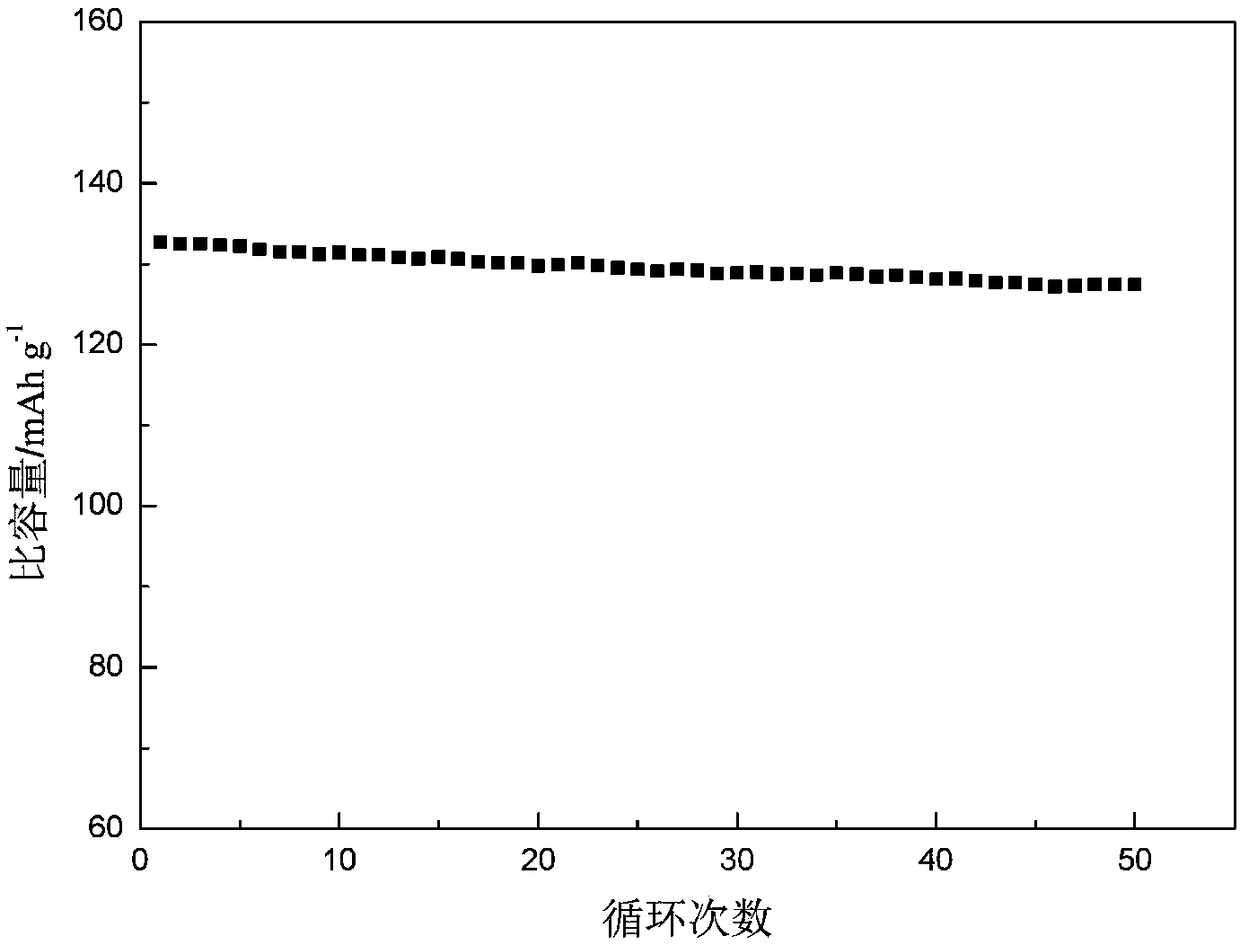
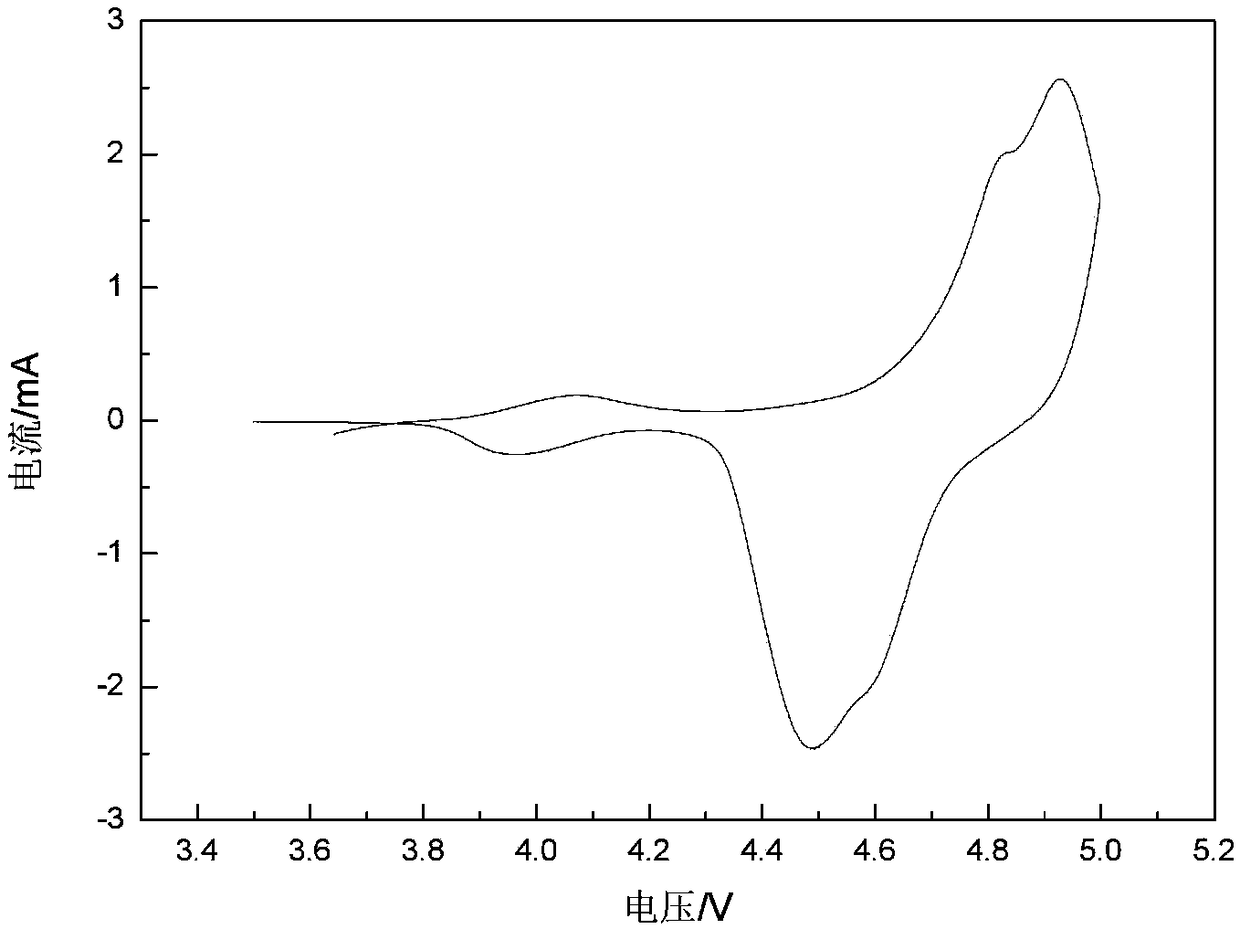
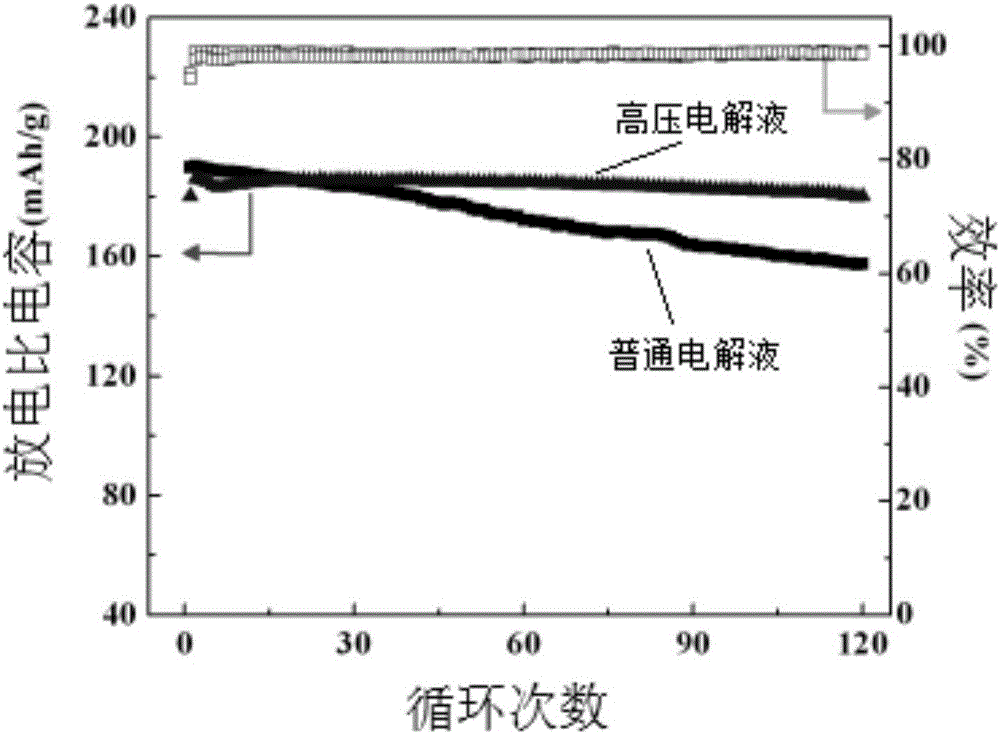
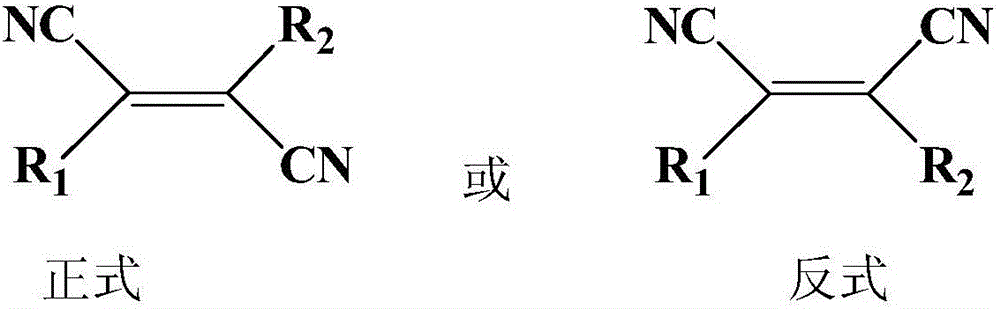
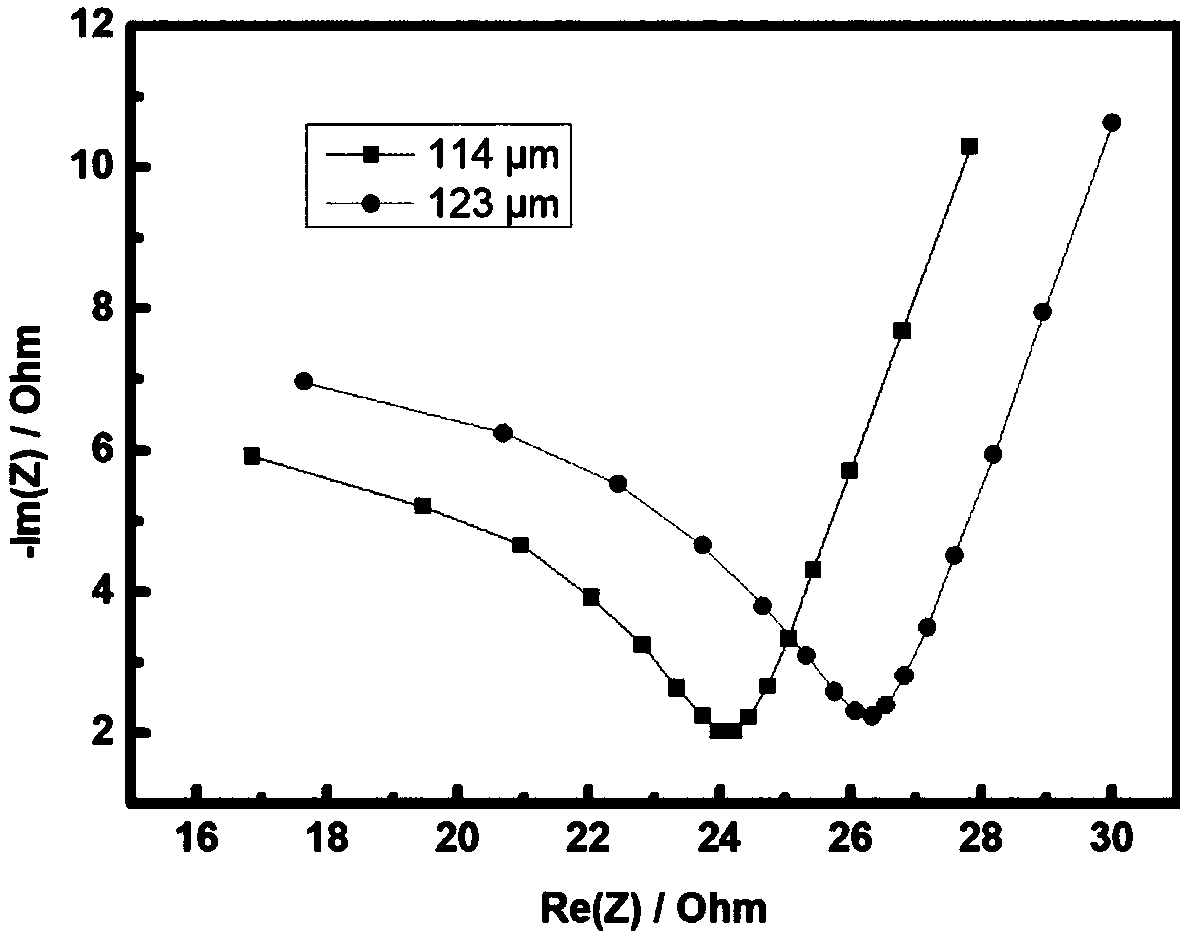
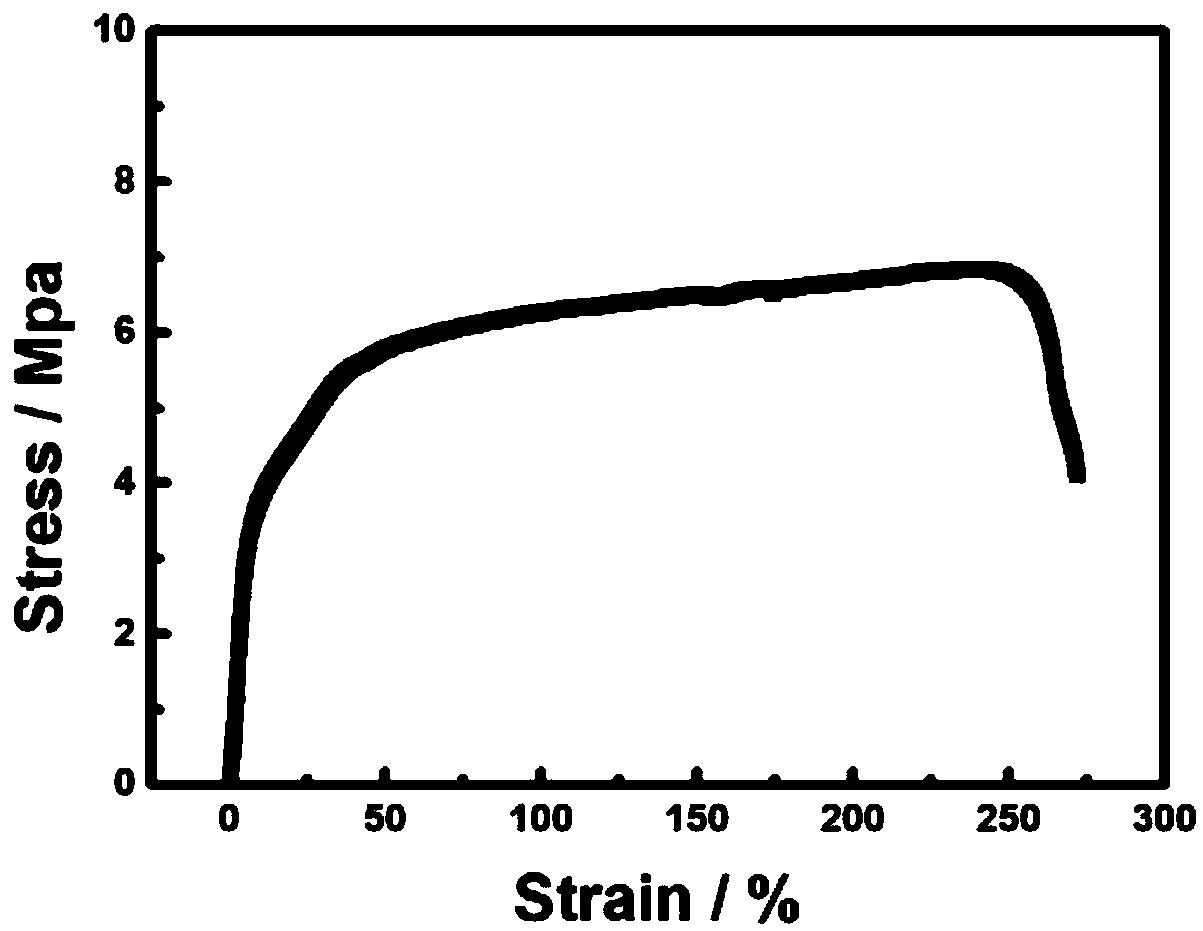
