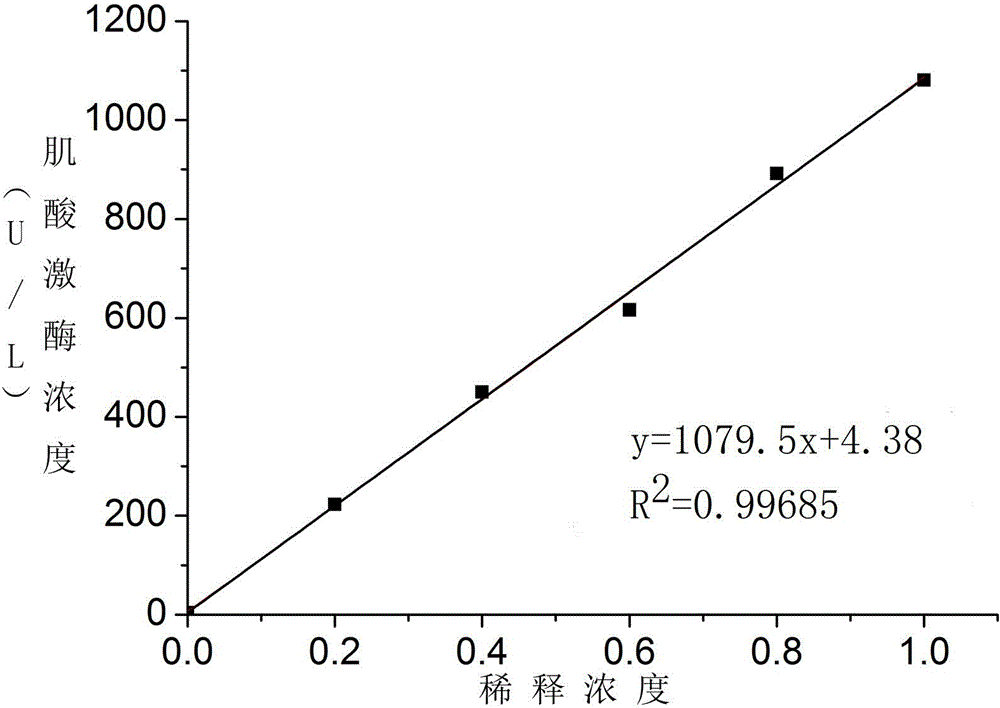
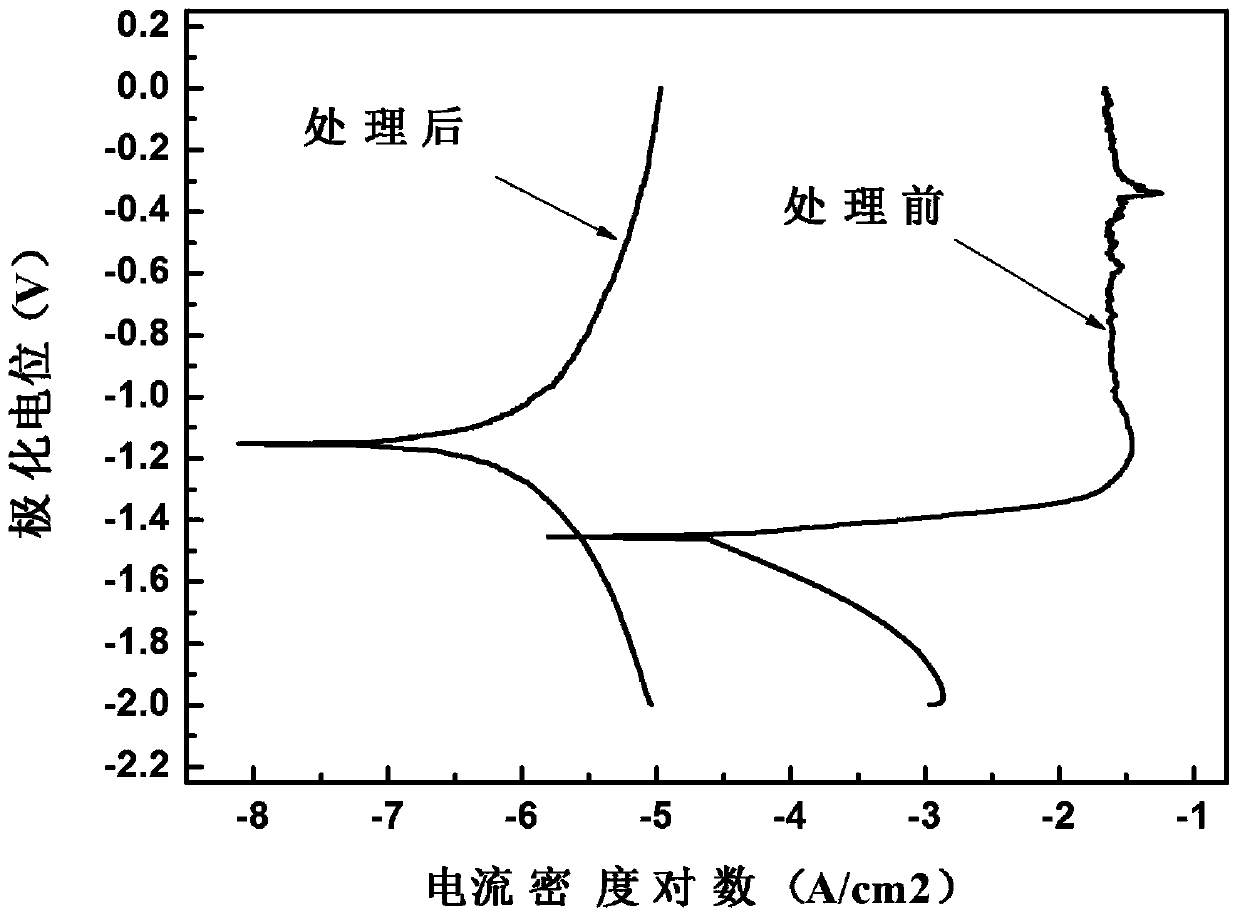
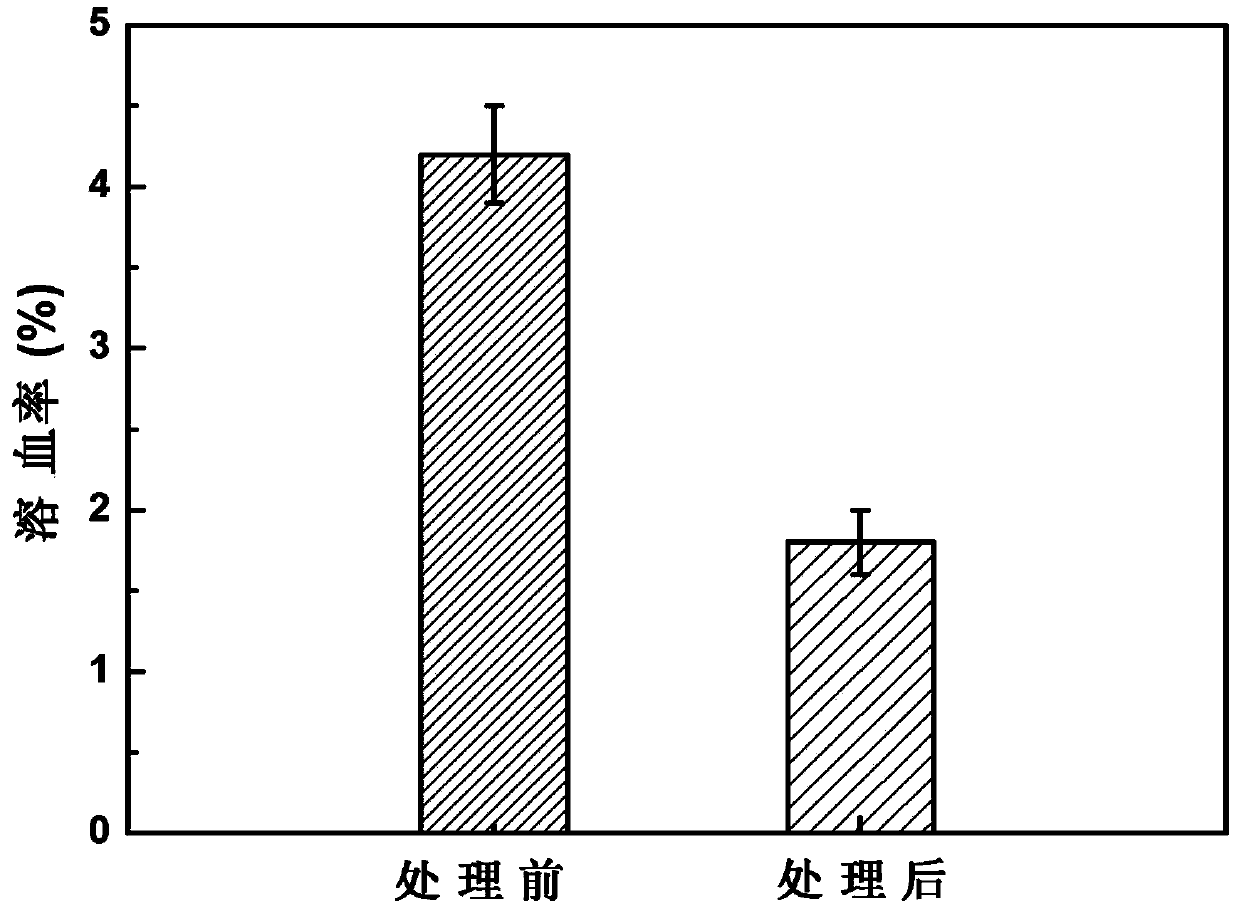
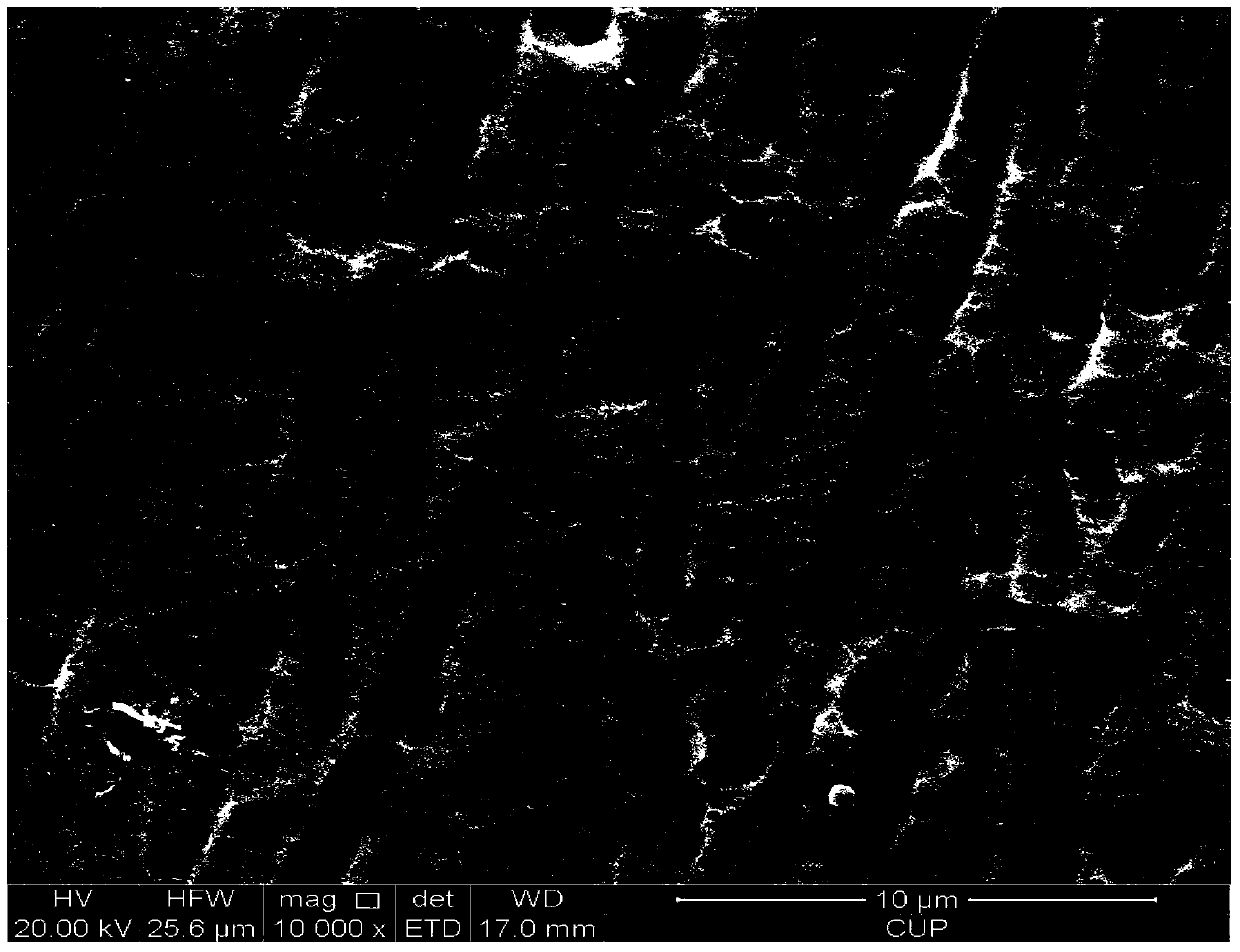
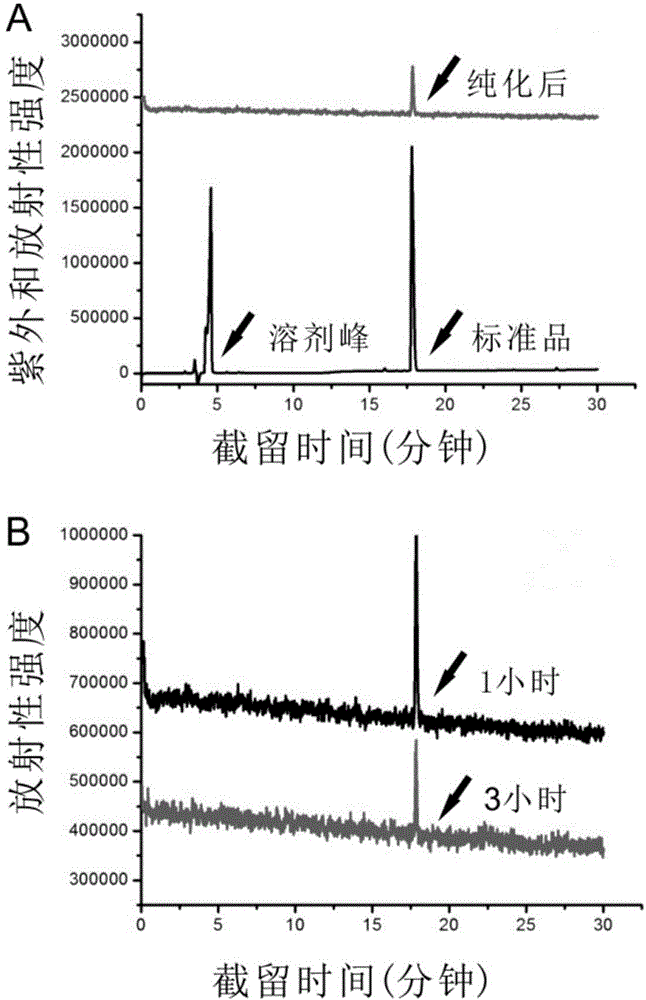
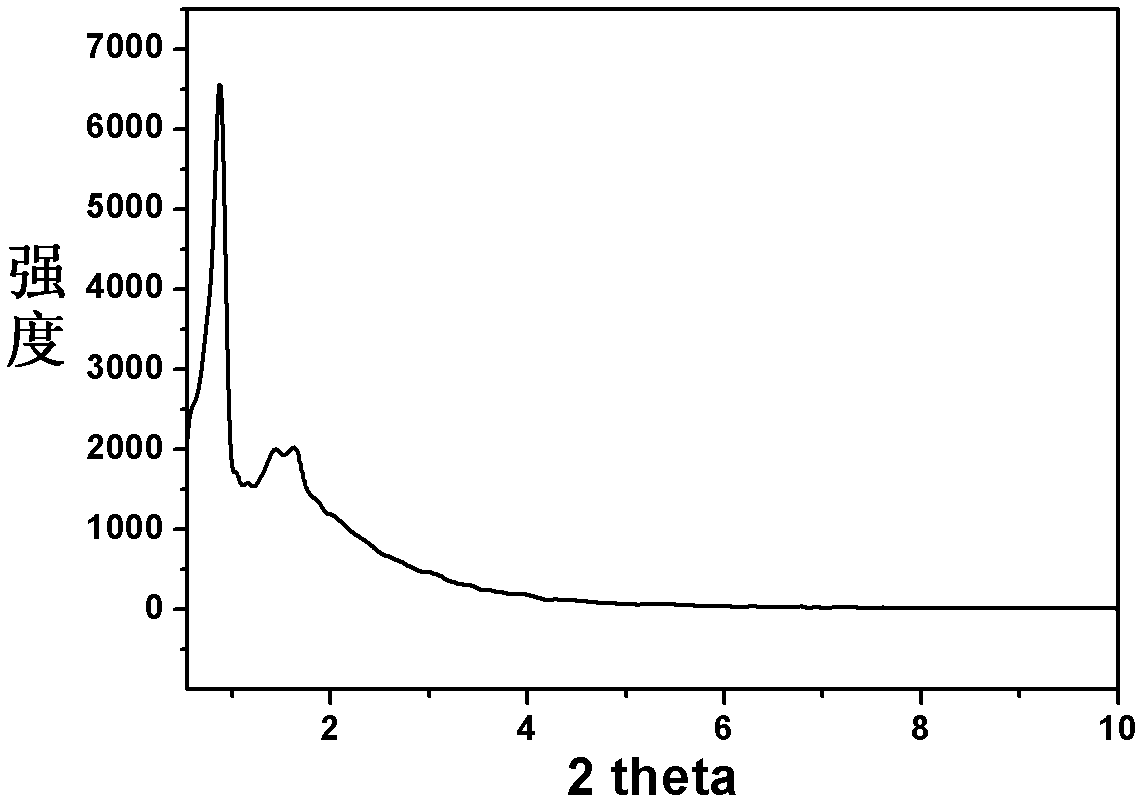
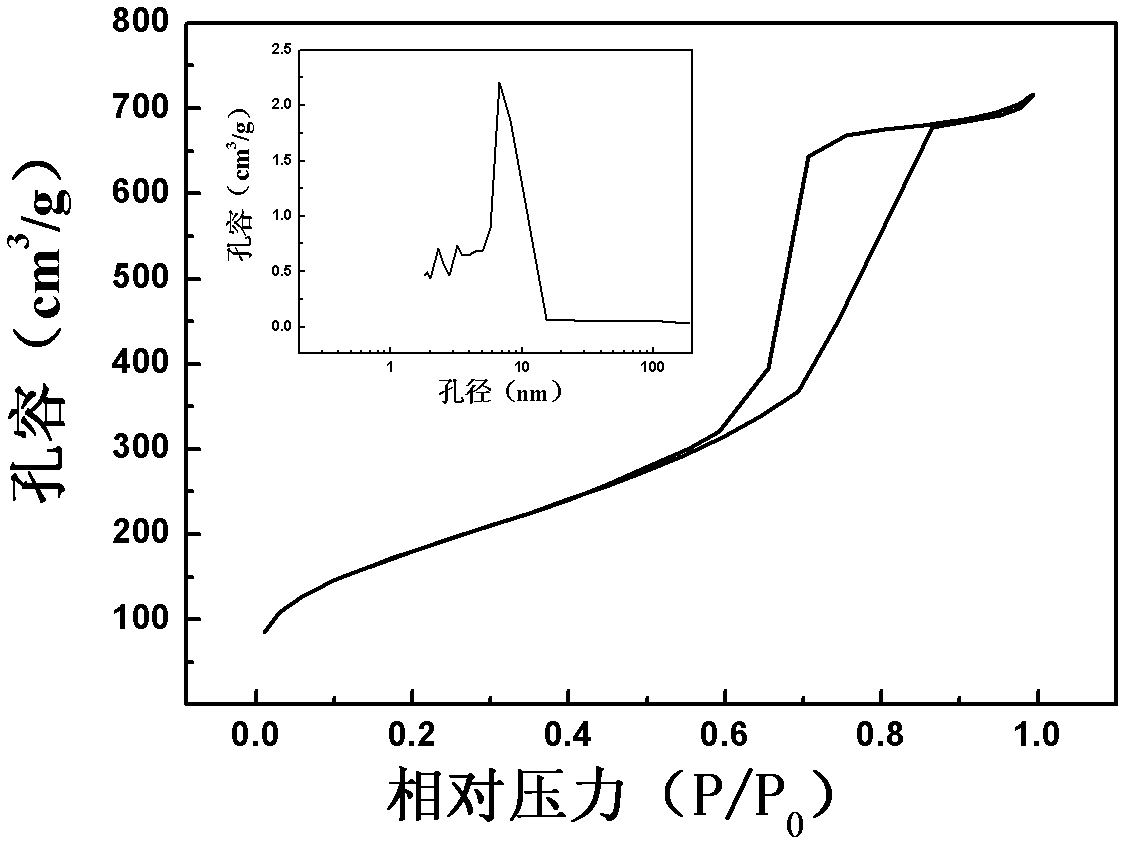
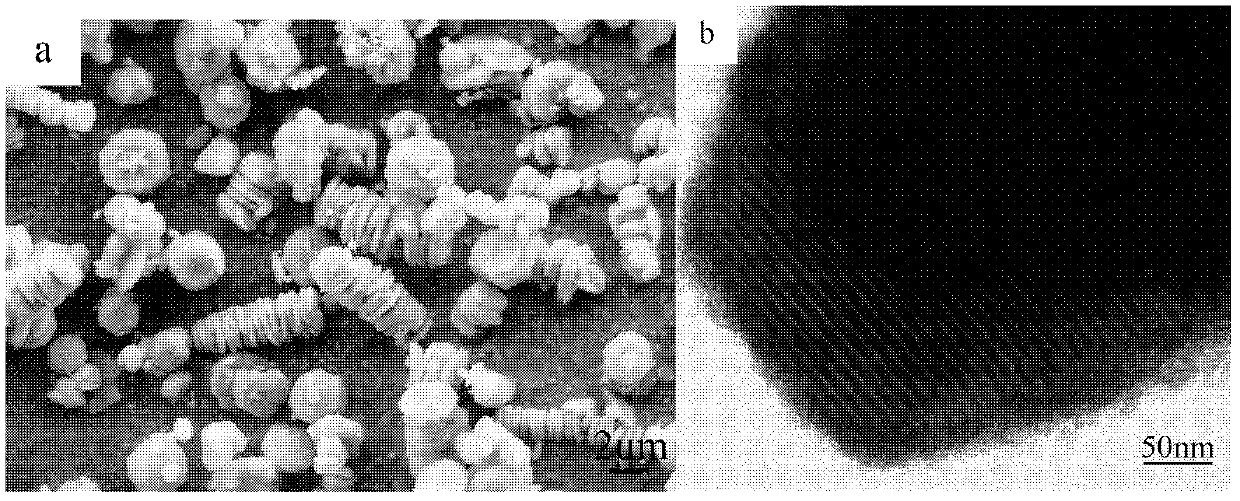
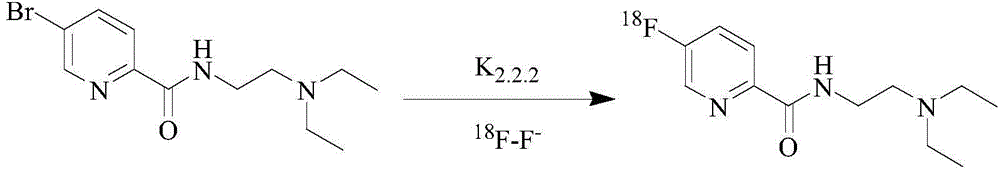Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47results about How to "Meet clinical application requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
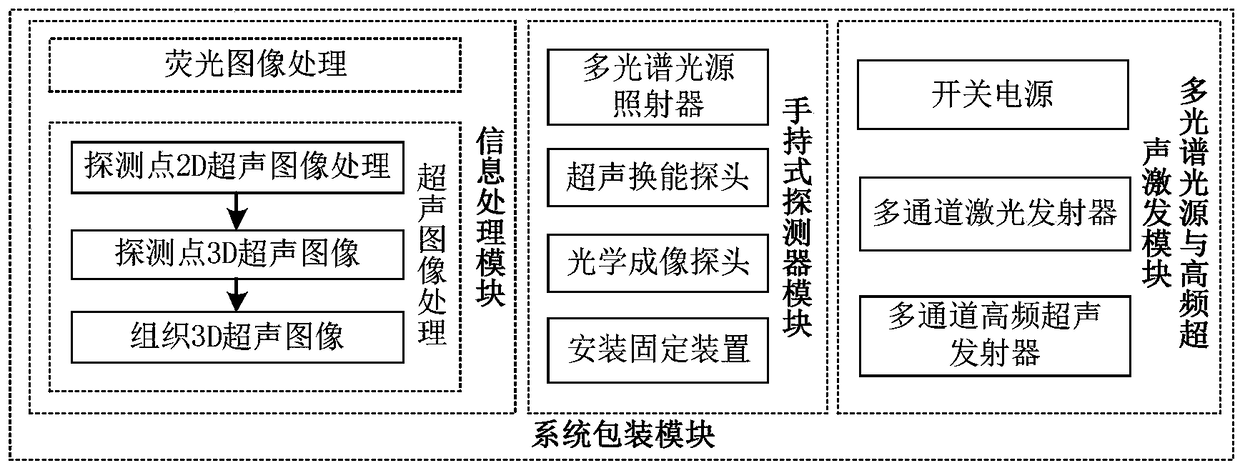
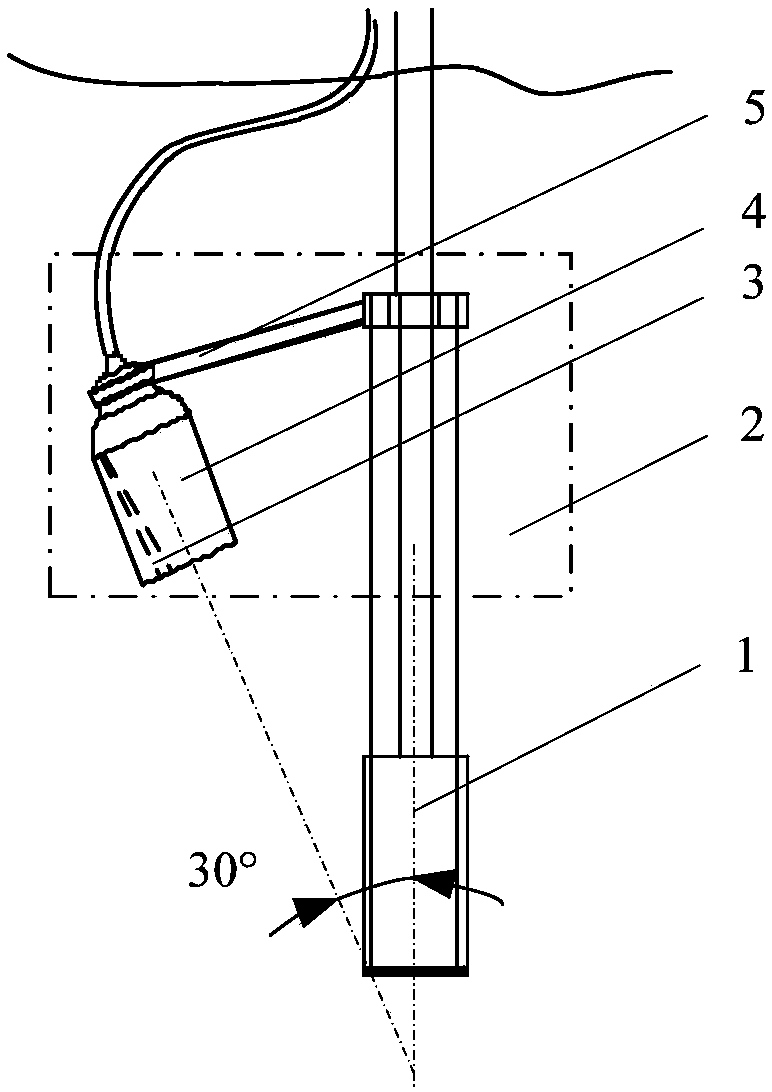
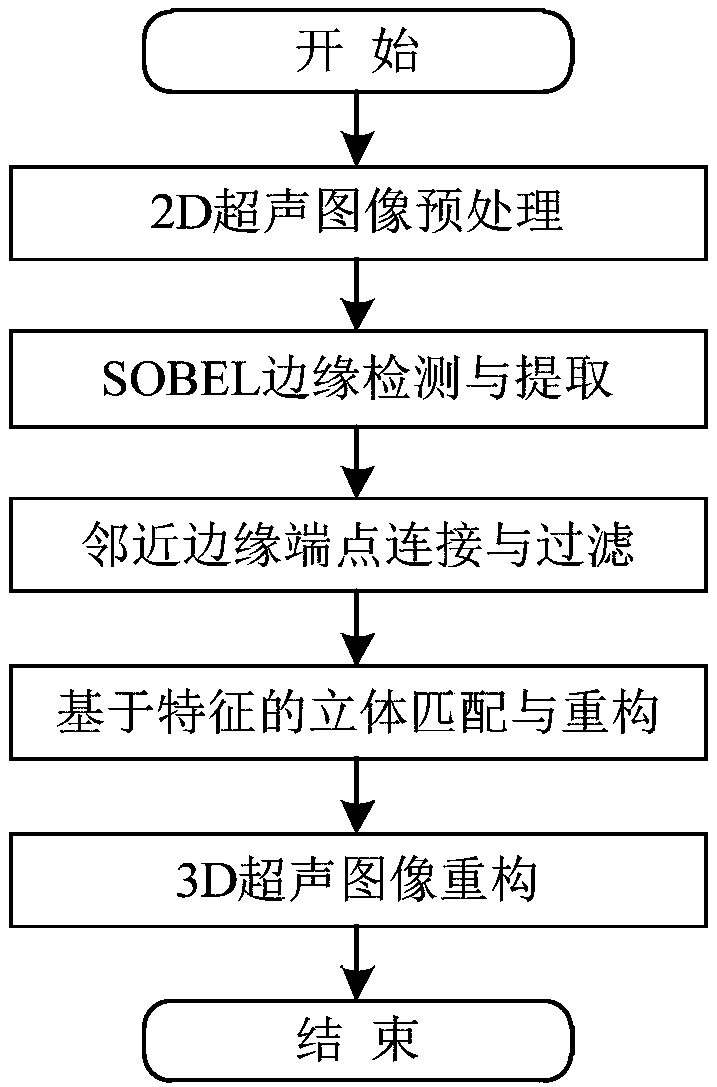
Handheld fluorescent ultrasonic fusion radiographic navigation system
ActiveCN108186115AFully reflect the shapeEasy to operateUltrasonic/sonic/infrasonic diagnosticsSurgical navigation systemsInformation processingFluorescence
The invention discloses a handheld fluorescent ultrasonic fusion radiographic navigation system. A multi-spectrum source exciting module excites near-infrared laser and visible white light; a handhelddetector module includes a fluorescent probe and an ultrasonic probe integrated to each other; the fluorescent probe irradiates tissue of an open living body to obtain a fluorescent image of a specific tissue part; an information processing module processes the fluorescent image to obtain an enhanced fluorescent image including position and boundary of diseased tissue; a high-frequency ultrasonicexciting module excites multi-frequency ultrasound according to the displayed position and boundary of the diseased tissue, an ultrasonic probe performs ultrasonic detection on the diseased tissue toobtain a continuous two-dimensional ultrasonic image of the diseased tissue; the information processing module performs 3D registered fusion on the continuous two-dimensional ultrasonic image to obtain a 3D ultrasonic image of the diseased tissue; the information processing module transmits the 3D ultrasonic image to a display for displaying, and the enhanced fluorescent image and the 3D ultrasonic image provide real-time surgical video navigation for surgeons.
Owner:BEIJING DIGITAL PRECISION MEDICAL TECH CO LTD
Induction ossified bio-active artificial tooth root implant material and preparing method thereof
