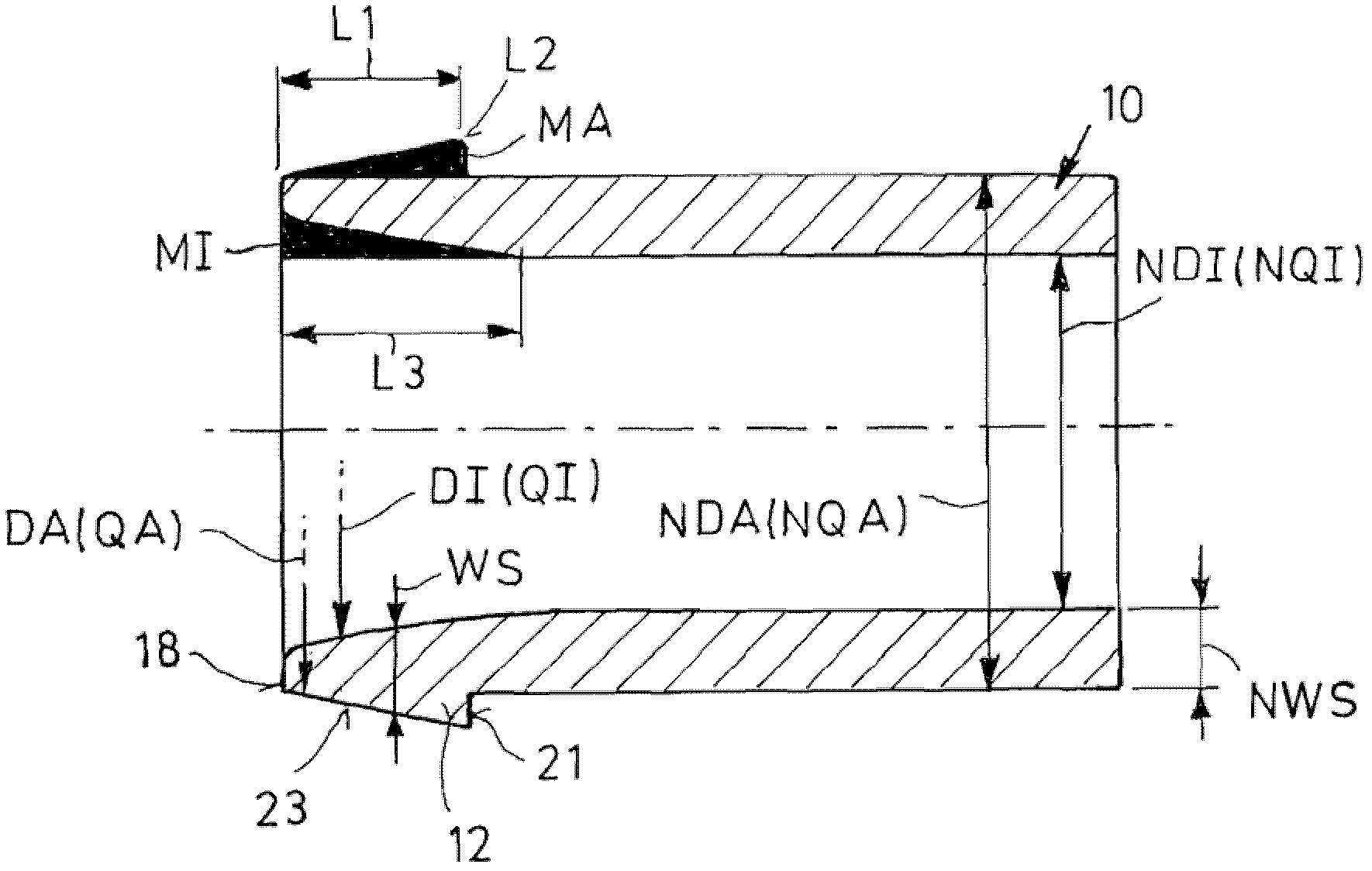
InactiveCN102483186AImprove Assembly PropertiesIncrease clamping forceJoints with sealing surfacesHollow articlesEngineeringScrew thread
The invention relates to a screwed pipe connection (1) for connecting a pipeline (10) comprising a connection end (12) having a formed wall region. The screwed pipe connection comprises a connection part (2) and a union joint part (4). Proceeding from the end face (18) of the connection end (12), an outside cross-section of the pipeline (10) increases and is larger than the nominal outside cross-section, then decreases again. Proceeding from the end face (18), a clear inside cross-section of the connection end (12) is larger than the nominal inside cross-section and decreases to the nominal inside cross-section, wherein the wall thickness on the end face (18) is smaller than the nominal wall thickness, and wherein an inner material difference (MI), resulting from the deviation of the inside cross-section from the nominal inside cross-section, and an outer material difference (MA), resulting from the deviation of the outside cross-section from the nominal outside cross-section, deviate from one another by a maximum of 30 percent.
Owner:VOSS FLUID
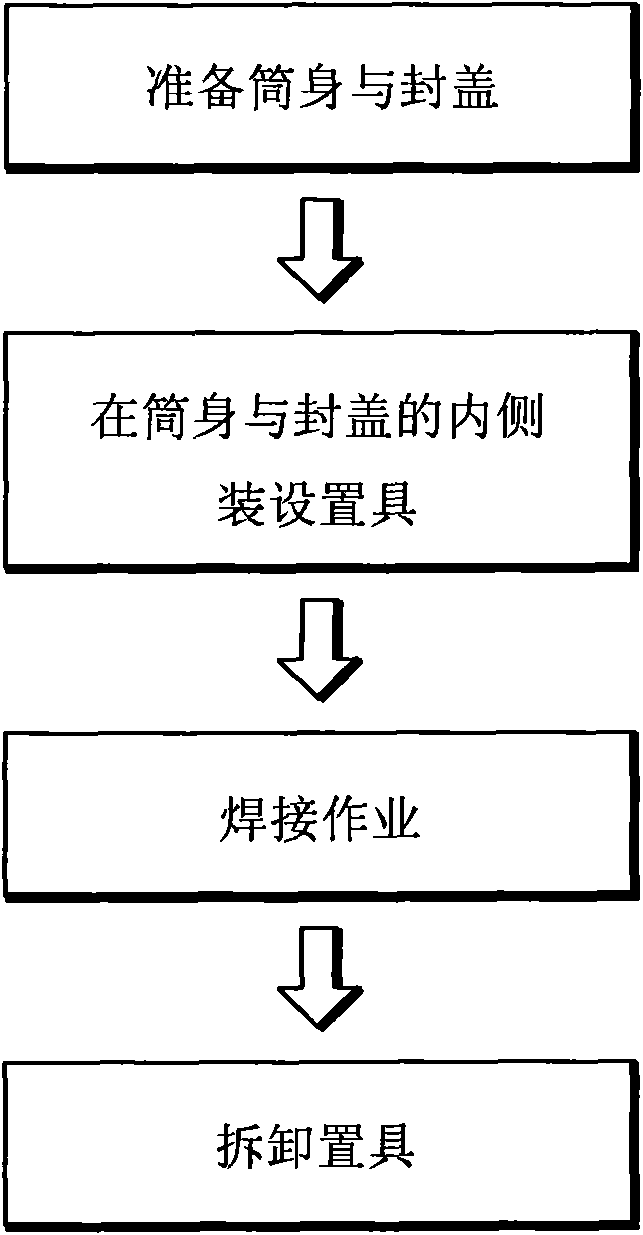
Method for welding cylindrical shell of water heater
InactiveCN102398119AImprove structural strengthThere will be no problem of leakage of molten iron at solder jointsWelding apparatusEngineeringCorrosion
The invention discloses a method for welding a cylindrical shell of a water heater. The method comprises the following steps of: preparing a cylinder body which has a shape of a circular pipe, preparing a sealing cover of which bottom edge has a shape matched with that of an end edge of the cylinder body, butting the sealing cover in a mode that the bottom edge faces the end of the cylinder body, arranging a placement tool on the inner sides of the end edge of the cylinder body and the bottom edge of the sealing cover in a detachable way, recessing a casting channel corresponding to the gaps of the sealing cover and the bottom edge on the outer circumferential surface of the placement tool, welding along the butting part of the sealing cover and the end edge of the cylinder body to ensure that a welding point is connected with the bottom edge of the sealing cover, and the end edge of the cylinder body in a melting way and is cast in the casting channel so as to form an inner welding pass; and by the welding means, an outer welding pass and the inner welding pass which have the supporting function can be formed on the outer and inner sides of the welding part of the sealing cover and the cylinder body respectively to ensure that weak points around the welding part are avoided, the cylindrical shell of the water heater, which is manufactured by welding can bear larger pressure, the inner and outer complete butting melting way is adopted by the welding part of the sealing cover and the end edge of the cylinder body and seams are avoided, and the corrosion, and change of electrical and chemical performance are avoided when water is filled in a cylinder.
