Rechargeable battery
A technology of rechargeable batteries and electrolytes, applied in secondary batteries, battery electrodes, circuits, etc., can solve the problems of minimum requirements for commercial production, not easy to decompose, hinder ion transport, etc., to avoid irreversible sulfation and improve cycle life. , the effect of improving reversibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Preparation of the positive electrode: first dissolve the binder (polyvinylidene fluoride) in N-methylpyrrolidone to prepare a dispersion with a mass fraction of 5%, and mix manganese dioxide, manganese oxyhydroxide, acetylene black, polyvinylidene Mix vinyl fluoride in a mass ratio of 70:5:15:10, stir evenly in a high-speed mixer, evenly coat the resulting mixture on the surface of graphite conductive paper, move it into a vacuum oven at 120°C, take it out after 12 hours, and cut it to get the positive electrode sheet .
[0081] Electrolyte preparation: 51.1 g of zinc methanesulfonate was dissolved in 100 mL of deionized water to prepare an aqueous solution of zinc methanesulfonate with a concentration of about 1.6 mol / L to obtain an electrolyte.
[0082] The prepared positive electrode sheet is used as the positive electrode, the zinc foil is used as the negative electrode, and the aqueous solution of zinc methanesulfonate containing 1.6 mol / L is used as the electroly...
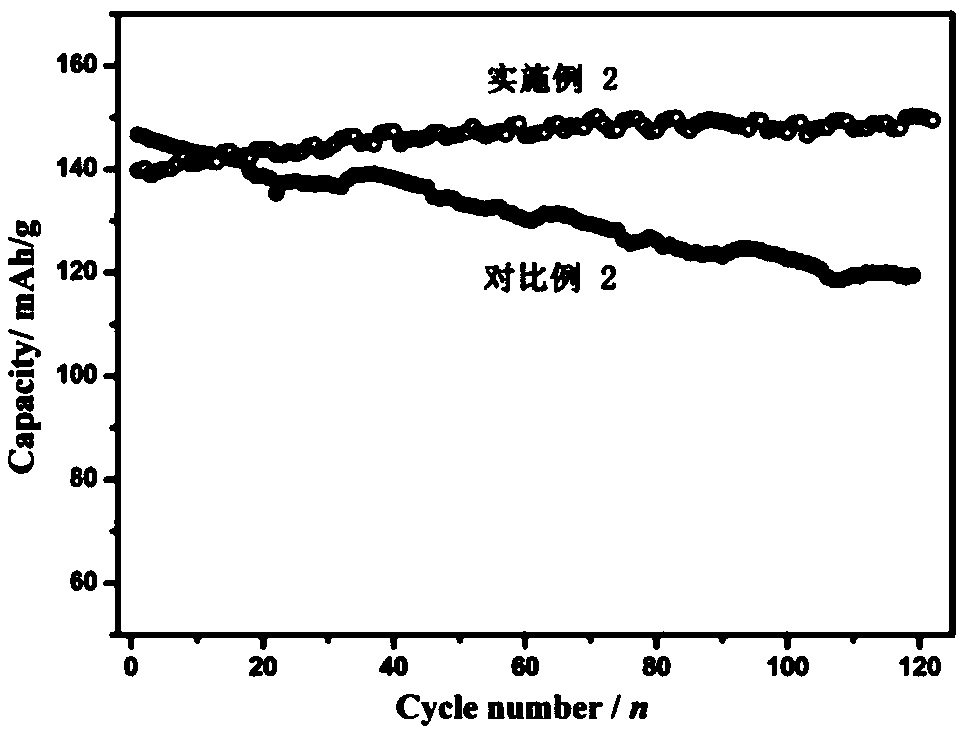
Embodiment 2
[0085] Preparation of the positive electrode: first dissolve the binder (polyvinylidene fluoride) in N-methylpyrrolidone to make a dispersion with a mass fraction of 5%, and mix manganese dioxide, manganese oxyhydroxide, acetylene black, polyvinylidene Mix vinyl fluoride in a mass ratio of 70:5:15:10, stir evenly in a high-speed mixer, evenly coat the resulting mixture on the surface of graphite conductive paper, move it into a vacuum oven at 120°C, take it out after 12 hours, and cut it to get the positive electrode sheet .