InactiveCN1451367APromote osseointegrationTo promote metabolismDental implantsImpression capsApatiteTitanium oxide
An artificial tooth root material with induced osteoplastic bioactivity is prepared through spraying a gradient bioactive layer on the surface of the metallic basic material which has excellent biologic compatibility and combining the bone morphogenetic protein, alkaline fibroblast growth factor, or conversion growth factor onto the surface of said coated layer. Said coated layer contains hydroxyphosphorite nano-powder, bioactive glass nanopowder, alumina nano powder and titanium oxide nano powder. Its advantages are high bioactivity and high adhesion of coated layer.
Owner:SOUTH CHINA UNIV OF TECH
Serum creatine kinase detection reagent
ActiveCN104374905AHigh activityEfficient removalMicrobiological testing/measurementBiological material analysisAcyl CoA dehydrogenaseSODIUM DODECYL BENZENE SULFONATE
The invention discloses a creatine jubase detection reagent. The reagent disclosed by the invention consists of a reagent R1 and a reagent R2 according to a volume ratio being 4 to 1, wherein the reagent R1 consists of an imidazole buffer solution, glucose, nano particles, N-acetylcysteine, ethylenediaminetetraacetic acid disodium salt, adenosine diphosphate, coenzyme I, adenine ribonucleotide, pyruvate decarboxylase, 6-phosphogluconic dehydrogenase, hexokinase and sodium dodecyl benzene sulphonate; and the reagent R2 consists of an imidazole buffer solution, phosphocreatine, a preservative and sodium dodecyl benzene sulphonate. Pyruvate decarboxylase and gamma-Fe2O3 nano particles are added into the creatine jubase detection reagent disclosed by the invention, so that interfering substances in a serum sample can be effectively eliminated, metal ions in the serum sample are chelated by matching sodium dodecyl benzene sulphonate and ethylenediaminetetraacetic acid disodium salt, the influence on reaction enzymes is alleviated, and the reagent is a stable, accurate and sensitive detection reagent with high interference resistance.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Method for carrying out surface modification upon biodegradable magnesium and magnesium alloy through iron ion implantation deposition