Owner:黄朝林
Plasma etching residual washing liquid
InactiveCN101561641ANo corrosion problemsReduce pollutionDetergent mixture composition preparationSemiconductor/solid-state device manufacturingHydroxylamineHydroxylamine Hydrochloride
The invention discloses a plasma etching residual washing liquid which contains hydroxylamine and derivate thereof, solvent, water and chelating agent. The plasma etching residual washing liquid is characterized by also comprising carboxylic polymer and polymer containing paint affinity groups and is compounded by the carboxylic polymer and the polymer containing paint affinity groups, thereby radically solving the problem of metal corrosion, particularly aluminum corrosion caused by rinsing with water during the washing in a wet method washing of the traditional hydroxylamine washing liquid in a process of semiconductor manufacture and omitting the solvent rinsing for avoiding the metal corrosion after the washing by the transitional hydroxylamine washing liquid. The invention keeps stronger washing property of the prior hydroxylamine washing liquid, causes metal corrosion, particularly aluminum corrosion resulted from direct washing by water after the washing of wafer, can remove the solvent washing procedure after the removal of plasma etching residual, and is beneficial to reduce the pollution and lower the cost.
Owner:ANJI MICROELECTRONICS (SHANGHAI) CO LTD
Limestone-gypsum wet flue gas desulfurization synergist and preparation method thereof
InactiveCN105251335AIncrease profitGood pH buffering capacityDispersed particle separationOrganic acidFood additive
The present invention discloses a limestone-gypsum wet flue gas desulfurization synergist, which comprises, by weight, 70-90 parts of an organic acid salt, 5-20 parts of an organic anhydride, and 5-15 parts of a surfactant. According to the present invention, the type of the component of the desulfurization synergist is simplified while the obtained synergetic desulfurization effect is good; due to the excellent and stable desulfurization effect, under the same conditions, the used raw materials are less, the production cost of the enterprise is low, and the performance price ratio is high; and the sodium diacetate accounting for the absolute proportion in the desulfurization synergist components is the internationally-recognized efficient, safe and inexpensive food additive, and has characteristics of good safety and good environmental protection during the production process, the storage and transportation process, the use process and other processes.
Owner:麦适(上海)化工有限公司
Construction dust depressor and preparation method thereof
ActiveCN106008851ANo secondary pollutionImprove securityOther chemical processesCarboxylic acidAqueous solution
The invention discloses a construction dust depressor and a preparation method thereof. According to the dust depressor, unsaturated carboxylic acid, unsaturated sulfonic acid and unsaturated polyether are adopted as monomer raw materials, a neutralizer and an initiator are adopted as auxiliary raw materials, and the dust depressor is prepared through an aqueous solution copolymerization technology. Ionic stability of the dust depressor is improved through the non-ionicity of unsaturated polyether monomers, the adaptability of the dust depressor to cement, concrete and residue soil dust is improved, and the prepared dust depressor can be diluted with water to be sprayed and used and is good in comprehensive performance, safe and environmentally friendly.