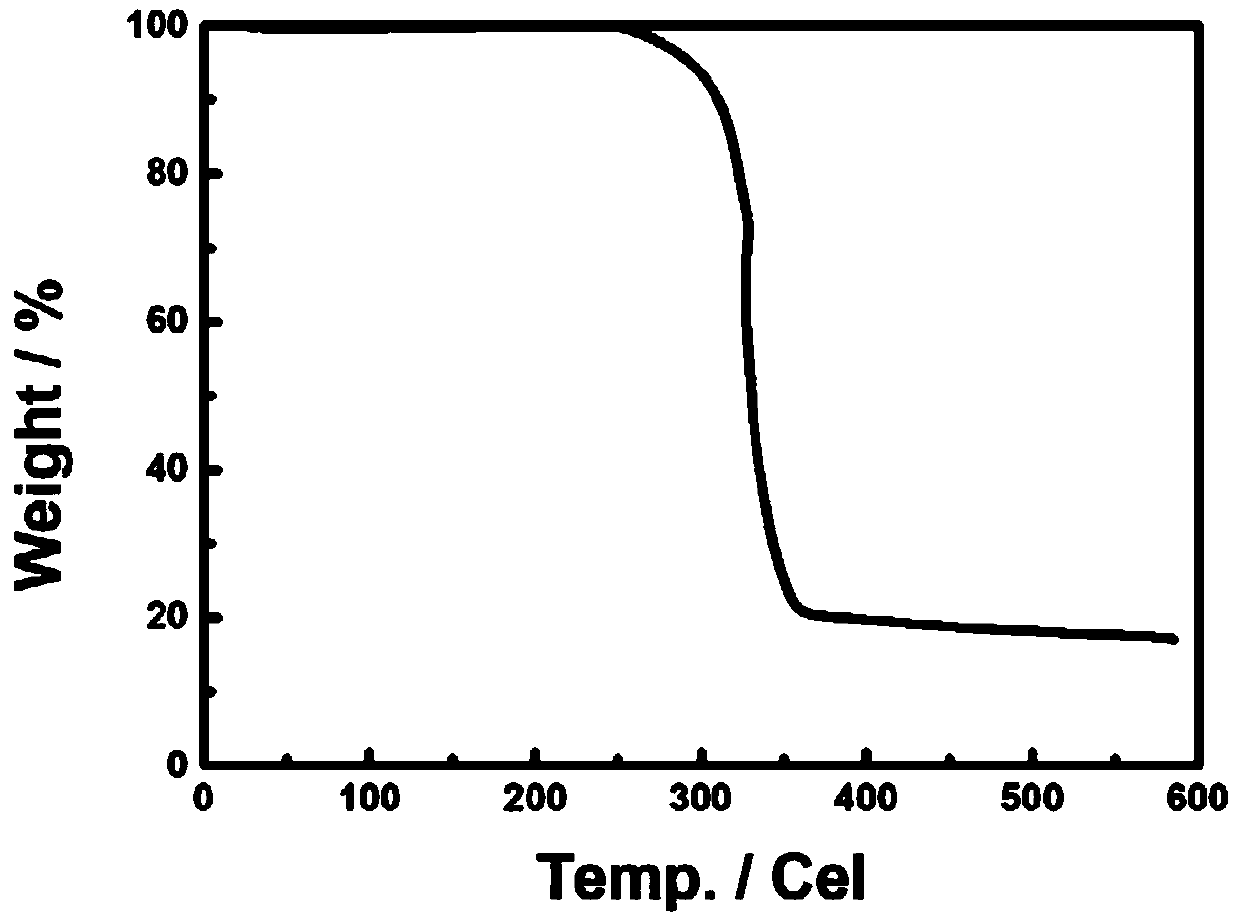
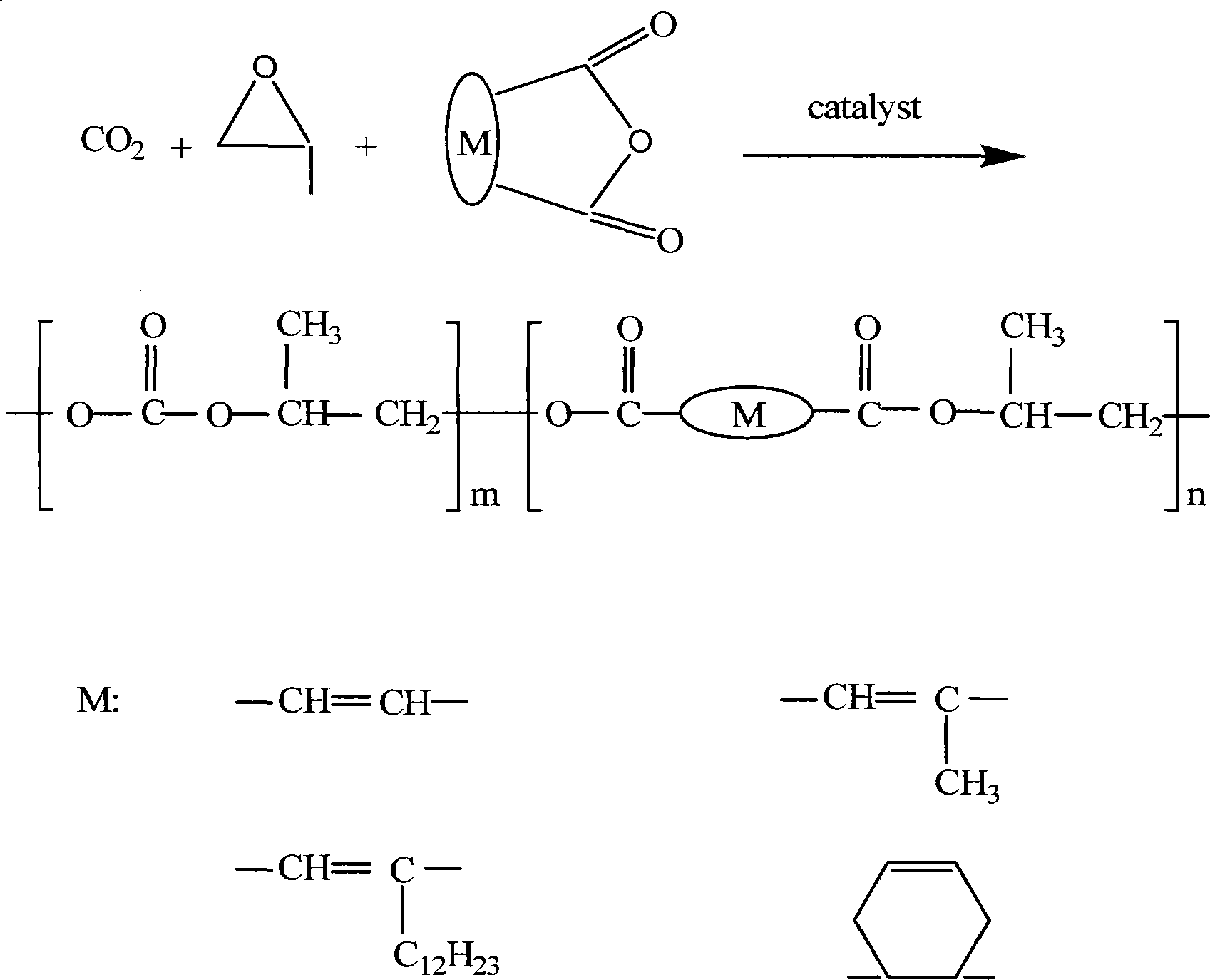
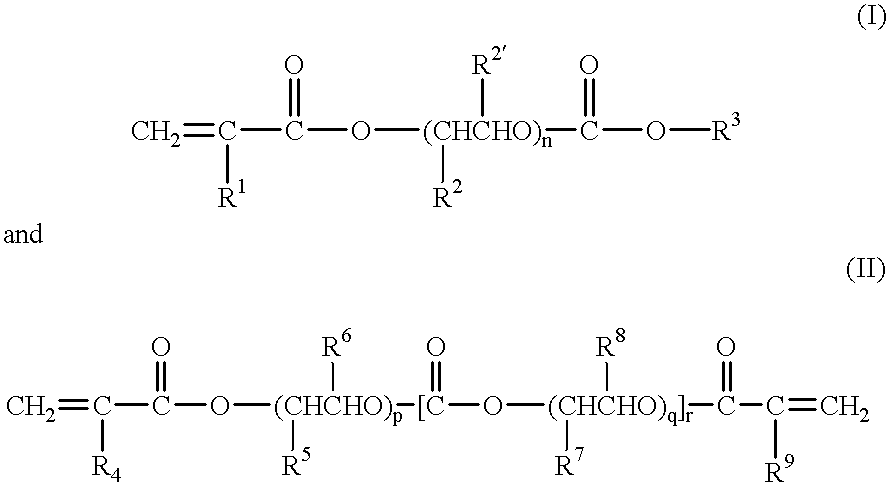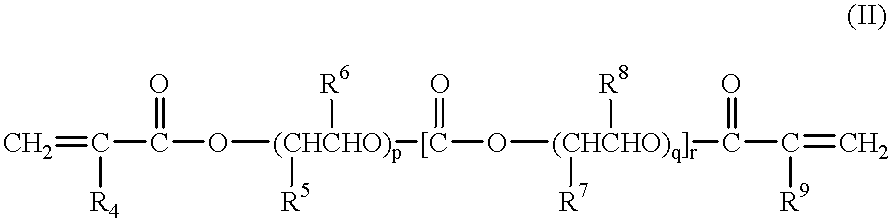Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61results about How to "Electrochemical stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
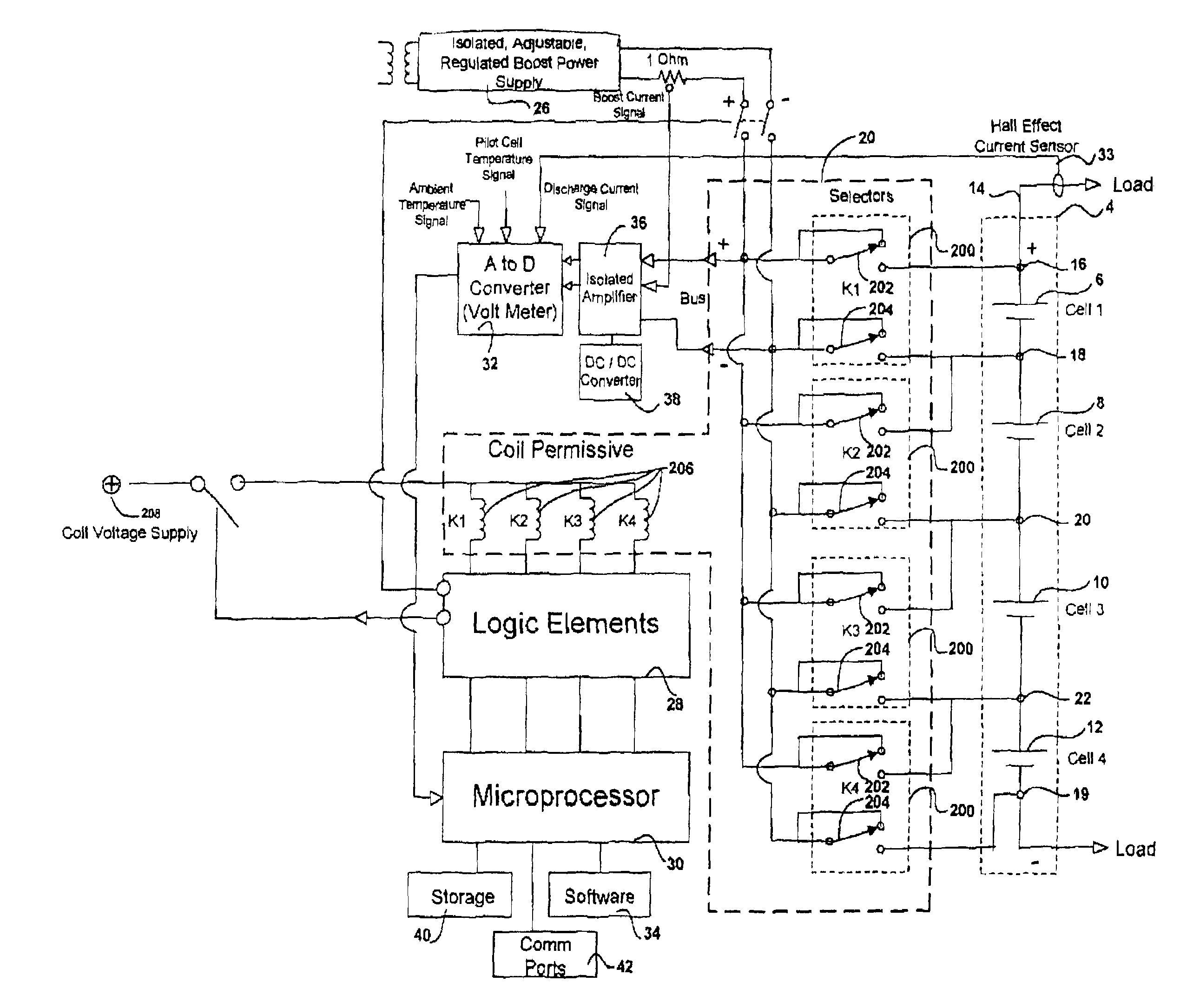
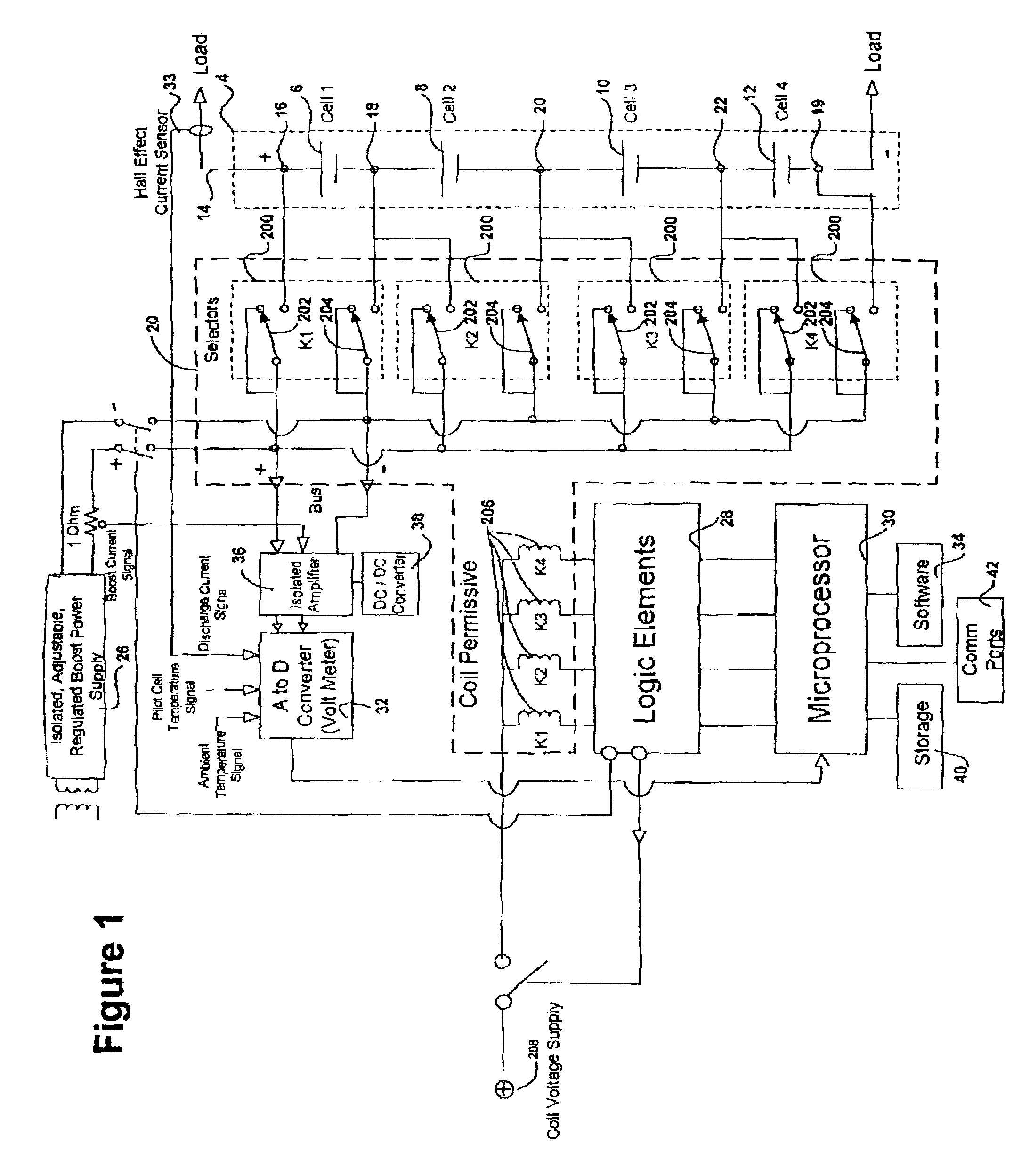
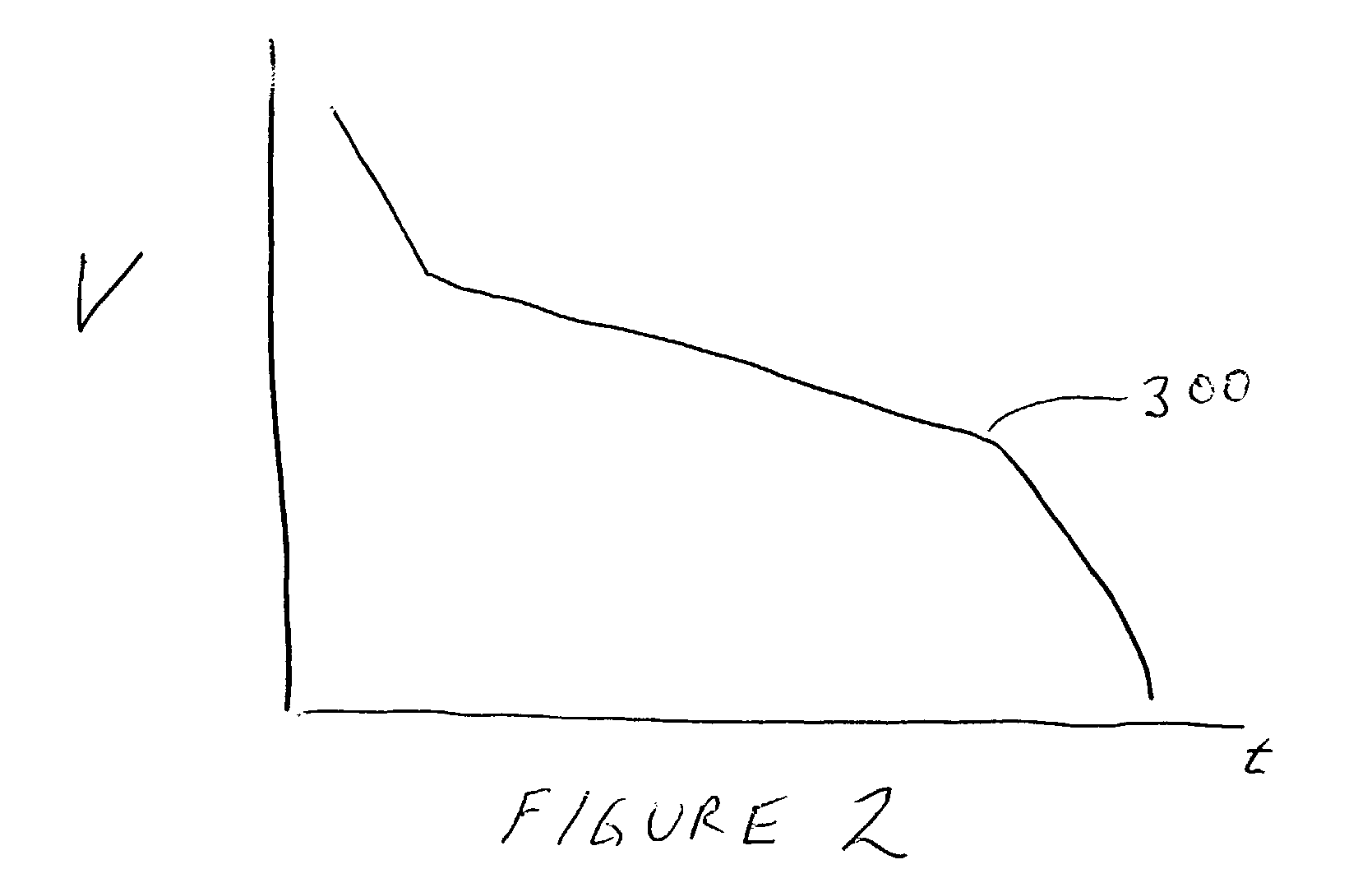
Battery management system and method
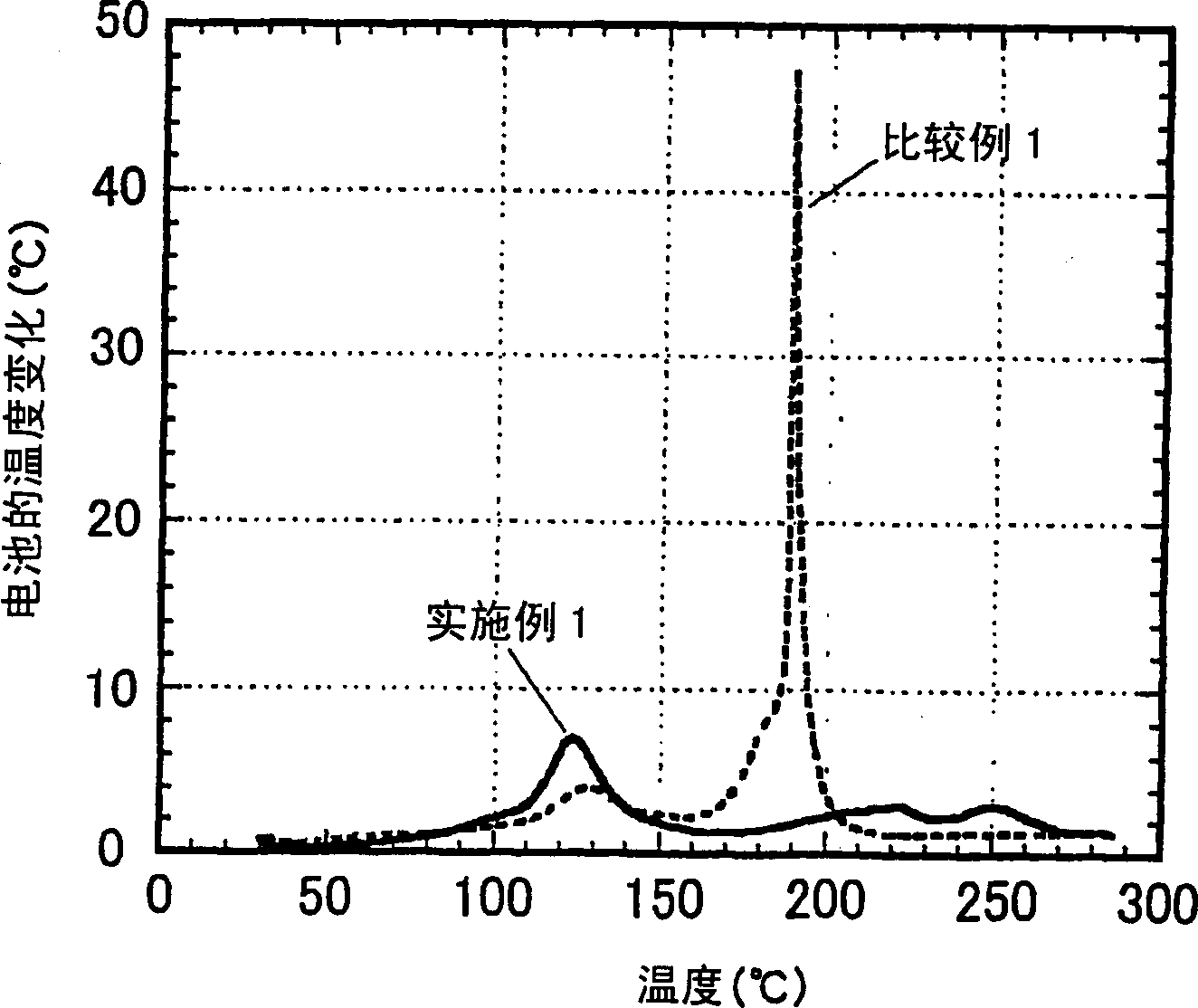