ActiveCN103498129AHigh bonding strengthImprove corrosion resistanceVacuum evaporation coatingSputtering coatingBiocompatibility TestingIon beam
The invention discloses a method for carrying our surface modification upon biodegradable magnesium and magnesium alloy through iron ion implantation deposition. The method belongs to the technical field of surface treatment. According to the invention, through ion implantation, a composite transition layer doped with iron ions is formed on the surface of magnesium and magnesium alloy. The transition layer is composed of Fe2O3 and MgO, and has a thickness of 20-50nm. An iron film with a thickness of 100-500nm is prepared on the transition layer with an ion-beam-assisted enhanced deposition technology. The transition layer preparation method provided by the invention assists in ensuring good bonding strength between the deposited iron film and the substrate. With the method provided by the invention, corrosion resistance, biocompatibility, and mechanical properties of magnesium and magnesium alloy are improved.
Owner:DONGGUAN MEIANMEIYE TECH CO LTD
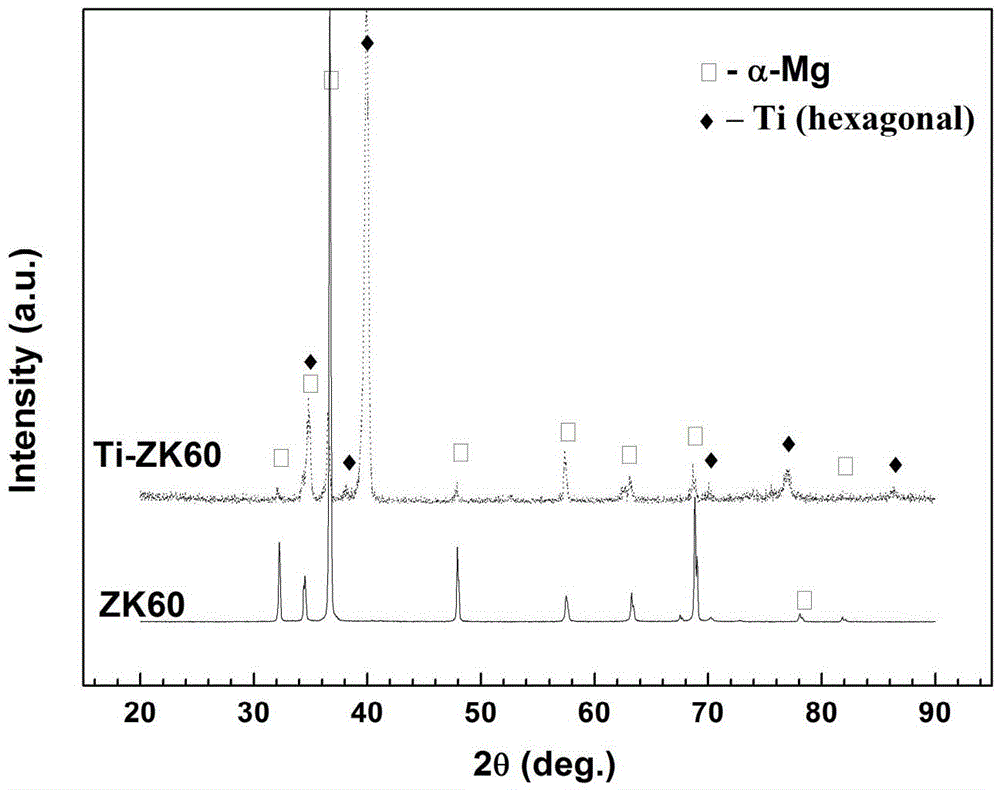
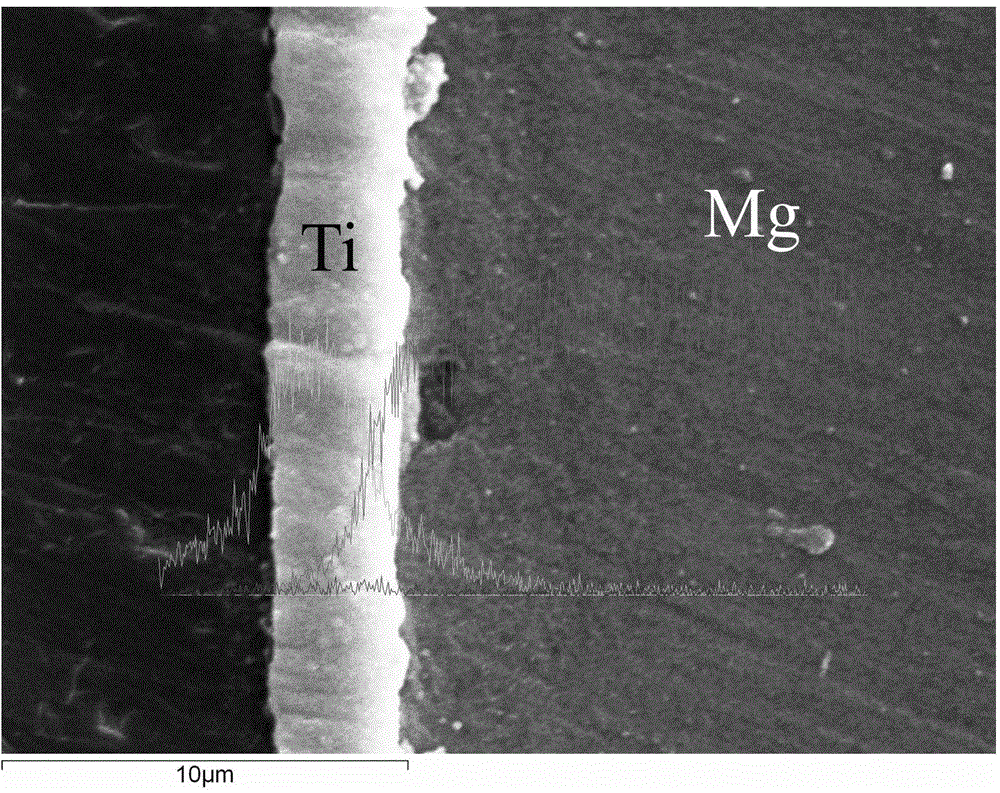
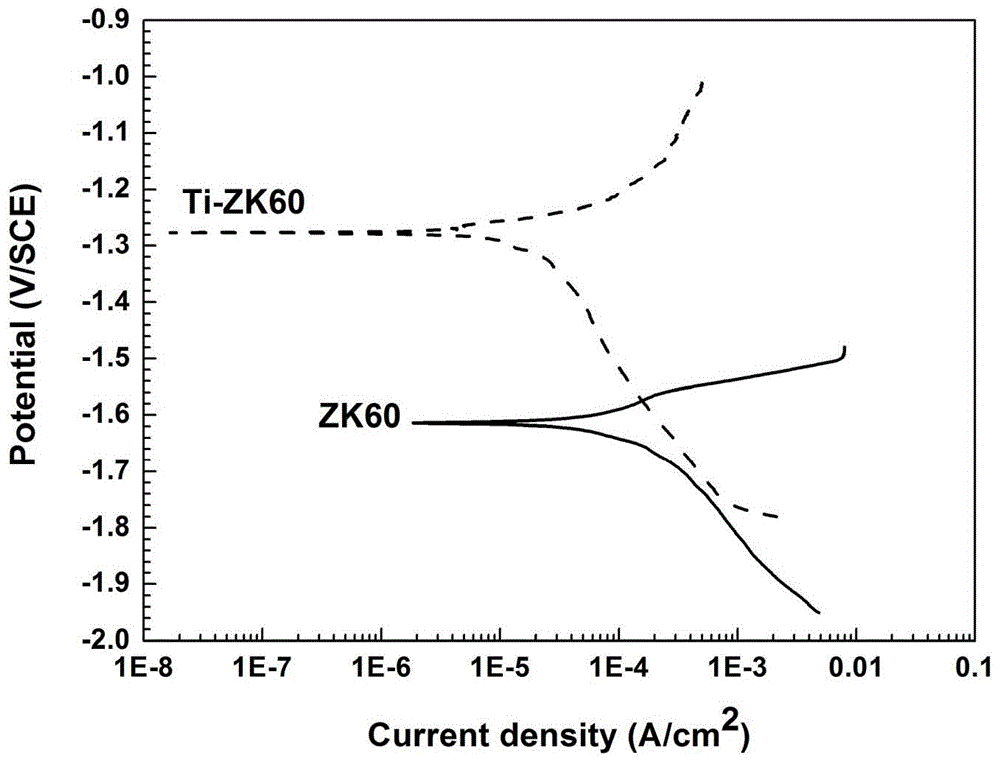
Method for modifying surfaces of biodegradable magnesium and magnesium alloy through titanium ion implantation and deposition
InactiveCN104878362AHigh bonding strengthReduce corrosion rateVacuum evaporation coatingSputtering coatingMixed oxideIon beam
The invention discloses a method for modifying surfaces of biodegradable magnesium and magnesium alloy through titanium ion implantation and deposition, and belongs to the technical field of surface treatment. The method is that mixed oxide transition layers are formed on the magnesium and magnesium alloy surfaces by the ion implantation method, wherein the transition layer is composed of titanium oxide and magnesium oxide and is 3 to 6 microns in thickness; then 2-4 microns titanium films are prepared on the transition layers by the ion beam reinforcing depositing technology. According to the method, the transition layers are prepared to ensure the high combination strength of the titanium depositing film layer and a matrix; the titanium deposition film and the transition layers can improve the corrosion resistance of the magnesium and the magnesium alloy.