Owner:加翎加(天津)环境科技有限公司
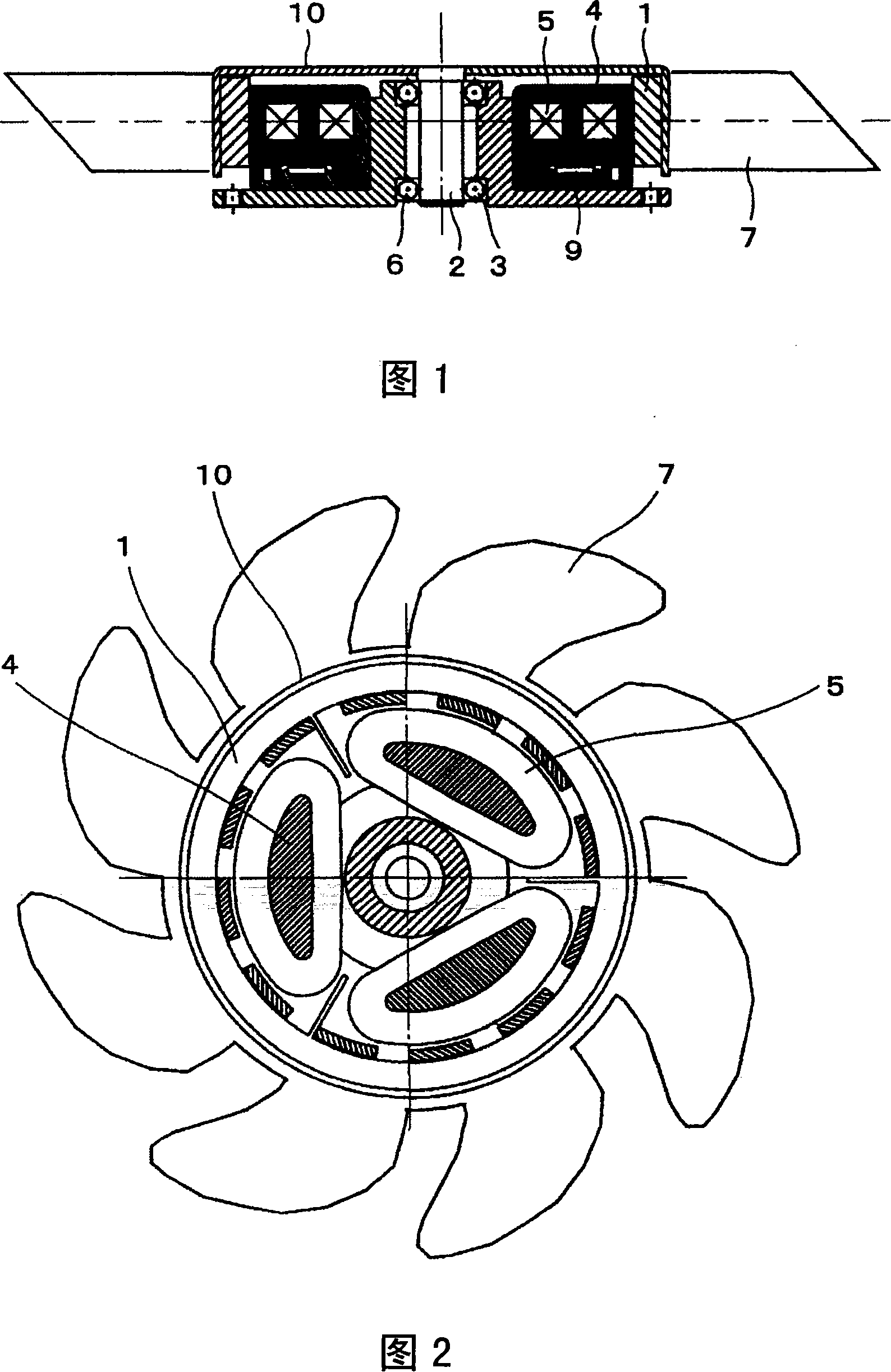
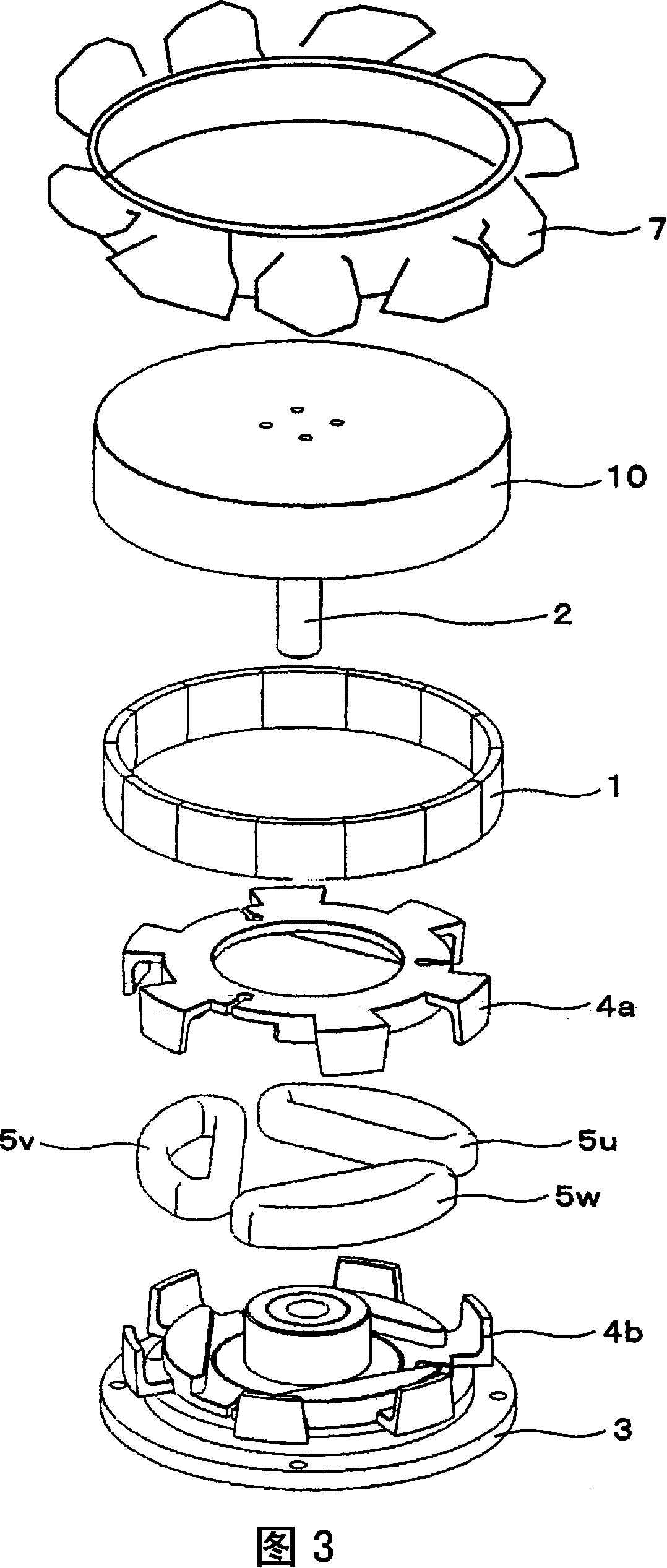
Fan system, electric motor, and claw-pole motor
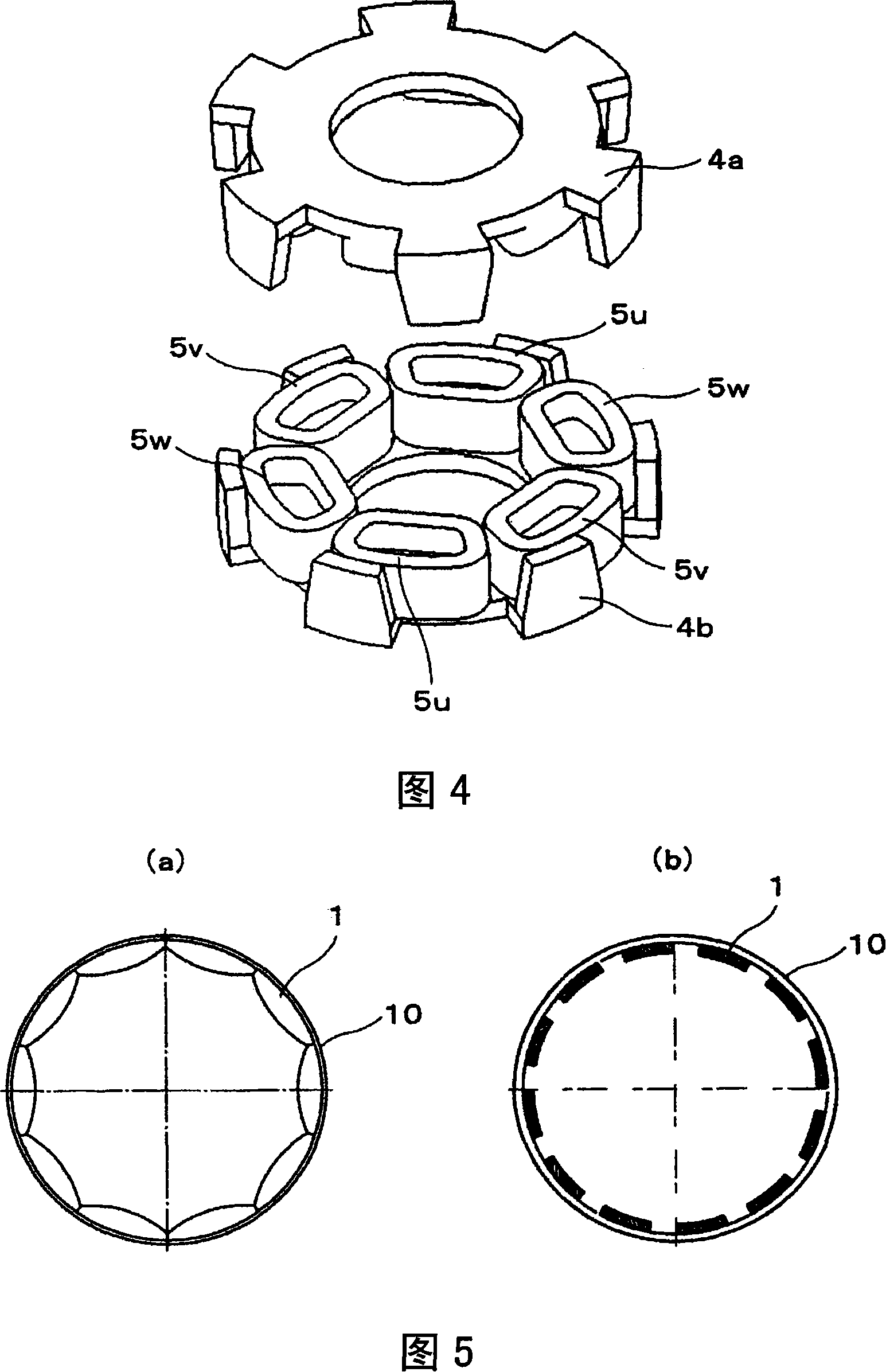
InactiveCN101154872AReduce vibration and noiseNo corrosion problemsMagnetic circuit rotating partsMechanical energy handlingStator coilEddy current
Owner:HITACHI IND EQUIP SYST CO LTD
Seawater desalination method for integrated membrane process
ActiveCN104909503AImprove conductivitySlow down the electrode reactionSeawater treatmentMultistage water/sewage treatmentUltrafiltrationHigh energy
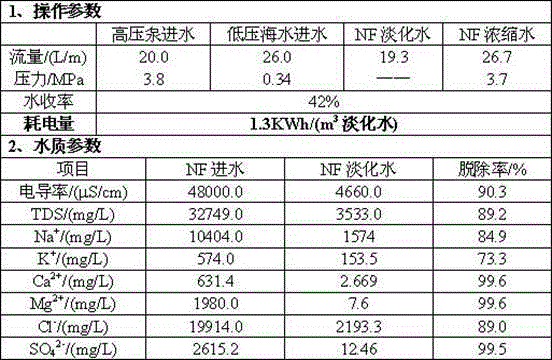
The invention provides a seawater desalination method for an integrated membrane process and belongs to a water desalination technology. Aiming at the defects of high investment, high pressure, high energy consumption and the like in an existing membrane-method seawater desalination process, 'nanofiltration / electrodialysis reversal' is taken as a core desalination process, wherein during nanofiltration, a high-desalination nanofiltration membrane with desalination ratio of 90% is adopted; during electrodialysis reversal, a thin-type electrode chamber is filled with a multistage multi-section energy-saving electroosmosis device provided with mixed-bed ion exchange resin. Seawater raw water is sequentially pretreated by virtue of a coagulative precipitation tank, a settling tank, a sand filter and an ultrafiltration membrane, and then is subjected to graded desaltination sequentially by virtue of a nanofiltration desalination device with an energy recovery device, and an energy-saving frequent electrodialysis reversal device; nanofiltration and energy-saving electrodialysis are respectively performed at low operation pressures which do not exceed 3.8MPa and 0.4MPa; the salt content of water produced by the system is 80-250mg / L, the total desalinization ratio can be mechanically regulated within a range of 99-99.75%, the body power consumption of per ton of water does not exceed 2.15KWh / m<3>, the investment and energy consumption of the whole seawater desalination process are obviously reduced, and the process operation is relatively safe and stable.
Owner:天津中领水系统技术有限公司
Air cooling/heating central air-conditioning system
InactiveCN1862119AImprove energy efficiency ratioAvoid disadvantagesCompression machinesAir conditioning systemsCold airCooling tower
The invention consists of an oil-free air compressor, an ice-ball cold-storage heat exchanger, an ice-storage tank, an ice-making system, a gas heater, a multi-functional cooling tower, an outdoor unit, a room unit, a winter-summer switcher and an adjustable timing change valve. The air intake duct of the oil-free air compressor is jointed on the top of the multi-functional cooling tower to absorb the cleaned air in the cooling tower and connected to the winter-summer switcher through the exhaust port of the compressor. In winter high temp. and pressure air is fed to the gas heater and then fed to the outdoor unit for heating and the heater air is fed again to the room unit for rising temp.. In summer the ice-ball cold-storage heat exchanger works and the output cold air is fed to the outdoor unit of the branch air pipe through the main pipeline and then fed again to the room unit for lowering temp. after separating of cold and hot air.