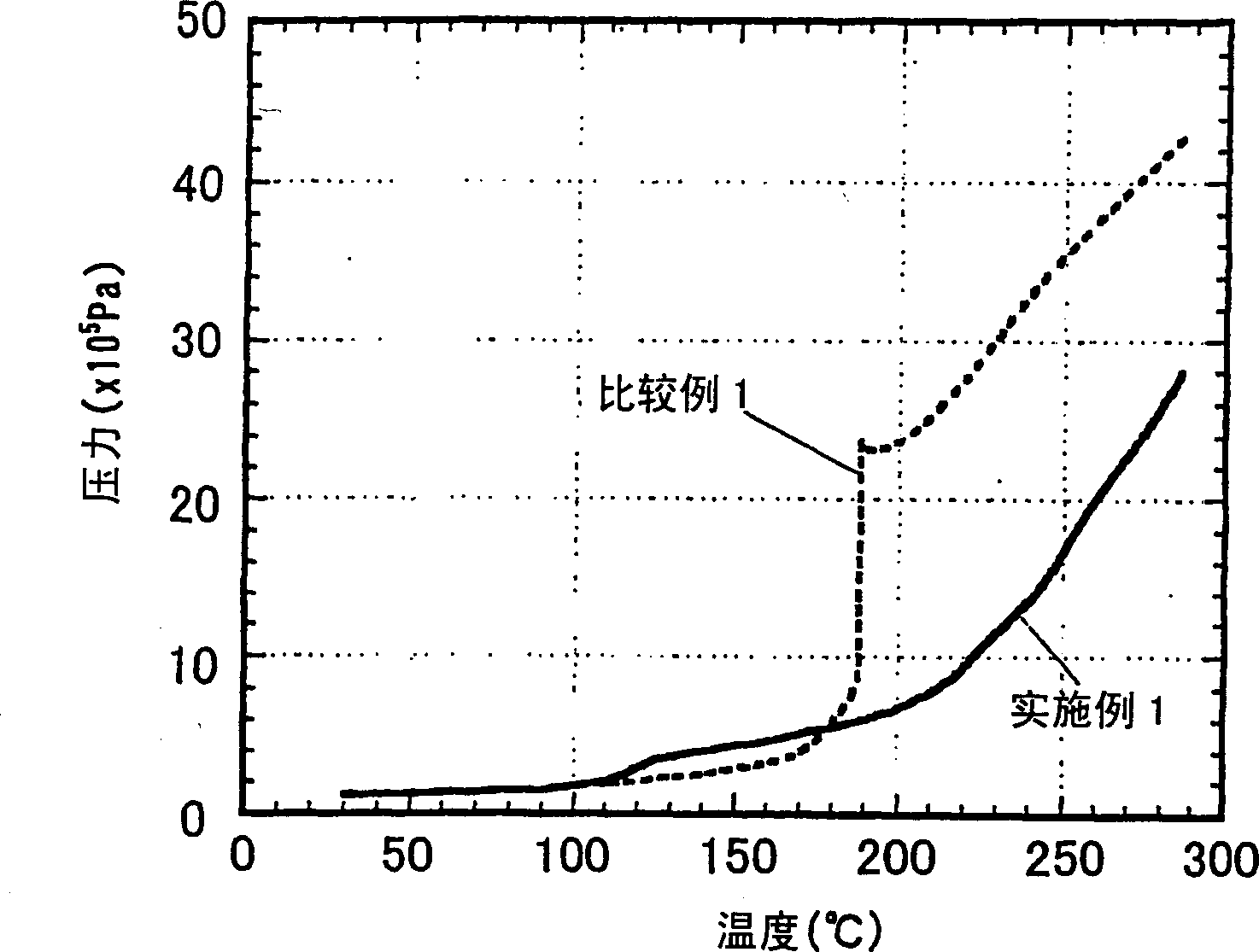
InactiveUS6983212B2Stable temperatureExtend battery lifeCircuit monitoring/indicationCharge equalisation circuitElectrical batteryVoltmeter
A battery management system is disclosed for control of individual cells in a battery string. The battery management system includes a charger, a voltmeter, a selection circuit and a microprocessor. Under control of the microprocessor, the selection circuit connects each cell of the battery string to the charger and voltmeter. Information relating to battery performance is recorded and analyzed. The analysis depends upon the conditions under which the battery is operating. By monitoring the battery performance under different conditions, problems with individual cells can be determined and corrected.