Owner:BEIHANG UNIV
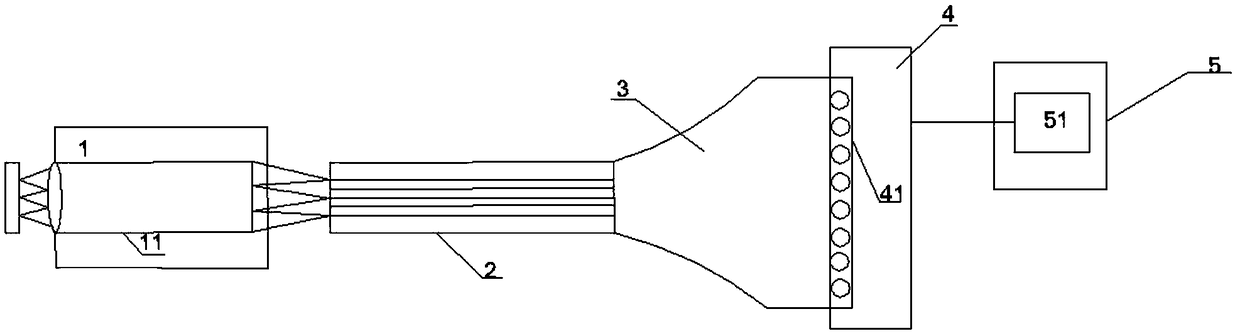
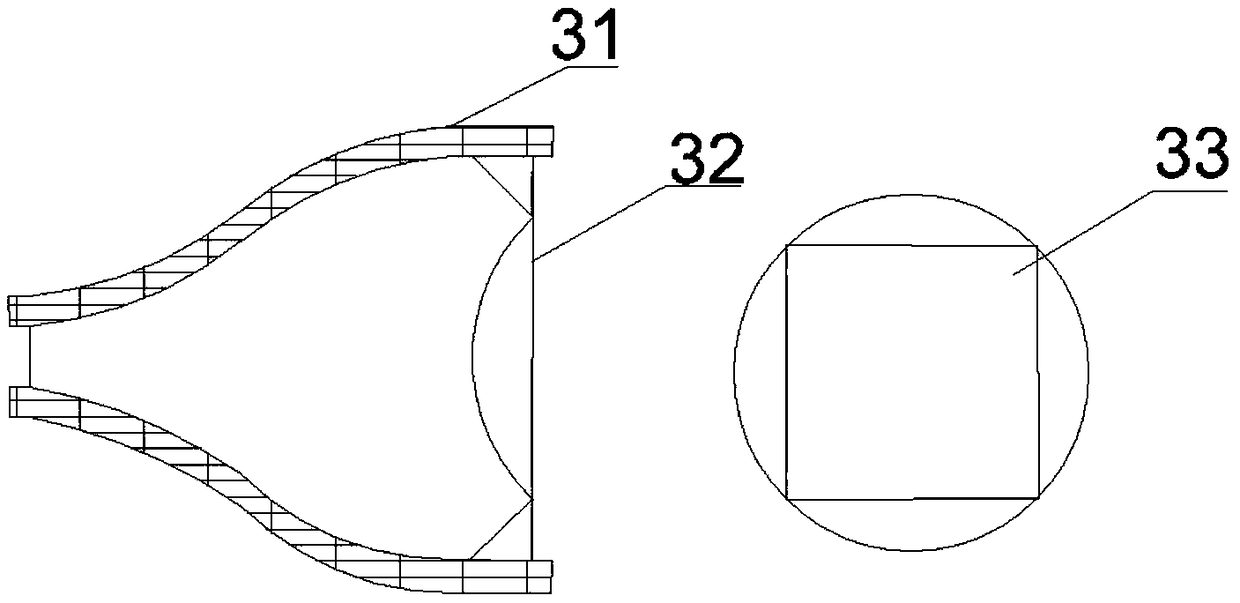
Cherenkov endoscope system based on conical optical fiber connection and medical imaging system
InactiveCN108888230ALight signal loss is smallSimple structureSurgeryEndoscopesMedical imagingEngineering
The invention belongs to the technical field of medical imaging, and discloses a Cherenkov endoscope system based on conical optical fiber connection and a medical imaging system. The Cherenkov endoscope system comprises an endoscope probe, an optical fiber image-transmitting bundle, a connecting device, a detecting device and a computing and imaging device, wherein the connecting device is a conical optical fiber and is used for connecting an endoscope with an imaging camera. The conical optical fiber is used as the connecting device for the endoscope and the imaging camera, and therefore theoptical signal loss of the Cherenkov endoscope system is effectively reduced; the Cherenkov endoscope system is simple in structure and meets clinical application requirements. The collecting rate ofCherenkov optical signals is high; the conical optical fiber is used for replacing an medical endoscope adapter to connect the optical fiber image-transmitting bundle and the imaging camera, a metalprotective bush completely wraps the conical optical fiber, in this way, the optical signal loss caused by the adapter and a coupling part is substantially reduced, the signal collection time for effective imaging of the Cherenkov endoscope is shortened, and clinical conversion of the Cherenkov endoscope is promoted.
Owner:XIDIAN UNIV
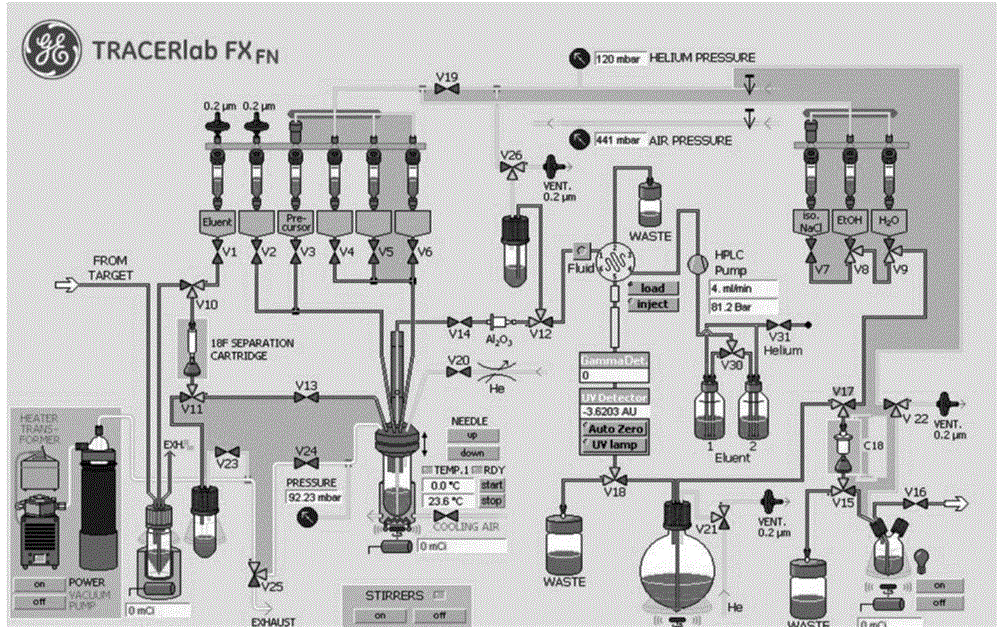
Preparation process of positron imaging agent 18F-5-floro-N-(2-(diethylamino) ethyl) pyridinecarboxamide
InactiveCN104645362AMeet clinical application requirementsSimple preparation processRadioactive preparation carriersEthyl phosphateMedical physics
The invention provides a preparation process of 18F-5-floro-N-(2-(diethylamino) ethyl) pyridinecarboxamide. The preparation process comprises the following steps: S1, capturing 18F-F<->; S2, dehydrating; S3, marking; and S4, purifying. The preparation process is simple, rapid and easy to automatically operate, and a target compound can be stably prepared. The 18F-5-floro-N-(2-(diethylamino) ethyl) pyridinecarboxamide provided by the invention is a positron imaging agent for diagnosing and periodizing malignant melanoma and is high in targeting, specificity and binding force with melanin; the imaging agent is quickly excreted through the kidney, rapid to shoot the tumor part, high in tumor / normal tissue ratio and good in imaging quality; and the imaging agent is high in sensitivity and can be used for detecting micro metastatic lesions. The preparation process of 18F-5-floro-N-(2-(diethylamino) ethyl) pyridinecarboxamide provided by the invention provides a basis for clinically diagnosing and periodizing malignant melanoma.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Amino-methyl dual-functionalized SBA-15 material as well as preparation method and application thereof
InactiveCN103303938AEasy to prepareHigh adsorption capacityCrystalline aluminosilicate zeolitesTetraethyl orthosilicateChemistry
The invention discloses an amino-methyl dual-functionalized SBA-15 material as well as a preparation method and an application thereof. The SBA-15 material has a submicron-grade short mesoporous channel, wherein the mesoporous channel ranges from 0.25 to 0.45 mu m in length; the mesoporous structure is a two-dimensional hexagonal structure; the specific surface area of the mesoporous structure ranges from 420 to 460 m<2> / g; the mesoporous diameter ranges from 5.0 to 5.1nm; the mesoporous volume ranges from 0.5 to 0.7 cm<3> / g; amino and methyl functional groups are uniformly distributed on the surface of the material. The preparation method comprises the following steps: adding surfactants P123 and KCl into a hydrochloric acid solution; then adding tetraethyl orthosilicate and aminopropyl diethoxymethylsilane; stirring for 24 hours at the temperature of 35 to 40 DEG C; standing in a self-pressure kettle; standing and performing a hydro-thermal treatment in a baking oven at the temperature of 90 to 100 DEG C; cooling, filtering, washing and drying; extracting an obtained product by using ethyl alcohol and drying, thereby obtaining the SBA-15 material. The SBA-15 material can be applied to bilirubin adsorption in PBS (Phosphate Buffer Solution) simulation plasma.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI +1
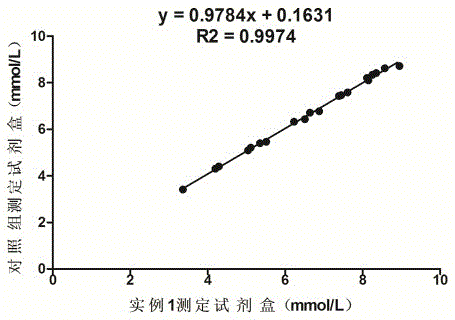
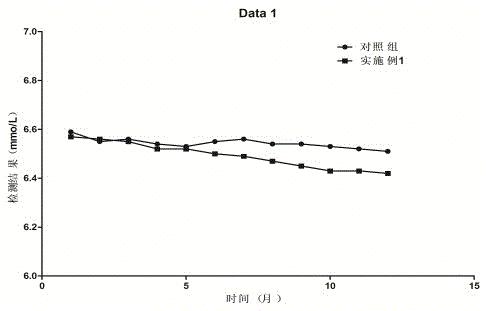
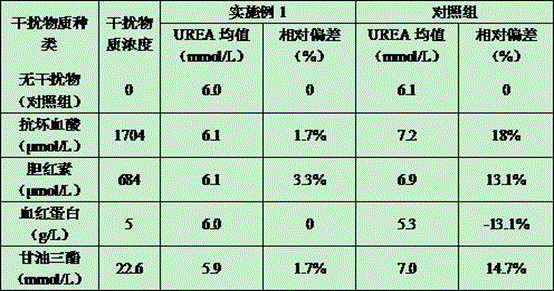
Urea detection reagent with excellent detection line and analysis sensitivity and detection method
The invention relates to the technical field of UREA detection, in particular to a UREA detection reagent. Reagent R1 contains tris buffer, NADPH, and a preservative; reagent R2 contains tris buffer, urease, gluten Acid dehydrogenase, alpha-ketoglutarate, XOD, POD, preservatives. The improved enzymatic urea reagent has superior detection limit and analytical sensitivity, and its own performance is good, which greatly enhances the stability of the reagent and meets the relevant clinical application requirements.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