Owner:杨波
Bamboo bridge
Owner:单波
Cold rolling apparatus for electromagnetic steel sheet and rolling method
InactiveCN102029289ANo cracking issuesEfficient heatingWork treatment devicesMetal rolling arrangementsBobbinEngineering
The invention discloses a cold rolling apparatus for electromagnetic steel sheet and a rolling method, solving the problem of plate rupture in a straightening machine which is used before a heating device. The cold rolling apparatus comprises a roller mill (2) for cold rolling an electromagnetic steel plate (9), a payoff bobbin (1) for discharging coiled materials (8) to a press (2), and a straightening machine (5) arranged between the press (2) and the payoff bobbin (1), wherein the payoff bobbin (1) is provided with a pre-heating device (6), and a heating device (7) is provided before the press (2). Since the electromagnetic steel plate (9) is preheated by a pre-heating device (6) on the payoff bobbin, and coiled materials (8) are heated by a heating device (7) before the press, the electromagnetic steel plate (9) passing through the straightening machine (5) before the heating device (7) is preheated in the pre-heating device (6) thereby effectively solving the problem of plate rupture in the straightening machine (5) before the heating device (7).
Owner:MITSUBISHI HITACHI METALS MASCH INC
Desulfurization wastewater treatment method
InactiveCN106986401AAchieve pollutionNo corrosion problemsGeneral water supply conservationWaste water treatment from gaseous effluentsGas phasePhase change
The invention discloses a desulfurization wastewater treatment method, and belongs to the field of desulfurization wastewater treatment. A hot air unit introduces hot air into a phase change generator; meanwhile, a wastewater system conveys desulfurization wastewater to an atomizer in the phase change generator for atomization, hot wind formed by the desulfurization wastewater and the hot air is changed into a gas phase from a liquid phase through mass and heat exchange and the gas phase is discharged from an outlet of the phase change generator along with the hot wind; a solid formed by impurity crystal in the desulfurization wastewater enters the hot air unit at a solid outlet of the phase change generator under the action of gravity. According to the desulfurization wastewater treatment method, hot air in the phase change generator and atomized desulfurization wastewater drops are subjected to mass and heat exchange, and the desulfurization wastewater drops are evaporated and crystallized and then subjected to gas-solid separation. The problems that an original desulfurization balance is broken and equipment corrosion is caused in the desulfurization wastewater treatment process in a flue are solved, so that zero pollution and zero emission of the desulfurization wastewater are achieved.