Owner:SCHNEIDER ELECTRIC IT CORP
Nonaqueous electrolytic liquid and lithium secondary battery employing same
InactiveCN1411619AExcellent charge and discharge characteristicsImprove conductivityOrganic electrolyte cellsNegative electrodesPhosphoric Acid EstersCarbamate
Nonaqueous electrolytic liquids for lithium secondary batteries which have flame retardancy (self-extinguishing characteristics) or incombustibility (no flash point), have a high conductivity, and are electrochemically stable. One of the nonaqueous electrolytic liquids comprises a nonaqueous solvent comprising as an essential ingredient at least one phosphate (a) selected among chain phosphoric esters (a1) and cyclic phosphoric esters (a2). The nonaqueous solvent may further contain a cyclic carboxylic ester (b1) and a cyclic carbonic ester (b2). Another nonaqueous electrolytic liquid comprises the nonaqueous solvent and incorporated therein at least either a vinylene carbonate compound (c1) or a vinylethylene carbonate compound (c2) and one or more compounds selected from the group consisting of cyclic amide compounds (d1), cyclic carbamate compounds (d2), and cyclic hetero-compounds (d3).
Owner:MITSUBISHI RAYON CO LTD
Porous membrane for a secondary battery and a secondary battery
ActiveUS20120189898A1Improve lithium ion conductivityHigh strengthFinal product manufactureActive material electrodesEpoxyLithium
Disclosed is a porous membrane used for a secondary battery, such as lithium-ion secondary battery, wherein strength is improved and break is difficult to occur while maintaining lithium-ion conductivity. The porous membrane for a secondary battery comprising a binder for the porous membrane and a nonconductive particle, wherein the binder for the porous membrane is a polymer particle having a hetero phase structure, in which internal layer is a polymer wherein vinyl monomer components are polymerized and outer layer is a polymer wherein monomer components containing a hydrophilic functional group are polymerized. The hydrophilic functional group is at least one selected from the group consisting sulfonic acid group, carboxyl group, hydroxyl group and epoxy group.
Owner:ZEON CORP
Preparation method of mesoporous carbon nanosheet and application of mesoporous carbon nanosheet as electrode material of super capacitor
ActiveCN102682928AImprove electrochemical performanceImproved magnification performanceElectrolytic capacitorsHybrid capacitor electrodesPolyethylene oxideElectric capacity
The invention relates to preparation of a mesoporous carbon nanosheet and application of the mesoporous carbon nanosheet as an electrode material in a super capacitor. According to the method, a porous magnesium oxide nanosheet is used as a template, dopamine is used as a carbon precursor, a carbon source is uniformly coated on the surface of the magnesium oxide nanosheet to form a complex, the complex is carbonized at a high temperature, and the magnesium oxide of the template is removed with excess sulfuric acid solution to obtain a two-dimensional carbon nanosheet. Triblock copolymer PEO-PPO-PEO (Polyethylene Oxide-Polyphenylene Oxide-Polyethylene Oxide) added in a preparation process is used as a structure directing agent to form a mesoporous structure, so that the specific surface area and the pore capacity of the carbon nanosheet are improved and transmission and diffusion of electrolyte ions are facilitated to improve the electric capacity of the material. The coating thickness of the carbon source on the surface of the template can be effectively controlled by adjusting the mass ratio of the template to the carbon source and the polymerization reaction time of the dopamine, and correspondingly the carbon nanosheets with different thicknesses are obtained, so that the electrochemical performance of the carbon nanosheet is affected.
Owner:EAST CHINA UNIV OF SCI & TECH
Gel-form solid polymer electrolyte
InactiveUS6296783B1Improve ionic conductivityElectrochemical stabilityHybrid capacitor electrolytesConductive materialPolymer scienceSolvent
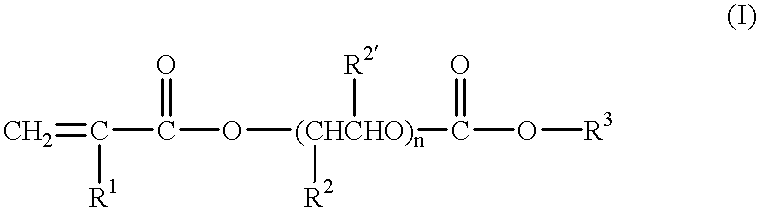
A polymeric gel electrolyte comprising:(a) an acrylic ester polymer matrix containing structural units derived from at least one acrylic ester selected from among acrylic esters represented by the general formulae:(wherein, in the formula (I), R1, R2 and R2' are identical with or different from each other and represent a hydrogen atom or an alkyl group having 1 to 4 carbon atoms, R3 represents an alkyl group having 1 to 4 carbon atoms, and n is an integer of 1 to 100; and, in the formula (II), R4 to R9 are identical with or different from each other and represent a hydrogen atom or an alkyl group having 1 to 4 carbon atoms, and p, q and r are identical with or different from each other and are an integer of 1 to 100); (b) a nonaqueous solvent of a carbonic ester; and (c) a salt of a metal of Group Ia of the periodic table. This polymeric gel electrolyte uses an acrylic ester polymer as a polymeric matrix and contains a specified nonaqueous solvent, so that it exhibits high ionic conductivity and is electrochemically stable. Thus, the polymeric gel electrolyte can suitably be used in electrochemical devices such as a primary battery, a secondary battery, a capacitor and an electrochromic display and in medical actuators.
Owner:MITSUI CHEM INC
High molecular electrolyte and lithium cell
InactiveCN1345897AElectrochemical stabilityImprove securitySolid electrolytesCell electrodesCross-linkPolymer electrolytes
A polymeric electrolyte and a lithium battery lithium employing the same. The polymeric electrolyte includes a cross-linked polyether urethane prepared by reacting a pre-polymer having a polyethylene oxide backbone and terminated with NCO, with a cross-linking agent, organic solvent and lithium salt. Since the polymeric electrolyte is electrochemically stable, a lithium battery having improved reliability and safety can be obtained by employing the polymeric electrolyte.