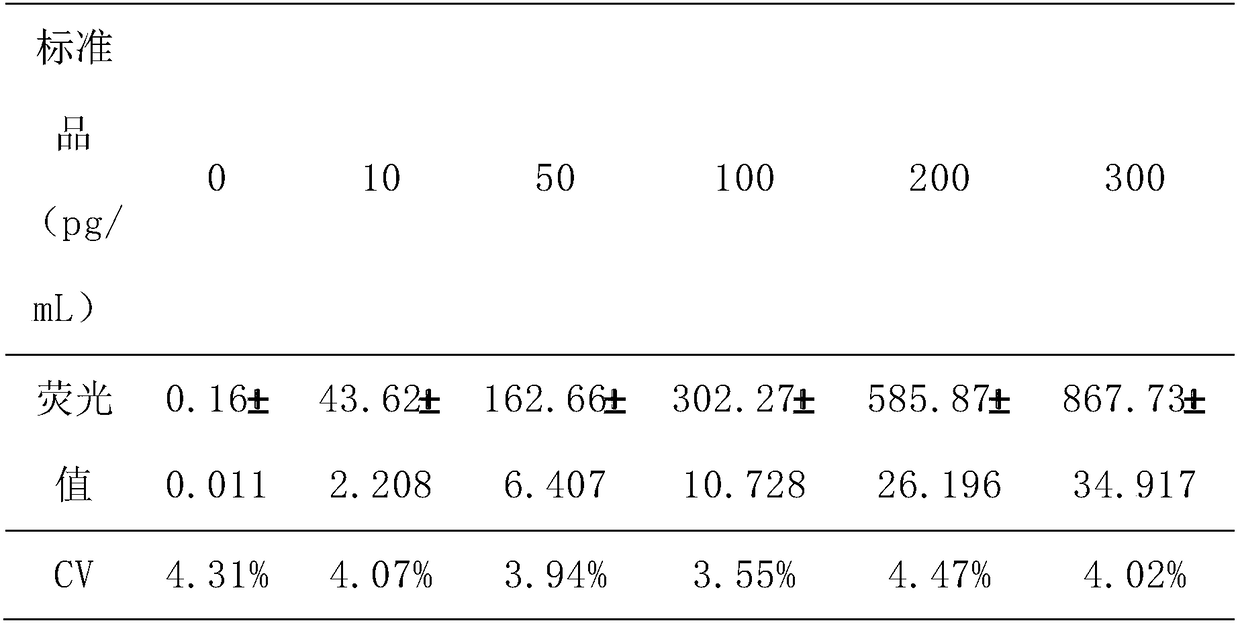
Fluorescent immunochromatographic test paper for quantitative detection of human parathyroid hormone and preparation method thereof
The invention discloses fluorescence immunochromatography test paper for achieving intraoperative judgment of parathyroid tissues through quantitatively detecting a human parathyroid hormone (PTH) and a preparation method of the fluorescence immunochromatography test paper. The test paper capable of detecting the human PTH through a double-antibody sandwich method and a fluorescence immunochromatography; the antibody is prepared by using a specific antigen peptide. The human PTH in a to-be-detected matter can be quickly and accurately detected, so that the fluorescence immunochromatography test paper is simple, convenient and fast to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE +1
Application of lipid compound combination in T cell culture
PendingCN113005080AClear chemical compositionNo Animal Origin IngredientsCulture processBlood/immune system cellsMolecular biologyStearic acid
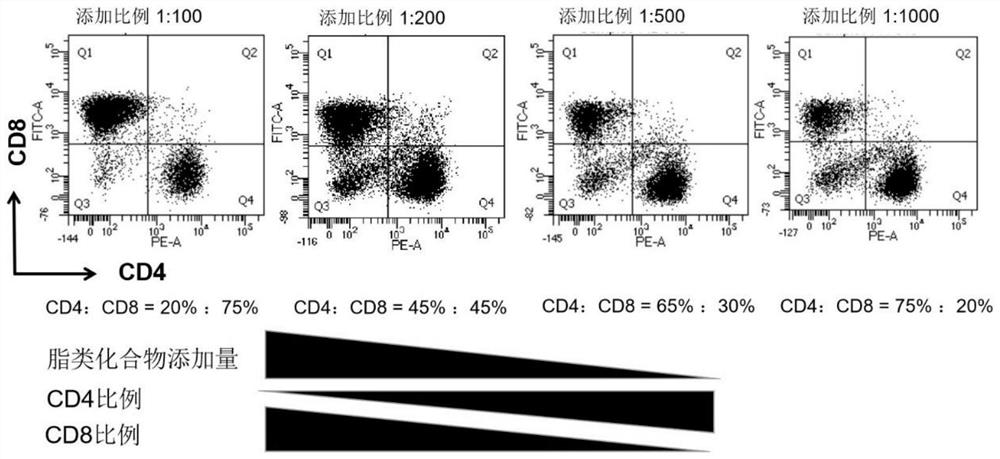
The invention relates to an application of a lipid compound combination in T cell culture. The lipid compound combination comprises any two or more lipid compounds of alpha-tocopheryl acetic acid, myristic acid, arachidonic acid, cholesterol, linoleic acid, oleic acid, palmitic acid, stearic acid, linolenic acid and palmitoleic acid. The lipid compound composition is added into a T cell culture medium according to different proportions, the proportion of CD4 and CD8 subgroups in a T cell population at the amplification end point can be adjusted at will, the requirements of scientific research or clinical application are met, and the lipid compound composition is definite in chemical component, the chemical component are a common nutrient substance in the cell culture process, safe and harmless, and free of human-derived and animal-derived components, and the clinical application requirements are met. The method provided by the invention has very high clinical application value.
Owner:苏州依科赛生物科技股份有限公司
Broadband light-proof infusion device
InactiveCN105944171ADark wavelength range value increasedReduce light transmittanceInfusion devicesNarrow rangeTransmittance
The invention relates to a broadband light-proof infusion device. The broadband light-proof infusion device comprises a puncture outfit, an infusion tube, a dripping bucket and a flow regulator and is characterized in that light-proof layers are arranged on the infusion tube and the inner wall of the dripping bucket; and the light-proof wavelength of the light-proof infusion device ranges from 250 to 550 nm. The broadband light-proof infusion device has following beneficial effects: the range of the light-roof wavelength of the light-proof infusion device can be increased to 250-550nm so that lower light transmittance is obtained; and problems of an existing light-proof infusion device such as light-proof narrow range (290-450nm) and high light transmittance in order to satisfy the higher application demand of the light-proof infusion device.
Owner:四川普瑞斯生物科技有限公司
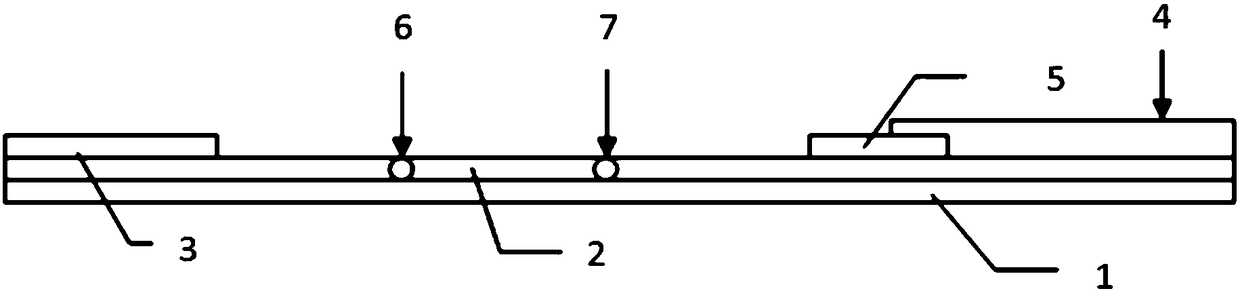
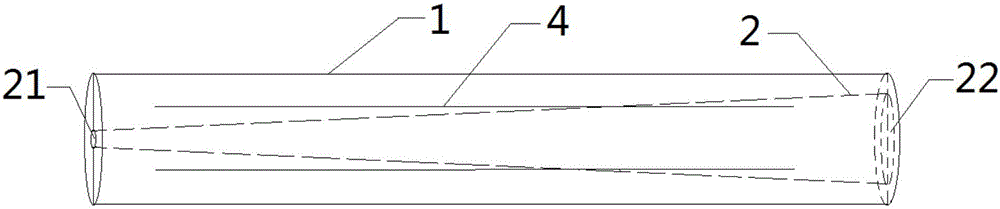
Double-cavity urethra piezometer tube connecting device and connecting method
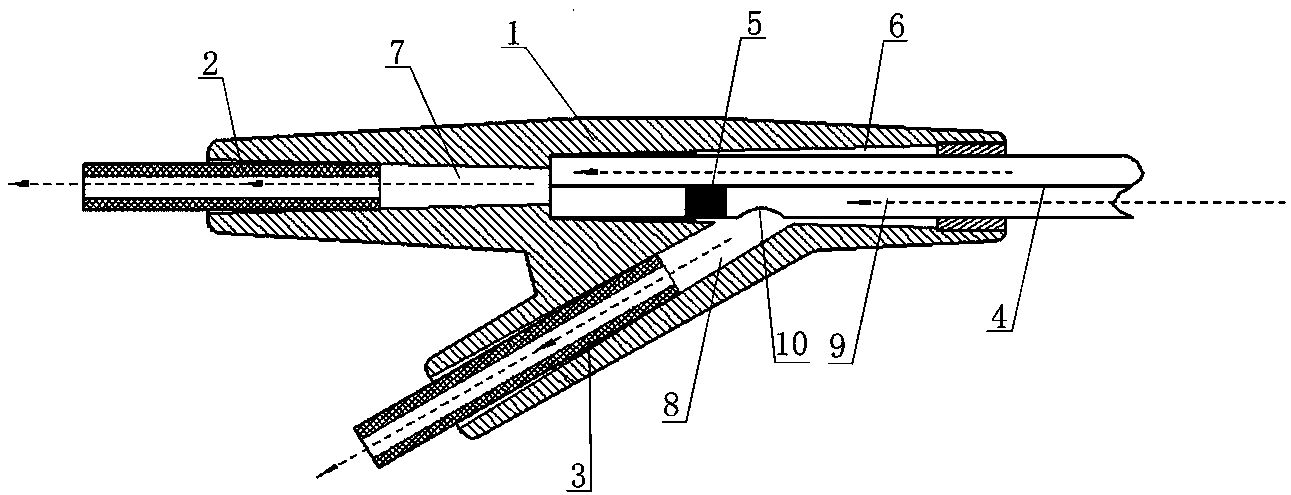
ActiveCN103815894AAvoid the defects of the production methodEasy to manufactureDiagnostic recording/measuringSensorsUrethraEngineering
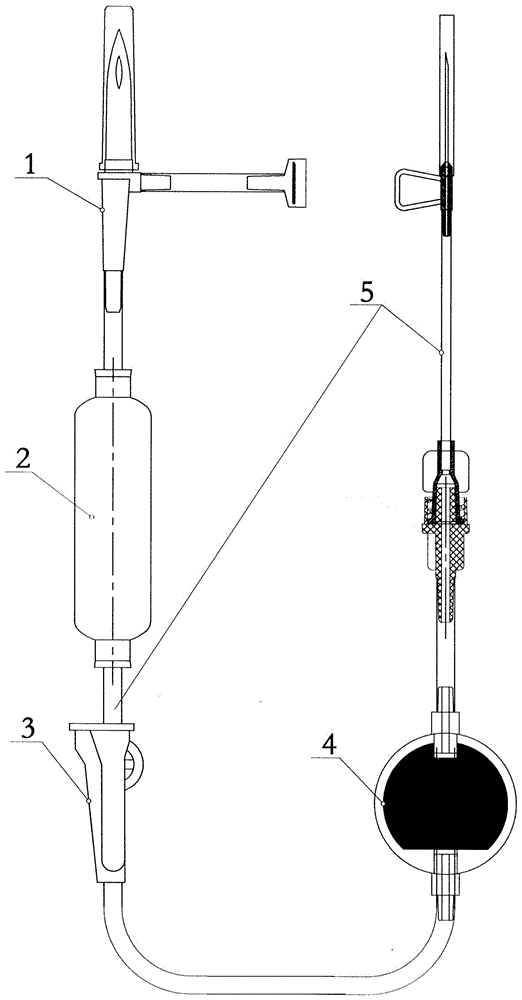
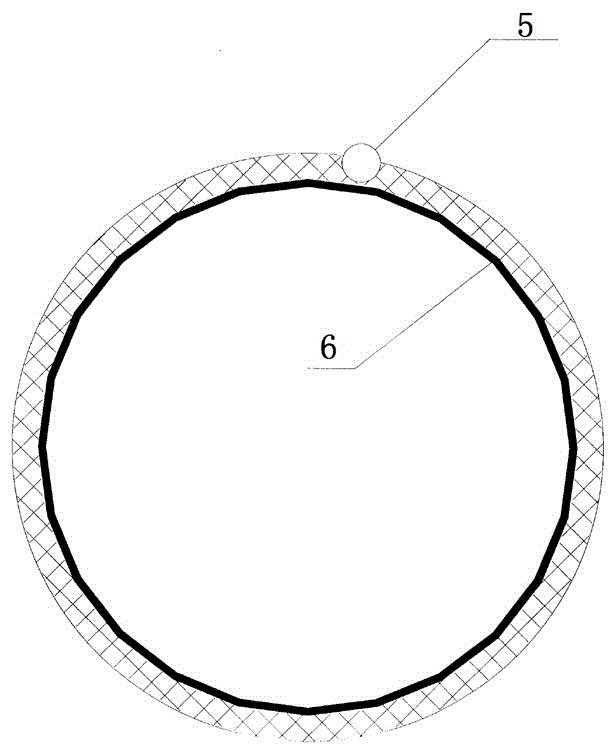
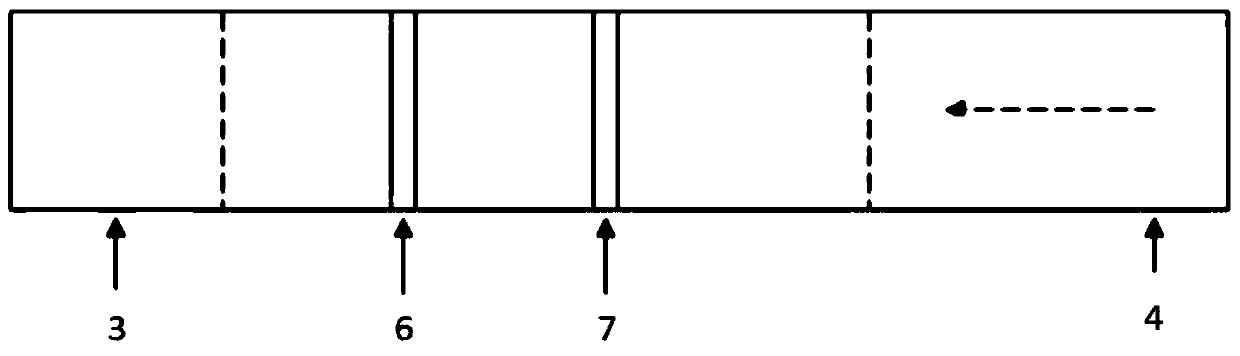
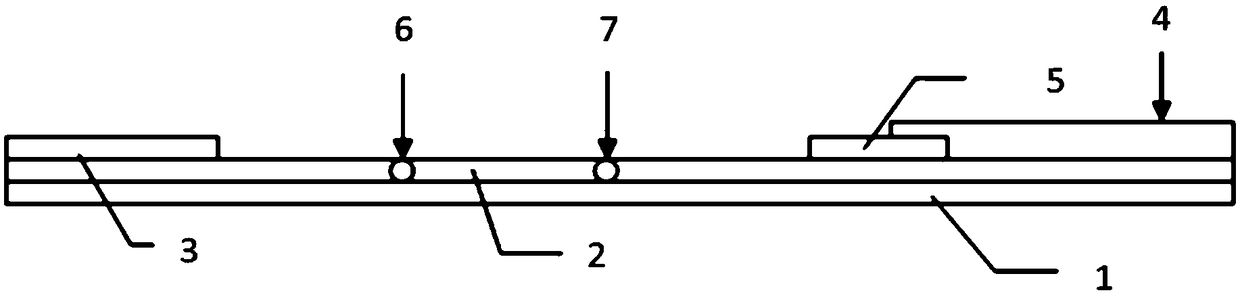
The invention discloses a double-cavity urethra piezometer tube connecting device which comprises a splitter (1), a single-cavity pipe A (2), a single-cavity pipe B (3), a double-cavity pipe (4) and a plug (5). A primary runner (6) and two secondary runners both communicated with the primary runner (6) are arranged inside the splitter (1), the double-cavity pipe (4) is arranged in the primary runner (6), the side wall of a cavity A (9), close to the secondary runner B (8), of two cavities in the double-cavity pipe (4) is provided with a side hole (10) communicated with the secondary runner B (8), and the plug (5) is arranged in the cavity A (9). The invention further discloses a double-cavity urethra piezometer tube connecting method. According to the double-cavity urethra piezometer tube connecting device and method, the technical method that the side hole and the plug are arranged in the double-cavity pipe is adopted, defects existing in existing connecting methods are ingeniously avoided, and the double-cavity urethra piezometer tube connecting device is simple in process, convenient to manufacture, high in finished product yield, and high in production efficiency and practicality; in addition, the double-cavity urethra piezometer tube connecting device is simple in structure, reliable in product performance and capable of fully meeting clinical application requirements.