Owner:张玉君
Device and method for achieving separation of negative and positive ions in solution through magnetic field
ActiveCN108160323AAchieve separationAchieve enrichmentLorentz force separationElectrochemical responseRelative motion
The invention provides a device and method for achieving separation of negative and positive ions in a solution through a magnetic field. The method can further be used for separation or enrichment ofelectronegative and electropositive particle groups in the solution. The device comprises a Lorentz force separation device, a magnetic field space, a liquid conveying part and a liquid collecting part. According to the device and method, the ions are separated or enriched through Lorentz force borne by the charged ions during movement of the ion solution in the magnetic field; the static magnetfield is changed into the rotating magnet field to increase the relative movement speed between the ions and the magnetic field so as to improve the separation effect; and the negative and positive ions in the solution are separated or enriched under the normal temperature and pressure, and compared with an electrochemical reaction method, the electrode corrosion problem is avoided. The device hasthe characteristics that the structure is simple, operation is convenient, the construction cost is low, energy consumption is low, the service life is long, the applicability is wide, and magnetic pollution is small.
Owner:UNIV OF SCI & TECH BEIJING
Preparation method and application method of HF electron gas deep purification material
ActiveCN111422870AWill not cause secondary pollutionHigh purityGas treatmentCarbon compoundsCarbonyl fluorideActivated carbon
The invention relates to the field of high-purity gas purification, in particular to a preparation method and application method of an HF electronic gas deep purification material. The preparation method produces a metal fluoride-loaded activated carbon material AC / MFx-nH2O. After deeply dehydrating the material by using a carbonyl fluoride / high-purity nitrogen mixed gas flow, the HF electron gasdeep purification material AC / MFx is obtained, the material is provided with fluoride, can form crystal water and hydrated metal fluoride and has high water absorption performance, anhydrous fluorideand activated carbon cannot be corroded by HF, collapse of a framework structure and secondary pollution of reaction products to HF cannot be caused, and the method has the advantages of being high inpurity and extremely low in water content when used for efficiently removing water from HF.
Owner:ZHEJIANG BRITECH CO LTD
Wall installation structure and installation method thereof
PendingCN107130711AImprove structural strengthThe snap-in method is firmWallsMechanical engineeringWall plate
Owner:BEIJING HENGTONG INNOVATION LUXWOOD TECH
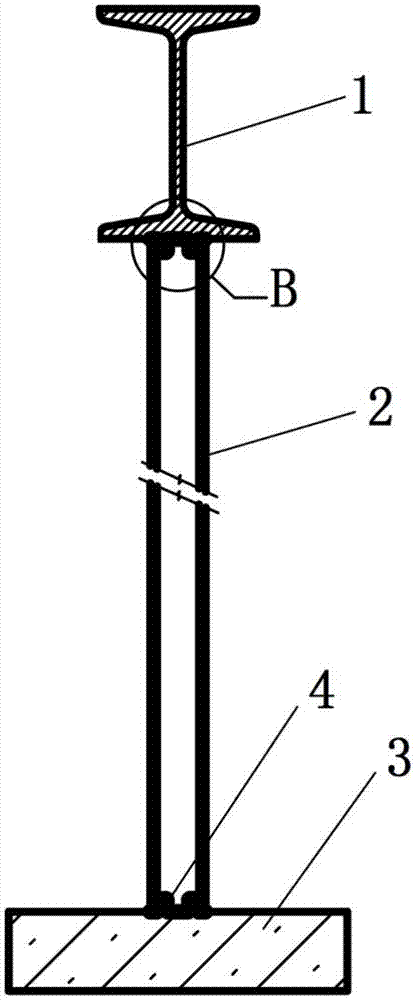
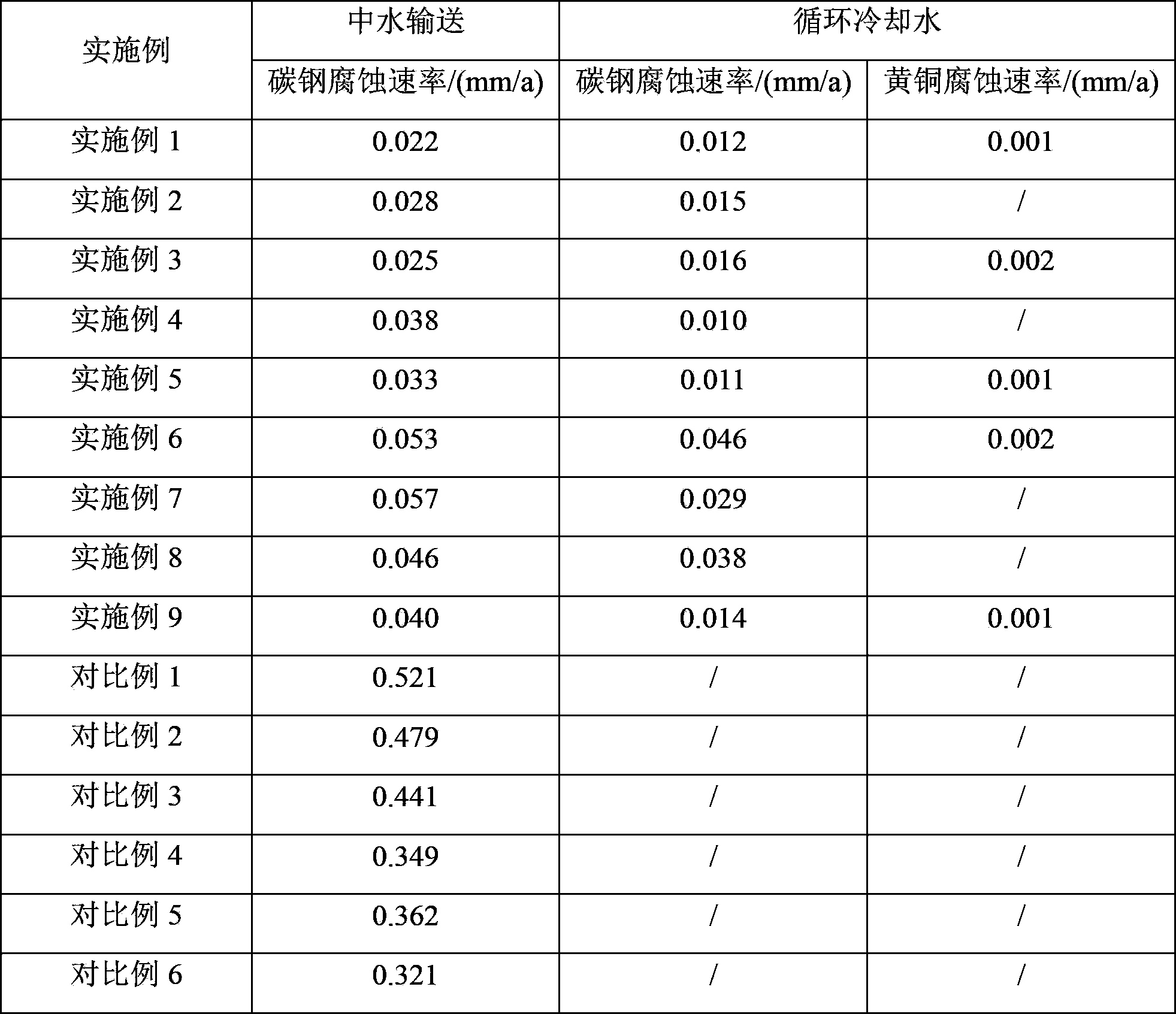
Method for recycling reclaimed water in circulating cooling water system
ActiveCN103803716APromote passivationAvoid corrosionTreatment using complexing/solubilising chemicalsReclaimed waterPolymer
The invention discloses a method for recycling reclaimed water in a circulating cooling water system. The method comprises the following steps: (1) adding lime to the reclaimed water to carry out softening treatment and then carrying out solid-liquid separation; (2) adding borate corrosion inhibitors to the reclaimed water obtained after solid-liquid separation in the step (1); (3) adding the reclaimed water to which the borate corrosion inhibitors are added in the step (2) to circulating cooling water of the circulating cooling water system as replenished water of circulating cooling water; (4) adding polymer scale inhibitors and dispersants to the circulating cooling water to which the reclaimed water is added in the step (3). By adopting the method provided by the invention, safety of reclaimed water transport can be guaranteed, the circulating cooling water treatment difficulty is reduced and introduction of phosphorus is avoided in the whole process.