[0086] Preparation of electrolyte solution: Dissolve 51.1 g of zinc methanesulfonate and 4.9 g of manganese methanesulfonate in 100 mL of deionized water to obtain an electrolyte solution. The concentration of zinc methanesulfonate in the obtained electrolyte is about 1.6 mol / L. The concentration of manganese sulfonate is 0.16mol / L.
[0087] The prepared positive electrode sheet is used as the positive electrode, the zinc foil is used as the negative elec...
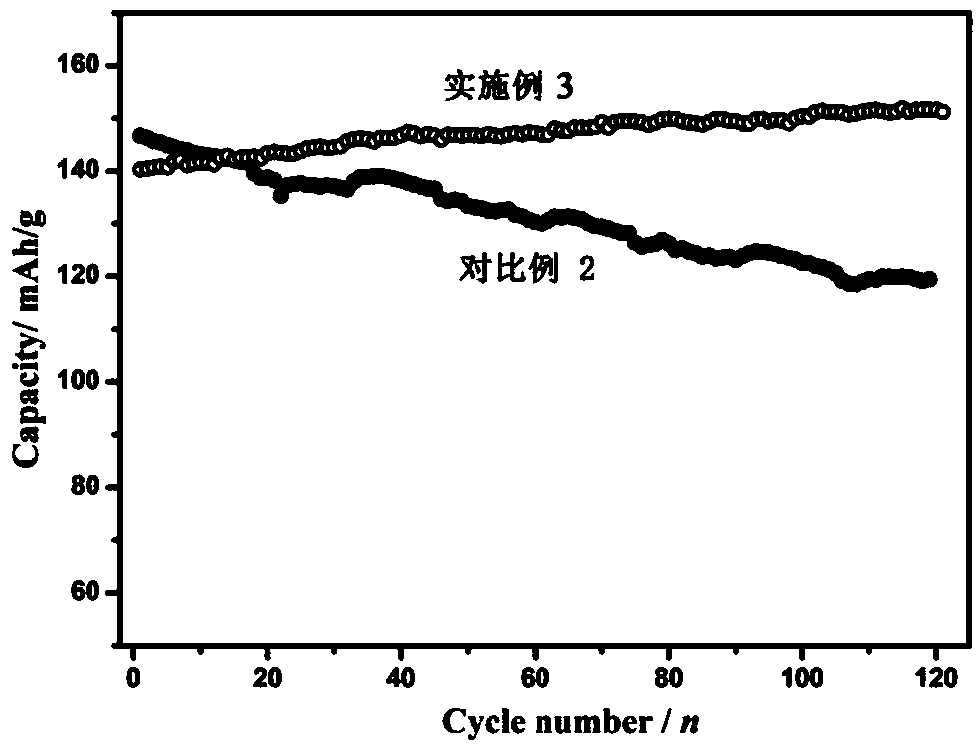
Embodiment 3
[0091] Preparation of the positive electrode: first dissolve the binder (polyvinylidene fluoride) in N-methylpyrrolidone to prepare a dispersion with a mass fraction of 5%, and mix manganese dioxide, manganese oxyhydroxide, acetylene black, polyvinylidene Mix vinyl fluoride in a mass ratio of 70:5:15:10, stir evenly in a high-speed mixer, evenly coat the resulting mixture on the surface of graphite conductive paper, move it into a vacuum oven at 120°C, take it out after 12 hours, and cut it to get the positive electrode sheet .
[0092] Electrolyte preparation: Dissolve 51.1 g of zinc methanesulfonate and 4.57 g of manganese fluoroborate in 100 mL of deionized water to obtain an electrolyte. The concentration of zinc methanesulfonate in the obtained electrolyte is 1.6 mol / L, and the concentration of manganese fluoroborate It is 0.16mol / L.
[0093] The prepared positive electrode sheet is used as the positive electrode, the zinc foil is used as the negative electrode, the aque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com