Owner:成都维信电子科大新技术有限公司
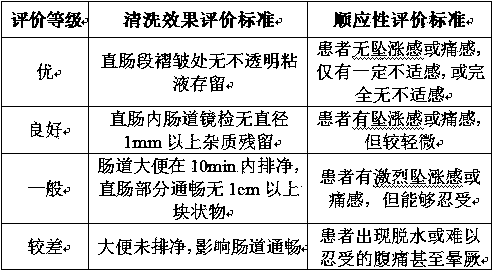
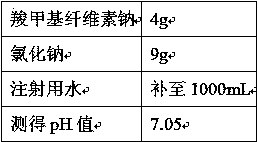
Plant polysaccharide cleaning fluid
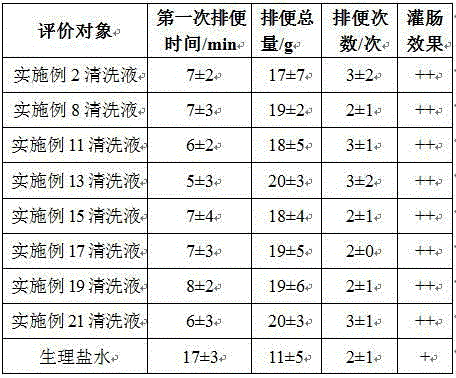
ActiveCN104622889AGood for bowel cleansingImprove use adaptabilityOrganic active ingredientsDigestive systemBiotechnologyCellulose
The invention relates to a plant polysaccharide cleaning fluid, which is used for intestinal lavage, and takes sodium carboxymethylcellulose as a main ingredient. The plant polysaccharide cleaning fluid comprises the following components in percent by mass: 0.1%-2% of sodium carboxymethylcellulose, 0.7%-1.2% of sodium chloride, 0.01%-0.1% of pH value buffer substances, 0.1%-5% of selectable magnesium chloride, 1%-10% of selectable mannitol, 0.01%-0.1% of selectable phenolphthalein and 0.1%-0.4% of selectable carboxymethyl chitosan, and the balance of water for injection, wherein the pH value of the polysaccharide cleaning fluid ranges from 6.5-7.8. The polysaccharide cleaning fluid is good in cleaning effect, free from adverse effect, and the cleaning fluid is stable and difficult to degrade.
Owner:河北柯瑞生物医药有限公司
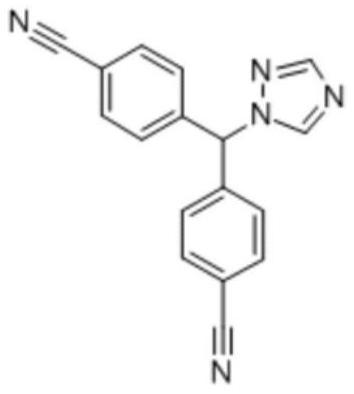
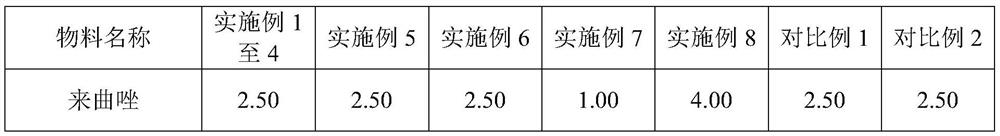
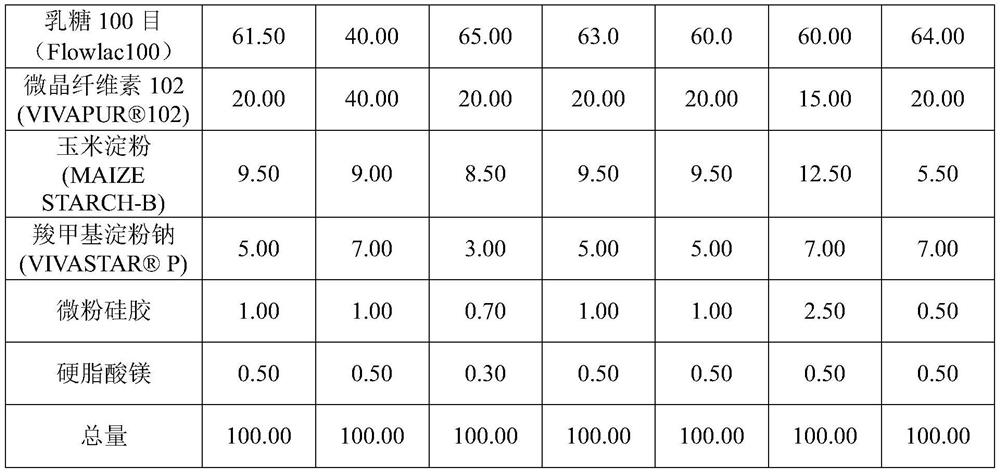
Letrozole tablet and preparation method thereof
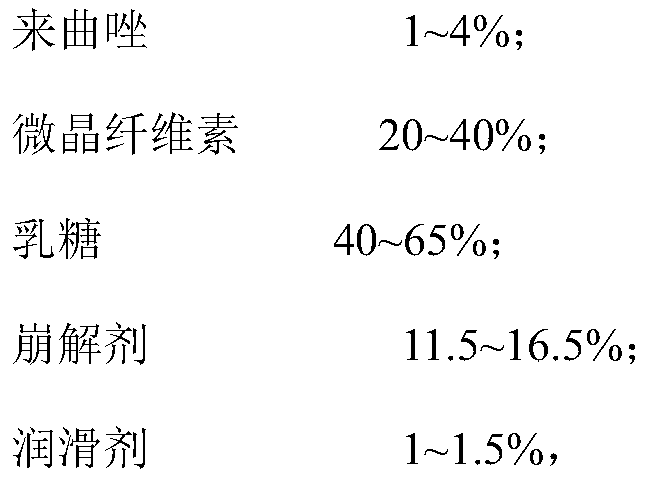
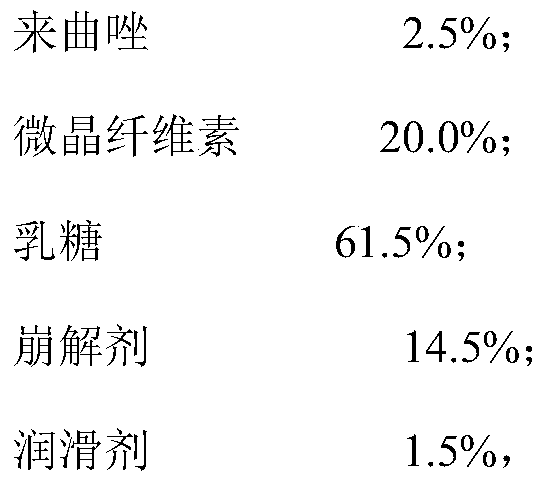
ActiveCN111012752AGuaranteed mix uniformityControl disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseLactose
The invention provides a letrozole tablet and a preparation method thereof. The letrozole tablet comprises a tablet core comprising the following components, by weight: 1-4% of letrozole, 20 to 40% ofmicrocrystalline cellulose, 40 to 65% of lactose, 11.5%-16.5% of a disintegrating agent, and 1 to 1.5% of a lubricating agent. On the basis of the total weight of the tablet core, the lubricating agent comprises 0.3%-0.5% of magnesium stearate and 0.7%-1.0% of superfine silica powder; and the disintegrating agent comprises 3%-7% of sodium carboxymethyl starch and 8.5%-9.5% of corn starch. After controlling of the use amounts of the corn starch and the sodium carboxymethyl starch, on the one hand, the disintegration time of the letrozole tablet is regulated and controlled;and on the other hand, the mixing uniformity of the superfine silica powder in the mixing and tabletting processes is ensured and the demixing phenomenon is avoided. The dosage ratio of the superfine silica powder, the lactose and the corn starch is strictly controlled, so that the mixing uniformity of the materials and the dissolution rate of the letrozole are guaranteed. The clinical application requirements are met.
Owner:瀚晖制药有限公司
Memory alloy tube for manufacturing expandable vertebral stent
InactiveCN106137366AMeet clinical application requirementsSolving Design Problems for Mechanical RequirementsInternal osteosythesisDilatorsAlloyVertebral bone
The invention discloses a memory alloy tube for making an expandable vertebral body stent, which is integrally made of nickel-titanium temperature memory alloy, and is characterized in that the memory alloy tube includes a cylindrical outer wall tube, and The inner lumen inside the tube; the outlets at both ends of the inner lumen are respectively located on the circular end faces of the first end and the tail end of the cylindrical outer tube wall; the inner lumen can be cut, molded, 3D printed or polished craft made. The advantage of the present invention is that the change of the internal structure of the bracket, the manufacture of brackets with different structural strengths and vertebral body structures, the rules of vertebral body fractures, and the connection between tail end material filling and injection of filling materials are solved at one time, and according to clinical conditions of patients of different ages The bone regeneration ability and mechanical strength of the vertebral body can be adjusted to obtain stents with different wall thicknesses.
Owner:刘小勇
A kind of letrozole tablet and preparation method thereof
ActiveCN111012752BGuaranteed mix uniformityControl disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseLactose
Owner:瀚晖制药有限公司
Pectin gynecological antibacterial gel and preparation method thereof
InactiveCN106511374ALow cytotoxicityImprove securityInorganic boron active ingredientsHydroxy compound active ingredientsSide effectPatient compliance
The invention discloses a pectin gynecological antibacterial gel and a preparation method thereof. The gel comprises the components in percentages by weight: 2-20% of pectin, 1-5% of a water soluble chitin derivative, 0.1-1% of alginate oligosaccharide, 0.01-0.5% of kathon, 0.1-3% of borneol, 0.1-2% of borax, 0.25-2% of carbomer and the balance of pure water. The gel disclosed by the invention overcomes the defects in the prior art, and is good in effect of treating gynecological diseases, convenient to use, good in patient compliance, simple in preparation process and free of side effects.