Owner:CHINA PETROLEUM & CHEM CORP +1
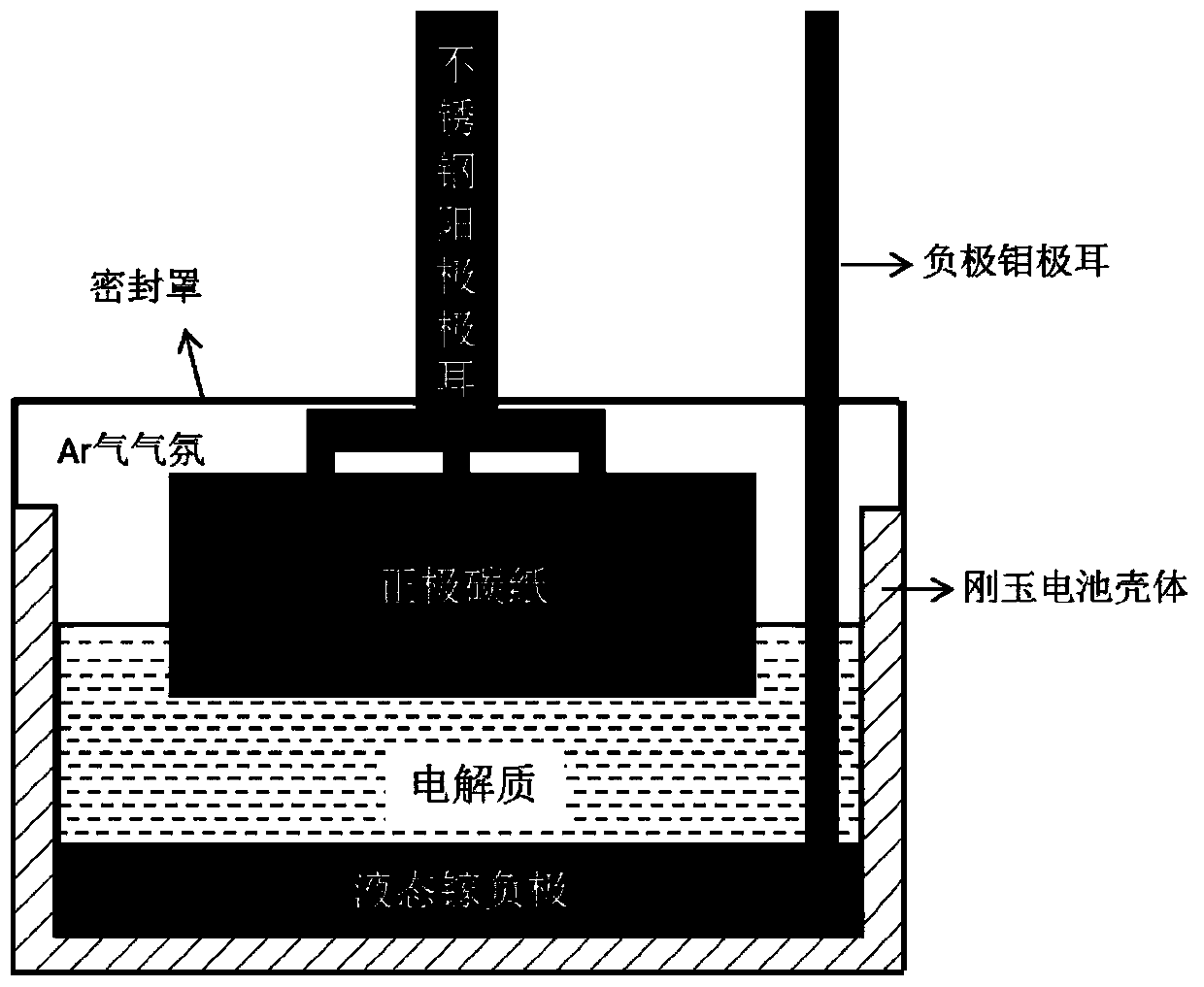
Aluminum ion battery using liquid metal gallium as negative electrode
InactiveCN110289444AImprove cycle lifeIncrease energy densityCell electrodesSecondary cellsAluminum IonHigh energy
The invention discloses an aluminum ion battery using liquid metal gallium as a negative electrode, which belongs to the field of electrochemical batteries. The battery is characterized by comprising a liquid gallium negative electrode with the purity of 99.9% to 99.999%, a positive electrode, an aluminum chloride and inorganic acid salt electrolyte and a separator selected according to specific situations. The liquid metal gallium is directly placed on the bottom layer of the battery as a negative electrode or placed in a crucible as a negative electrode. A solid conductive material is used as the lead of the liquid gallium negative electrode. During the charging process, aluminum ions are reduced to aluminum atoms on the surface of the liquid gallium negative electrode and dissolved and diffused into the liquid gallium body phase. During the discharging process, the aluminum atoms dissolved in the liquid gallium are diffused to the surface of the gallium and are oxidized to the aluminum ions to enter the electrolyte. The charging and discharging voltage range is 0.1 to 2.4 V, and the current density range is 0.01 to 10 Ag<-1>. The liquid gallium negative electrode adopted in the invention has the characteristics of corrosion resistance, inhibition of Al dendrites and recycling. The aluminum ion battery based on the negative electrode has the advantages of long cycle life, high energy density, safety and stability, and can be used for large-scale energy storage or automobile power batteries.
Owner:UNIV OF SCI & TECH BEIJING +1
Coal mine explosion-proof frequency converter
InactiveCN104113188ALow priceLow costCooling/ventilation/heating modificationsPower conversion systemsEngineeringPower unit
The invention discloses a coal mine explosion-proof frequency converter. The coal mine explosion-proof frequency converter comprises an explosion-proof housing composed of a rear housing plate, a housing maintenance frame and a door; a rectification power unit is arranged in the explosion-proof housing, the rear housing plate is provided with a heat sink base plate partially stretching into the explosion-proof housing, the rectification power unit is fixed to the heat sink base plate, the external portion of the heat sink base plate extends outwards from the heat sink base plate to be provided with a plurality of cooling fins in parallel, and a cooling gap is arranged between the cooling fins. The coal mine explosion-proof frequency converter is long in service life, even in cooling and high in cooling efficiency. A cooling cycle of the coal mine explosion-proof frequency converter is among an IGBT (Insulated Gate Bipolar Transistor)-thermally conductive silicone grease-the base plate-the cooling fins. The attenuation of the transferred heat is less due to fewer heat transfer steps. The heat can be transferred rapidly due to no gas-liquid state change in the heat transfer process. The red copper cooling fins are high in heat conduction efficiency, so that more heat can be transferred to the air in the same time.