Owner:ANHUI YUNING PECTIN CO LTD
Intracranial Pressure Monitor Based on Ultrasonic Acoustoelastic Effect
InactiveCN103190930BEnables non-invasive monitoringAccurate measurement dataUltrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsAcoustoelastic effectIntracranial pressure monitoring
Owner:CHONGQING UNIV
Colchicine compound, preparation method, preparation, applications and pharmaceutical composition thereof
InactiveCN110790675AImprove solubilityFast dissolutionOrganic active ingredientsAntipyreticGout arthritisChemical compound
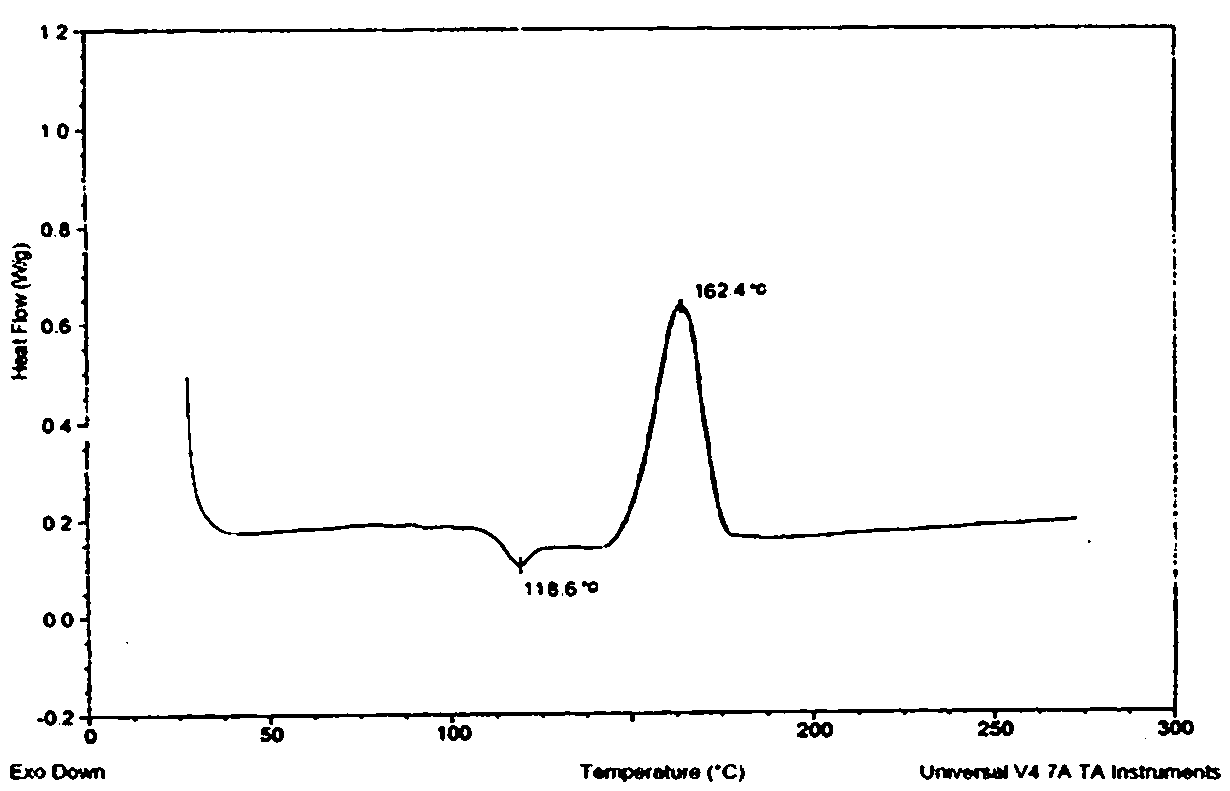
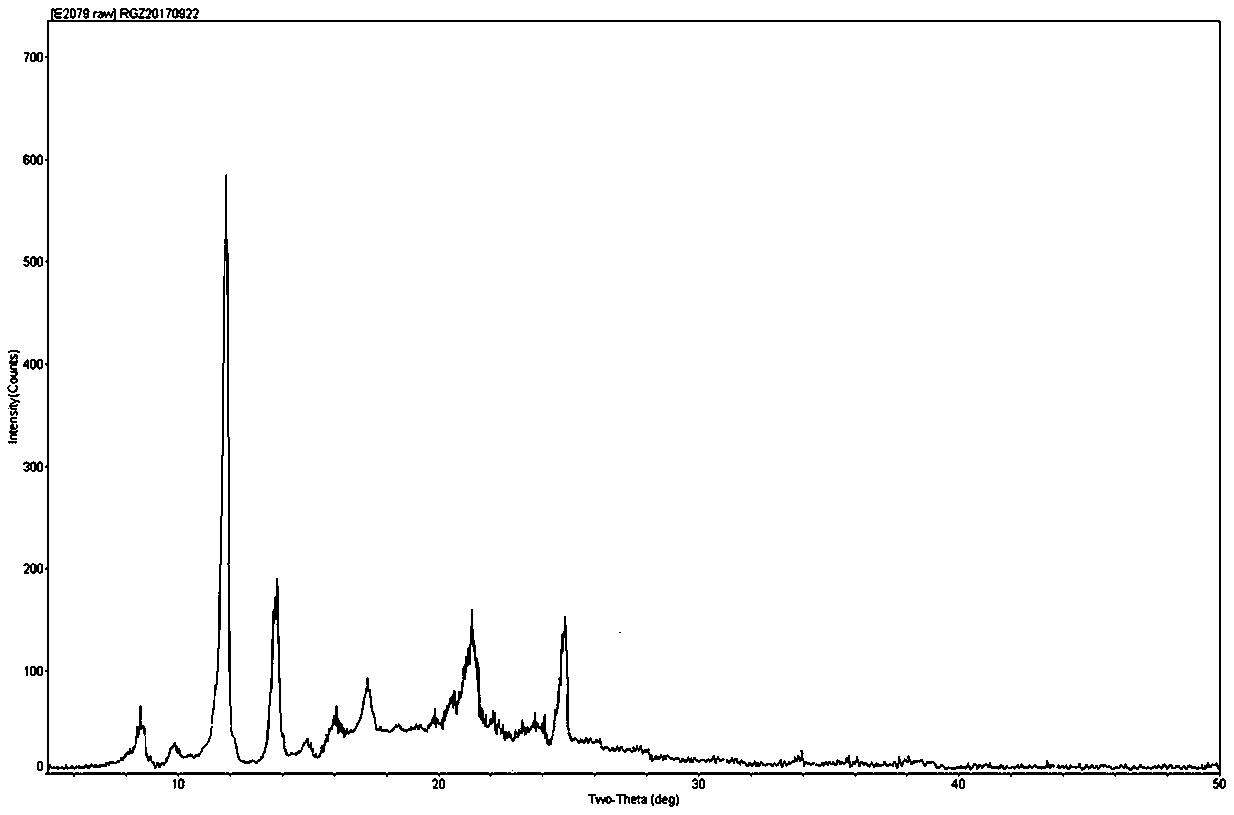
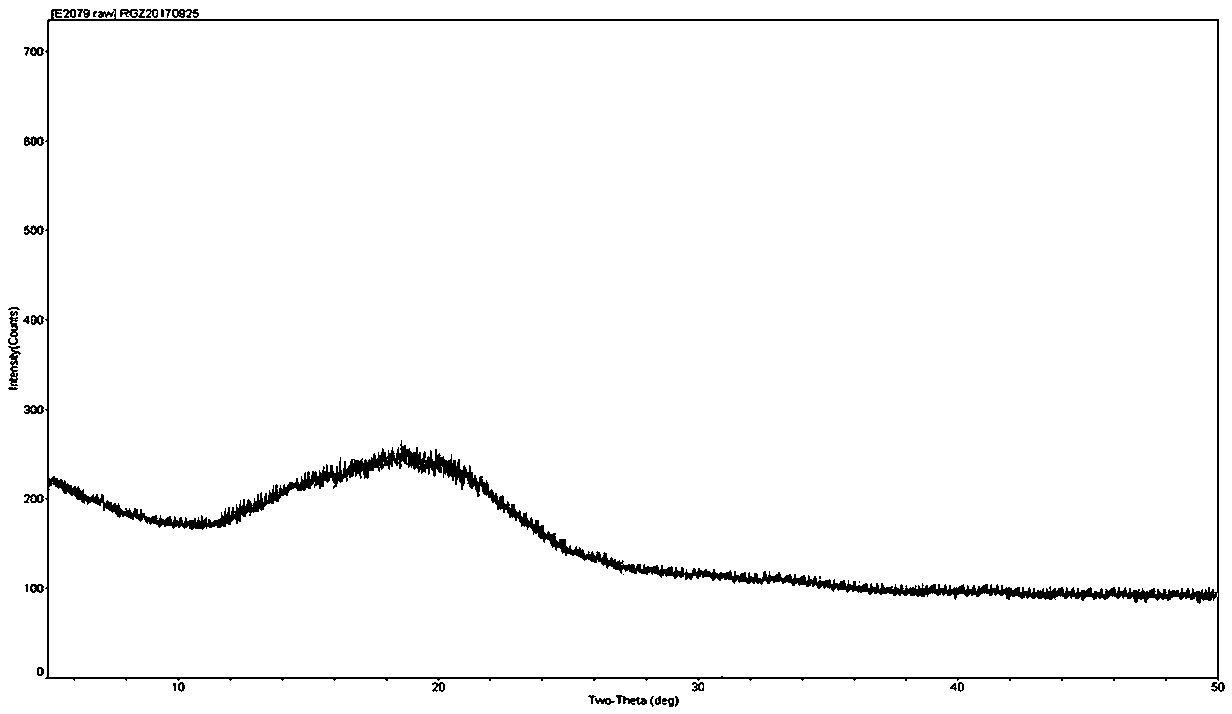
The invention relates to a colchicine compound, a preparation method, a preparation, applications and a pharmaceutical composition thereof, wherein the colchicine compound is amorphous and has a structure represented by a formula I, the X-ray powder diffraction pattern is represented by figure 2, the differential scanning calorimetry analysis curve is represented by figure 3, and the colchicine compound has an exothermic peak at a temperature of about 118.6 DEG C and an has an endothermic peak at a temperature of about 162.4 DEG C. According to the invention, the colchicine has good treatmenteffects on gout, arthritis, tumors, liver fibrosis and pulmonary fibrosis; compared with the products and the technologies in the prior art, the colchicine disclosed by the invention is amorphous, hasadvantages of goof heat resistance, good moisture resistance, stable properties and high bioavailability, and can well meet the requirements of clinical medication; and the preparation method provided by the invention is simple in process, mild in condition, convenient to operate, stable and controllable in quality and suitable for industrial large-scale production.
Owner:KPC PHARM INC
Method for rapidly identifying thyroid papillary carcinoma lymphonodi cervicales metastasis in operation
The invention discloses fluorescence immunochromatographic test paper for rapidly detecting human thyroglobulin (Tg) so as to rapidly identify thyroid papillary carcinoma lymphonodi cervicales metastasis. According to the test paper, a double-antibody sandwich method and a fluorescence immunochromatography technology are adopted to detect human Tg; and antibodies are prepared through specific antigen epitope peptides. With the method adopted, human Tg in an object to be detected can be rapidly and accurately detected. The test paper has the advantages of simple, convenient and rapid operation, wide detection range, high specificity and high sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
A kind of orthopedic adhesive and preparation method thereof
ActiveCN104399114BImprove performanceMeet clinical application requirementsSurgical adhesivesPhosphateAdhesive
The invention provides an adhesive for the department of orthopaedics and a preparation method of the adhesive. The adhesive comprises the following components: silicate, phosphate, sodium alginate, magnesium oxide, calcium carbonate, kaolin, propylene glycol, glycerin, a retarder and deionized water. The preparation method comprises the following steps: weighing all the raw materials in parts by weight; performing ball-milling on silicate, phosphate, magnesium oxide, calcium carbonate and kaolin into powders, and uniformly mixing the five powders to obtain a mixture; sequentially adding sodium alginate, propylene glycol, glycerin and the retarder in the deionized water, uniformly stirring to obtain a settled solution, slowly adding the mixture in the settled solution under the condition of continuously stirring, and performing ultrasound at the room temperature to obtain the adhesive. The adhesive for the department of orthopaedics, prepared by the preparation method, is high in comprehensive performance, finally sets for about 60 minutes, has the compressive strength of 54.6-58.3 MPa, can meet the clinical application requirement, and is suitable for the treatment of osteoporosis and the fracture fixation and treatment in the department of orthopaedics and the spine surgery.
Owner:BEIJING ALLGENS MEDICAL SCI & TECH
Pancreatic color doppler transforming agent and preparation method thereof
InactiveCN109620975AReduce misdiagnosisShort detection timeEchographic/ultrasound-imaging preparationsSolution deliveryTapioca starchPancreatic structure
The invention discloses a pancreatic color doppler transforming agent and a preparation method thereof. The pancreatic color doppler transforming agent consists of the following raw materials, by weight, 10%-17% of wheat flour, 28%-32% of rice, 2%-6% of wild rice shoot, 2%-6% of citrus peel, 2%-7% of tapioca, 17%-25% of soybean, 5%-12% of Chinese yam, 5%-12% of mung bean and 1%-5% of stevioside, and the total content of the above raw materials is 100%. The pancreatic color doppler transforming agent can well stay in the stomach to be matched with the color doppler for ultrasonic penetration, so that an imaging interface similar to the liver tissues can be formed after the stomach and the duodenum, pancreatic organs can be observed clearly to reduce misdiagnosis caused by unclear imaging ofthe pancreas due to the interference of the stomach and the duodenum, and the agent has the advantages of short detection time, high imaging efficiency and stable effect.
Owner:贾皓然
A rapid method for identifying human parathyroid glands
The invention discloses a method for rapidly identifying human parathyroid glands. The method is realized by using parathyroid hormone (PTH) fluorescence immunochromatography test paper to determine the content of PTH in liquid to be detected; the test paper is used for detecting human PTH by using a double-antibody sandwich method and a fluorescence immunochromatography; the antibodies are prepared by using specific antigenic epitope peptide. The method can be used for accurately detecting the human PTH in a matter to be detected, and is simple and convenient to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:无锡市江原实业技贸有限公司
High-strength calcium phosphate composite nano material bone cement and preparation method thereof
The invention discloses a high-strength calcium phosphate composite nano material bone cement which is composed of a solid-phase powder and a liquid phase, wherein the solid-phase powder is composed of the following raw materials in parts by weight: 5-20 parts of tricalcium phosphate, 30-70 parts of hydroxyapatite and 1-10 parts of gamma-aluminum oxide nanotube; the liquid phase is a water solution containing 1-10 wt% of citric acid, 0-6 wt% of chitosan and 5-15 wt% of glucose; and before use, the solid-phase powder and liquid phase are blended according to the liquid phase:solid phase ratio of 1ml:(0.3-0.8)g. The invention also discloses a preparation method of the bone cement. The bone cement material has the advantages of favorable comprehensive properties and high compression strength, and is convenient to operate; the maximum compression strength can reach 120 MPa, which is much higher than that of the existing calcium-phosphate-base bone cement; and the bone cement material with favorable injectability can satisfy the clinical application requirements, and is suitable for restoring hard tissue bone defects, treating osteoporosis, and fixing and treating fractures.
Owner:连云港格兰特化工有限公司
Novel plant polysaccharide cleaning fluid
ActiveCN104606222AGood for bowel cleansingImprove use adaptabilityOrganic active ingredientsDigestive systemBiotechnologyMicrobiology
The invention relates to a novel plant polysaccharide cleaning fluid which is used for cleaning intestinal tracts and uses oxycellulose as a main ingredient. The novel plant polysaccharide cleaning fluid comprises the following components in percentage by weight: 0.1-2% of oxycellulose, 0.8-1.0% of sodium chloride, 0.01-0.1% of pH value buffering substances, optional components (0.1-5% of magnesium chloride, 1-10% of mannitol, 0.01-0.1% of phenolphthalein and 0.1-0.4% of carboxymethyl chitosan), and the balance of water for injection. The pH value range of the novel plant polysaccharide cleaning fluid is 6.5-7.8. The novel plant polysaccharide cleaning fluid provided by the invention has a good cleaning effect and zero bad influences.
Owner:河北柯瑞生物医药有限公司
A kind of plant polysaccharide cleaning liquid
ActiveCN104622889BGood for bowel cleansingImprove use adaptabilityOrganic active ingredientsDigestive systemMANNITOL/SORBITOLPhenolphthalein
Owner:河北柯瑞生物医药有限公司
Preparation method and application of biomedical degradable zinc alloy capillary tube
ActiveCN107838222BHigh dimensional accuracyImprove the finishSurgeryCatheterCapillary TubingIntravascular stent
The invention discloses a preparation method and application of a biomedical degradable zinc-alloy capillary tube. The preparation method comprises the following steps: subjecting cast zinc alloy to heat treatment and machining so as to form a cylinder and carrying out hot extrusion on the cylinder to obtain an extruded bar; processing the extruded bar to form an extruded tube blank and carrying out hot extrusion on the extruded tube blank to obtain an intermediate tube with an external diameter Phi of 3 to 8 mm; carrying out a plurality of passes of room-temperature rolling until the capillary tube blank has an external diameter Phi of 2 to 3 mm and wall thickness of 0.10 to 0.20 mm; and carrying out room-temperature drawing to eventually obtain a capillary tube with a diameter Phi of 1 to 3 mm and carrying out annealing treatment so as to obtain the capillary tube for cutting stents and with yield strength Rp0.2 of 250 to 450 MPa, tensile strength of 280 to 460 Pa and elongation percentage of 11 to 72%. The biomedical degradable zinc-alloy capillary tube prepared in the invention has appropriate strength and excellent elongation percentage and is applicable to preparation of intravascular stents, nerve conduits and other medical implant products.
Owner:SHANGHAI JIAOTONG UNIV
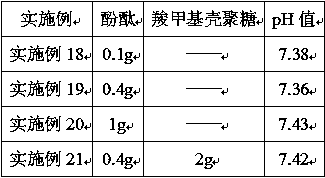
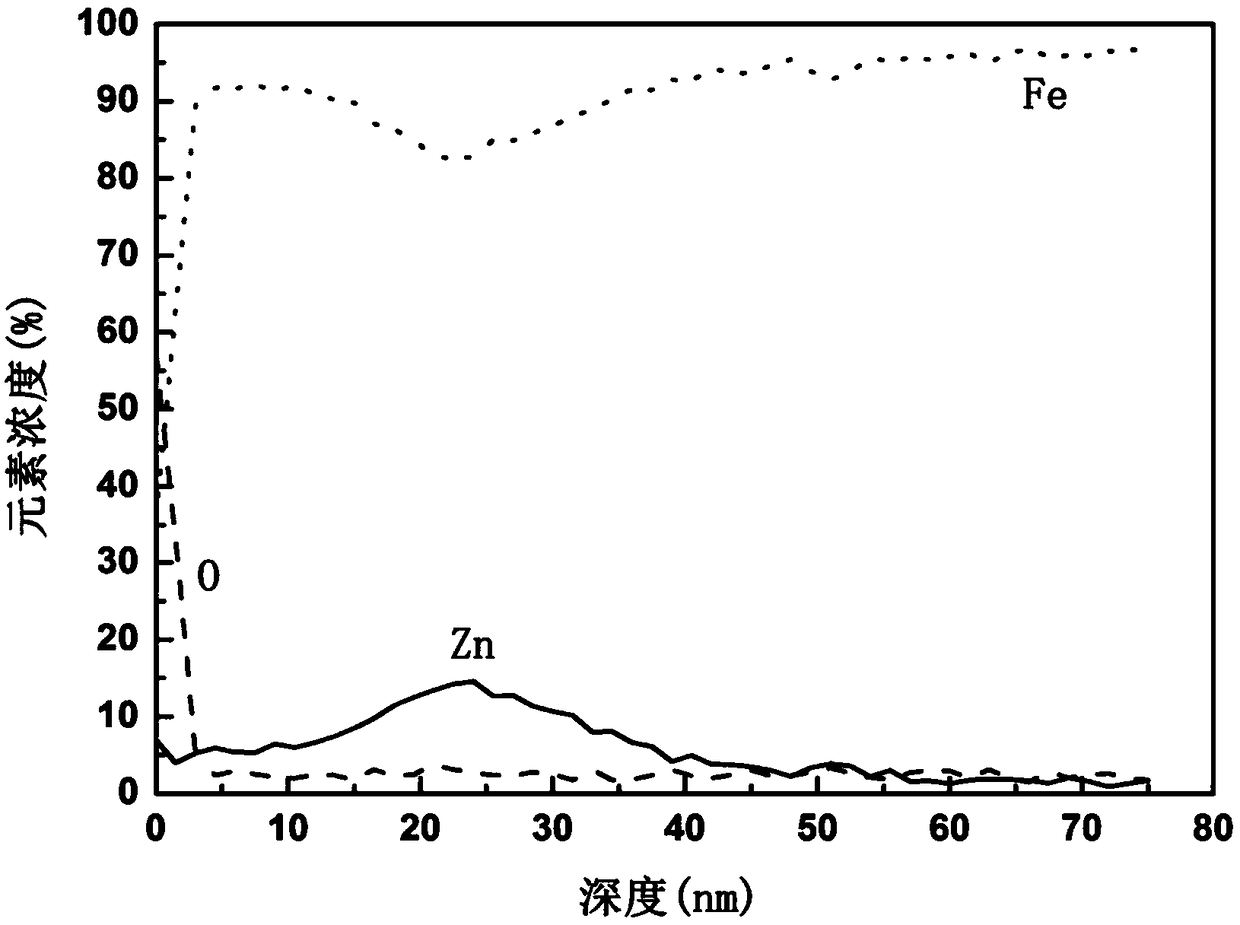
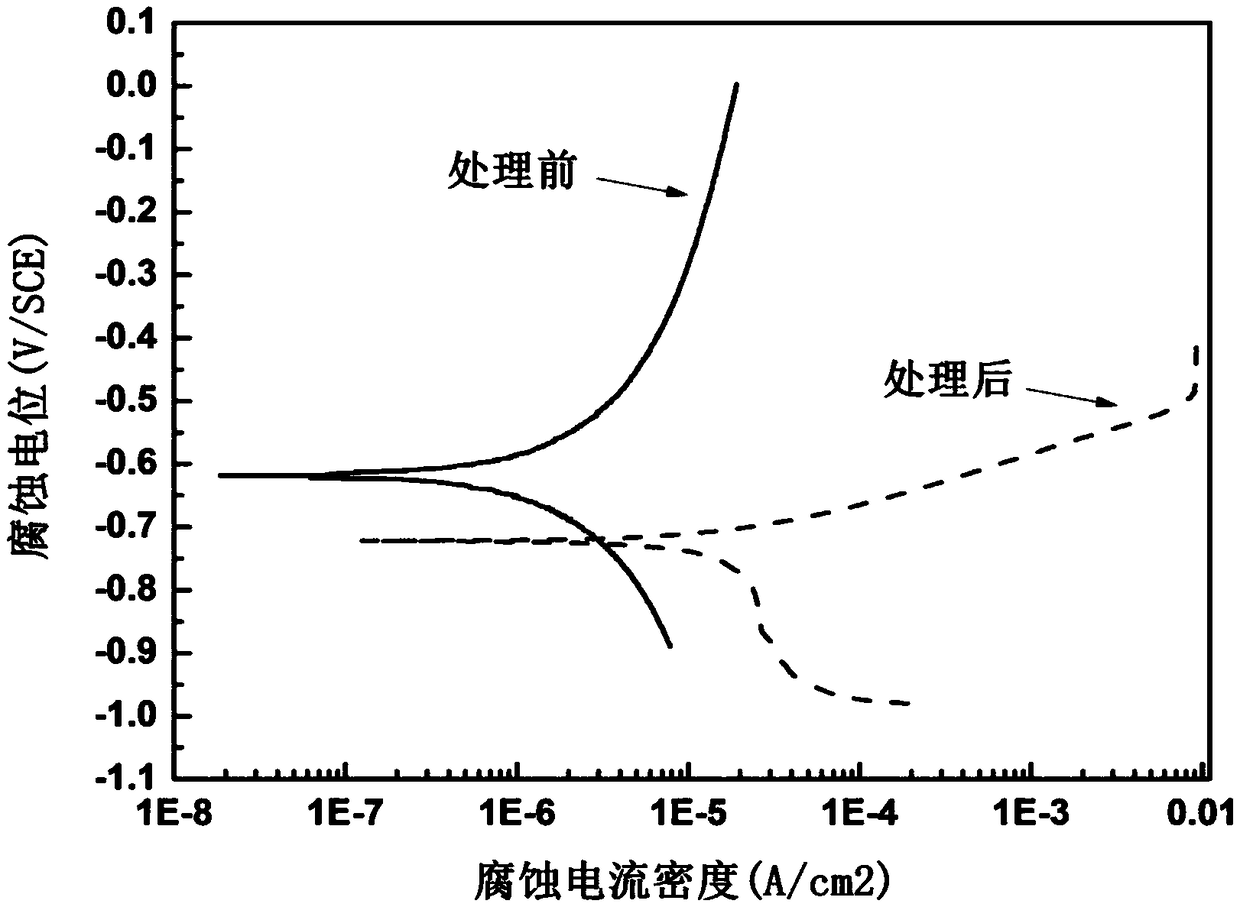
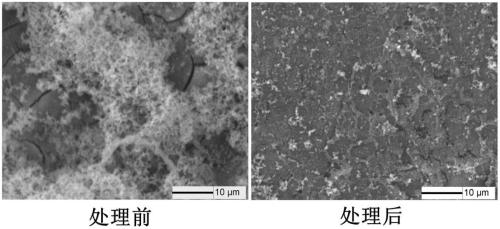
A method for surface modification of biodegradable iron and iron alloys by zinc ion implantation
ActiveCN105839067BHigh bonding strengthReduce corrosion potentialSurgeryVacuum evaporation coatingZinc ionBiocompatibility
The invention discloses a method for surface modification of biodegradable iron and iron alloy by zinc ion implantation, which belongs to the technical field of surface treatment. In the present invention, a modified layer doped with zinc is formed on the surface of iron and iron alloy by zinc ion implantation. In the surface modified layer, a mixture phase of iron-zinc second phase, iron oxide and zinc oxide is formed, with a thickness of 50 ~100nm, the implanted element zinc shows a Gaussian distribution. The zinc surface modification layer prepared by the invention has good bonding strength with the substrate, improves the corrosion rate of iron and iron alloy, and has good biocompatibility at the same time.
Owner:BEIHANG UNIV
A stabilizer for clinical-grade lentivirus and its application method
ActiveCN107604006BGuaranteed stabilityEnsuring the ability to infect cellsFermentationGenetic engineeringSucroseLentivirus
The invention relates to the field of biochemical medicines, in particular to a stabilizer for clinical lentivirus and a use method of the stabilizer. The stabilizer for clinical lentivirus comprises10-20 g / ml of sucrose, 40-100 mg / L of tocopherol, 10-20g / ml of human albumin, and the balance of 0.01 mol / L of a PBS buffer with the pH value being 7.4, and is obtained by filtering with a 0.22-micronfilter. The use method of the stabilizer for clinical lentivirus comprises the following steps: gently and uniformly mixing a clinical lentivirus suspension with the stabilizer for clinical lentivirus at a volume ratio of 9:1; sub-packaging; and storing the sub-packaged clinical lentivirus suspension in an environment at -80 DEG C. After verification, the clinical lentivirus using the stabilizerprovided by the invention has no drop in titer after being stored at -80 DEG C for 6 months, has no significant drop in titer after one freeze-thaw cycle, and has a titer loss of only 10% after threefreeze-thaw cycles, thereby satisfying storage requirements in production, transportation and use processes of lentivirus. The stabilizer provided by the invention has simple components and few use steps, does not need to use additional instrument and equipment, is easy to control quality and meets requirements of clinical application.
Owner:上海埃秀马生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com