Owner:QINGDAO VECCON ELECTRIC
Large concrete block for lifting a crane, method for manufacturing same, and method for installing same
ActiveCN103429519ASolving problems caused by the use of lifting ring componentsNo corrosion problemsBuilding componentsLoad-engaging elementsEngineeringSynthetic resin
Disclosed herein is a large concrete block for crane lifting. The large concrete block includes a large concrete block body (110) made of concrete, and a connection-wire-rope insert tube (120) made of synthetic resin. The connection-wire-rope insert tube is embedded at a medial portion thereof in the concrete block body in such a way that opposite ends of the connection-wire-rope insert tube are disposed at an upper surface of the concrete block body and oriented upwards. The present invention having the above construction can solve problems which have been caused in the conventional technique that uses a lifting eye member.
Owner:YUJOO CO LTD
Clean aerosol composition and preparation method of aerosol
ActiveCN107603755AEasy to removeEasy to useOrganic detergent compounding agentsSurface-active detergent compositionsEngineeringAerosol composition
The invention relates to the technical field of cleaning supplies and in particular to a clean aerosol composition suitable for a screen window, a filter screen and the like and a method for preparingaerosol by using the clean aerosol composition. The aerosol can form abundant foam at the moment of spraying a feed liquid, and the foam is extremely easily intercepted by the screen window during being sprayed towards the screen window and can be used for uniformly covering the surface of the screen window; and meanwhile, the foam sufficiently acts on dust on the surface of the screen window, sothat the dust originally attached to the surface of the screen window is floated on the surface of the foam by virtue of the self-cleaning capacity of the foam and microvibration caused during foam breakdown. By using the aerosol provided by the invention, a user only needs to uniformly spray the aerosol on the surface of the screen window and wipe the surface of the screen window by using a drywiping towel, a scouring pad or an analogue thereof after the foam is eliminated, and the problems that a common cleaning screen window needs to be dismounted, a mist of a common cleaning liquid directly penetrates through the screen window and the screen window is required to be cleaned by using a great deal of water after being cleaned are solved.
Owner:广州超威生物科技有限公司
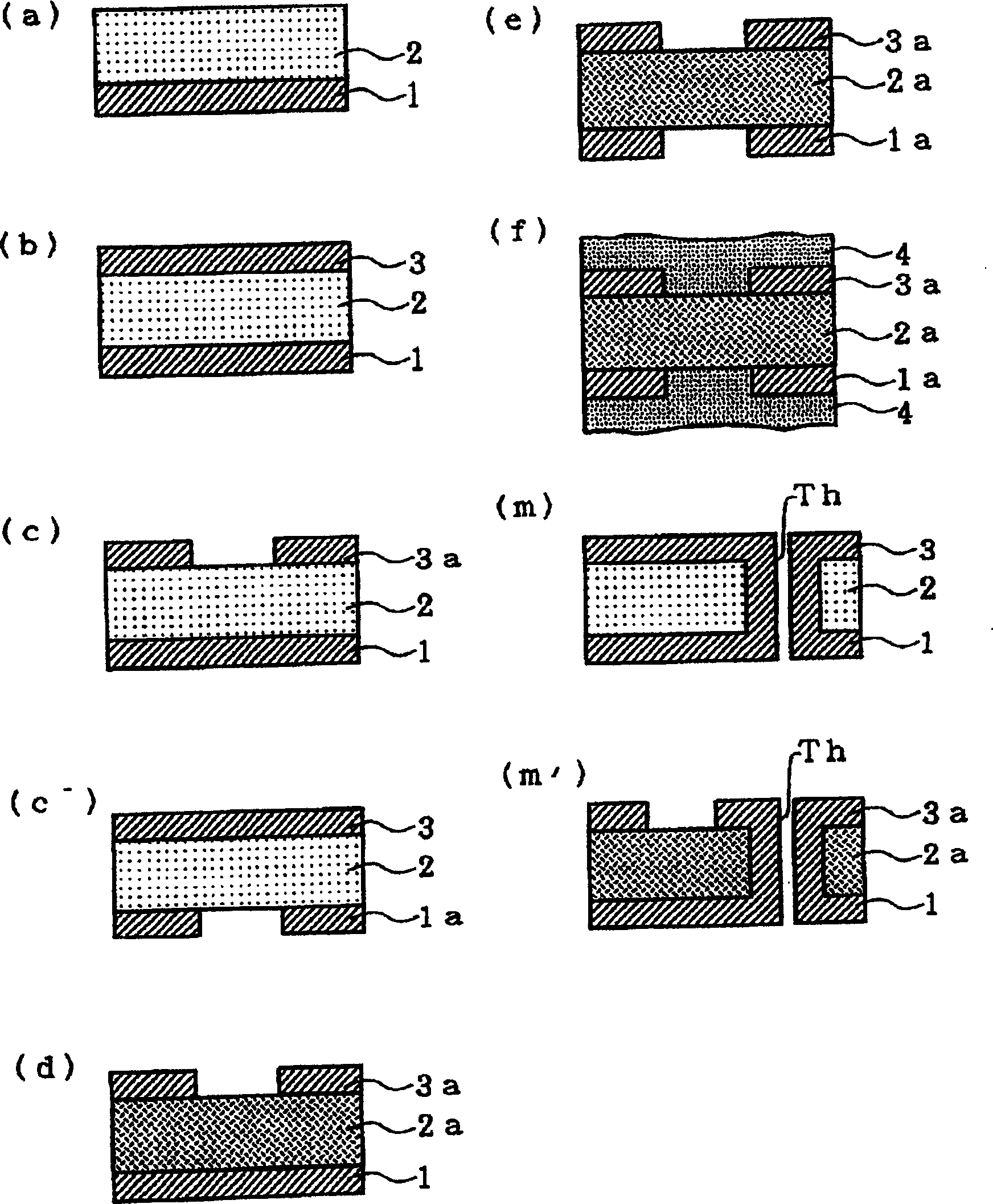
Method for manufacturing double-side flexible printed circuit board
InactiveCN1183812CImprove heat resistanceGood dimensional stabilityInsulating substrate metal adhesion improvementPrinted circuit aspectsHeat resistanceFlexible electronics
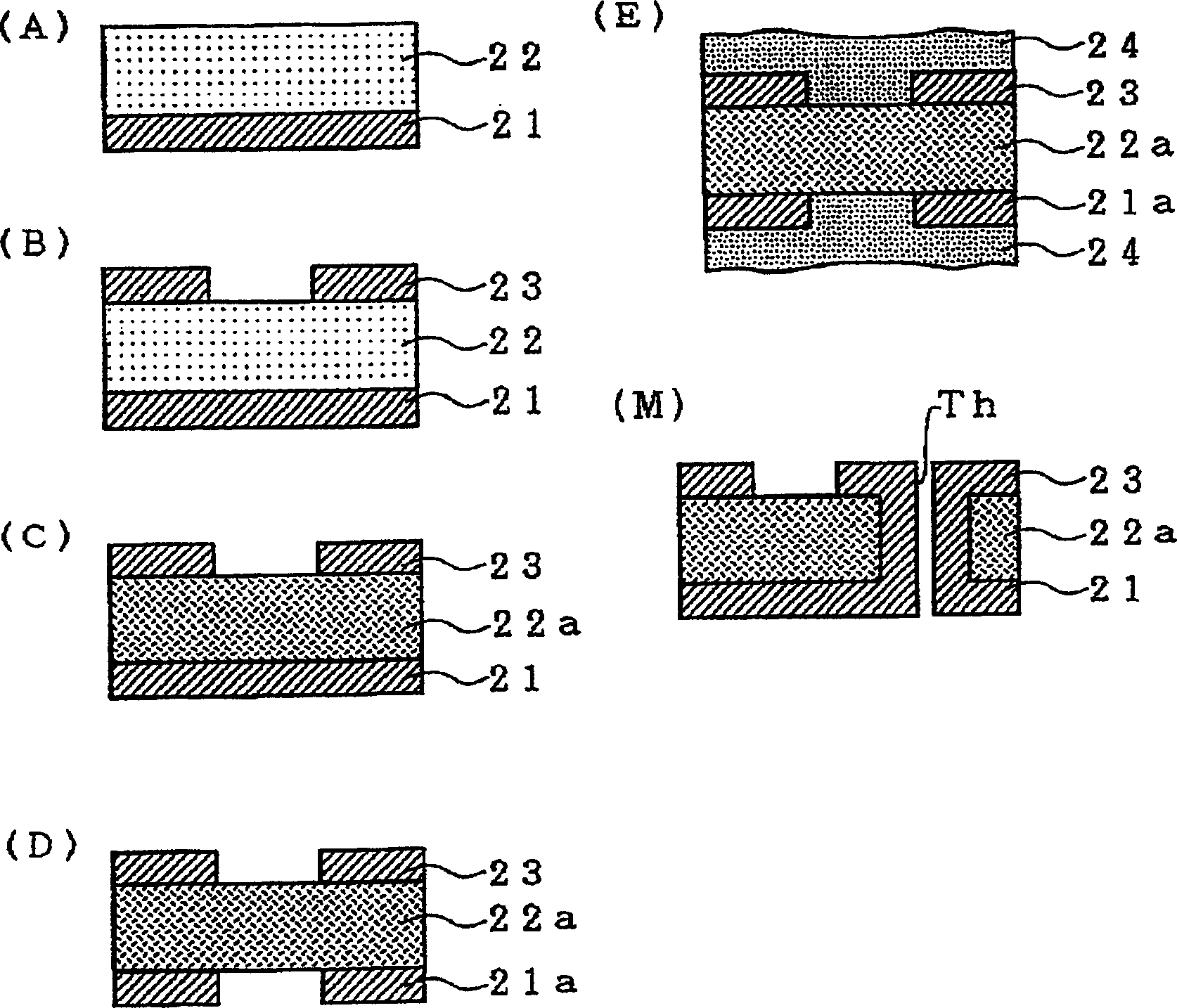
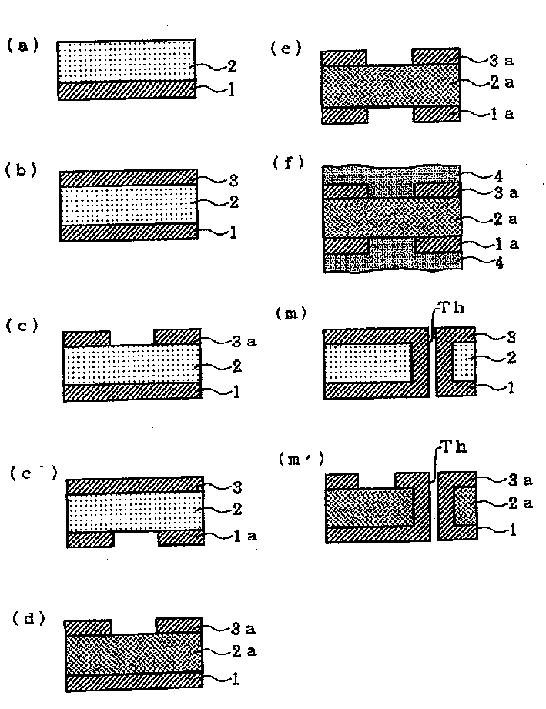
A double-sided flexible printed board is manufactured by the following steps of: (a) forming a polyimide precursor layer 2 on a first metal layer 1; (b) forming a second metal layer 3 on the polyimide precursor layer 2; (c) patterning the second metal layer 3 to form a second circuit layer 3a or (c') patterning the first metal layer 1 to form a first circuit layer 1a; and (d) imidating the polyimide precursor layer 2 to form a polyimide insulating layer 2a.
Owner:SONY GRP CORP
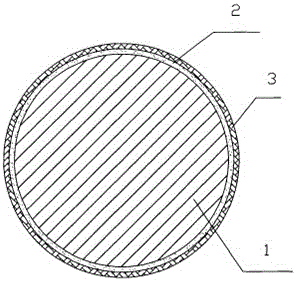
Copper steel composite material for seawater enclosing aquaculture fishing net and manufacturing method for copper steel composite material
InactiveCN105881992AGood mechanical propertiesAvoid Galvanic CorrosionAgricultural articlesClimate change adaptationFishing netSeawater
The invention provides a copper steel composite material for a seawater enclosing aquaculture fishing net. The outer layer of the copper steel composite material is a copper alloy layer; the core part of the copper steel composite material is steel; a cementing layer is arranged between the core part and the copper alloy layer; the thickness of the copper alloy layer is 1 to 3mm; the mass percent of the copper in the copper alloy layer is at least 70 percent or above; the strength of the steel is greater than 600 MPa; the material of the cementing layer is epoxy resin or phenolic resin; the thickness of the cementing layer is 10 to 30 micron. The invention also provides a manufacturing method for the copper steel composite material for the seawater enclosing aquaculture fishing net. According to the copper steel composite material for the seawater enclosing aquaculture fishing net and the manufacturing method for the copper steel composite material, the copper and the steel are compounded, and the copper alloy coats the surface layer of the steel, so that the advantages of the corrosion resistance and the biofouling preventing property of the copper alloy can be fully played; the core part is made of the steel with high strength, so that the advantages of high mechanical properties of the steel are fully played; the steel and the copper are combined, so that the requirements on the fishing net materials of sea farming can be met better; the steel is coated with the copper, so that the steel is not exposed in seawater, and the problem of corrosion is avoided.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
A kind of polymer dispersion and preparation method thereof
InactiveCN104693345BImprove securityPrevent gelationOther chemical processesBuffering agentPolymer chemistry
The invention discloses a polymer dispersion and a preparation method thereof. The polymer dispersion is obtained through the soap-free emulsion copolymerization technology. The polymer dispersion comprises a negative ion monomer, a non-ionic monomer, a pH buffering agent, an initiator, an antalkali and water, wherein the negative ion monomer accounts for 3-10 parts of the raw materials, the non-ionic monomer accounts for 10-25 parts of the raw materials, the pH buffering agent accounts for 0.3-1.0 part of the raw materials, the initiator accounts for 0.3-2 parts of the raw materials, and the water accounts for 100 parts of the raw materials. A proper amount of the antalkali is used for adjusting the pH value of a reaction system to range from 6.0 to 9.0. The obtained polymer dispersion is used as a dust depressor, an effective shell can be formed on the surface of dust, and the construction safety and application safety of the polymer dispersion are higher. The polymer dispersion is applied as the chemical dust depressor, can be diluted with water to be sprayed for use, and is good in overall performance, safe and environmentally friendly.
Owner:HEBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com