Owner:SAMSUNG SDI CO LTD
Method for preparing semi-interpenetrating network gel polymer electrolyte thin film
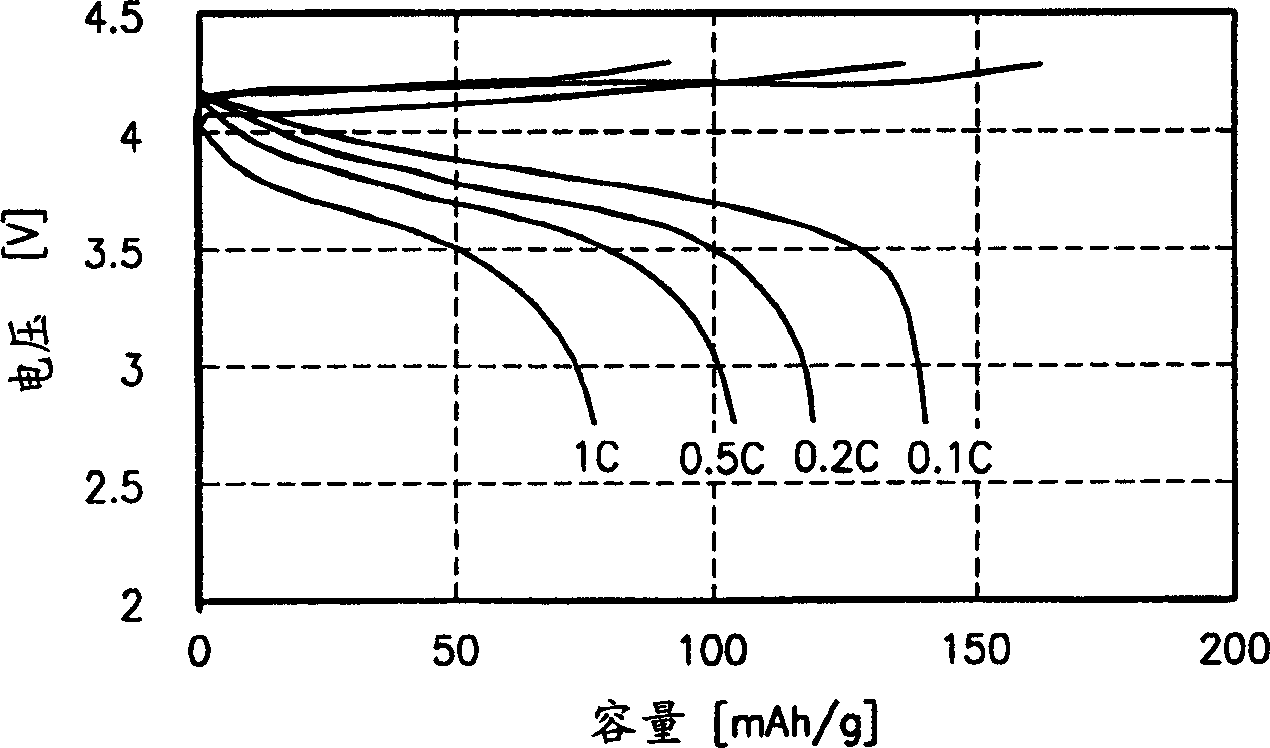
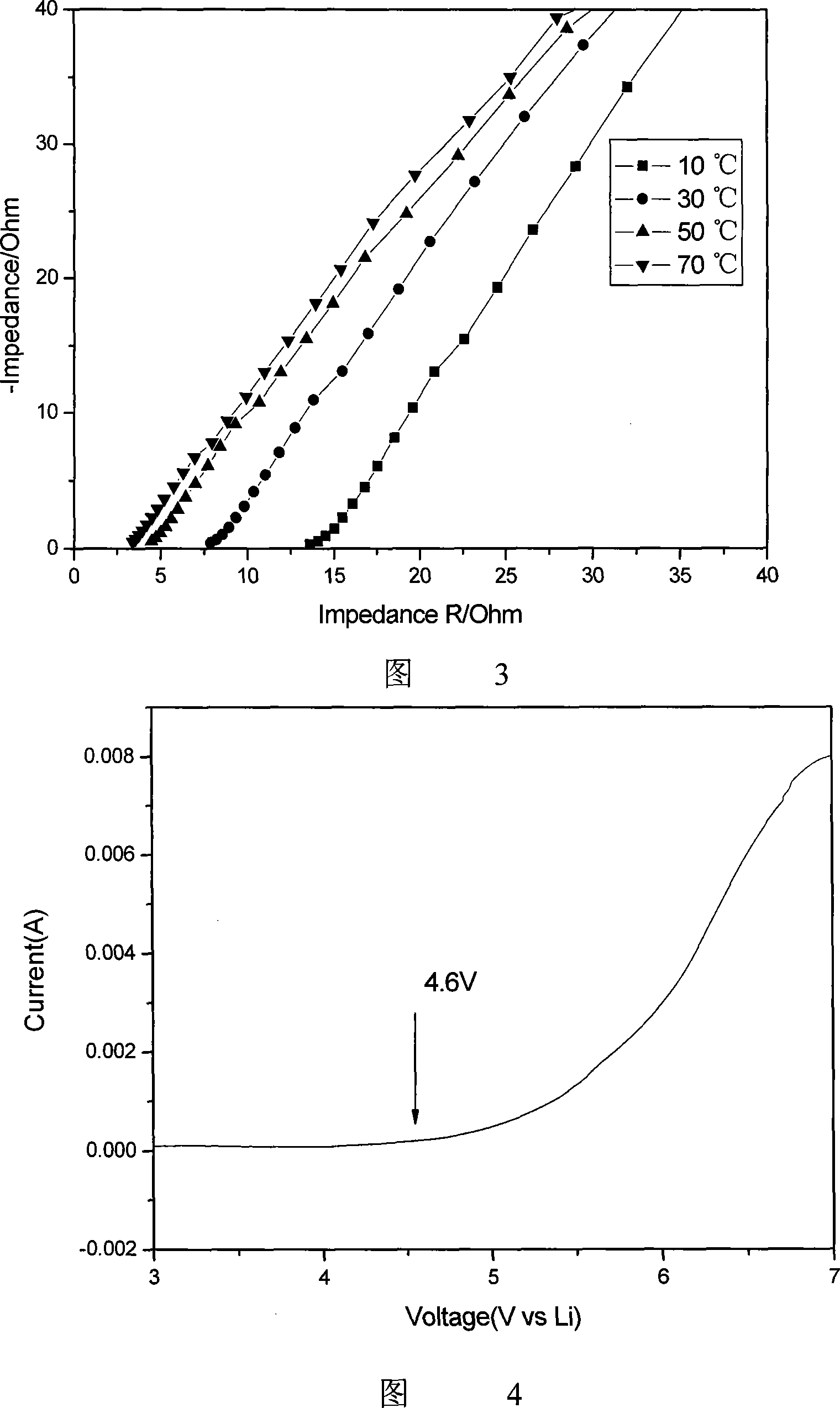
The invention discloses a preparation method of semi-interpenetrating network gel polyelectrolyte film. The preparation steps are that: firstly, methacryloxypropyl trimethoxy silane is hydrolyzed and crosslinked through a sol-gel method and dimethacrylate ethylene glycol is added to get a mixture; the mixture is mixed with tetrahydrofuranm solution of nitrile rubber and the solution is evaporated to get pre-polyblend film, then pre-polyblend film is solidified by UV-light irradiation to absorb 1M hexafluorophosphate lithium electrolyte solution to prepare the semi-interpenetrating network gel polyelectrolyte film directly. The semi-interpenetrating network ensures the system with better mechanical property, better compatibility between medium polar components in the mixing polymer system and liquid electrolyte, thus the liquid electrolyte is easy to be absorbed for gelation and has relatively high ionic conduction rate. The ionic conduction rate of the prepared semi-interpenetrating network gel polyelectrolyte film can be up to 2.28 is multiplied by 10-3S cm-1 at 30 DEG C and the electrochemical stability is up to 4.6V at room temperature. The invention has simple preparation technique and quick production speed by UV-light irradiation, thereby being applicable to mass industrial production.
Owner:JIANGSU UNIV OF SCI & TECH
Method for processing silicon base material, article processed by the method, and processing apparatus
InactiveCN101680106ALarge processing methodImprove energy efficiencyAnodisationMachining electrodesElectrolysisElectrical battery
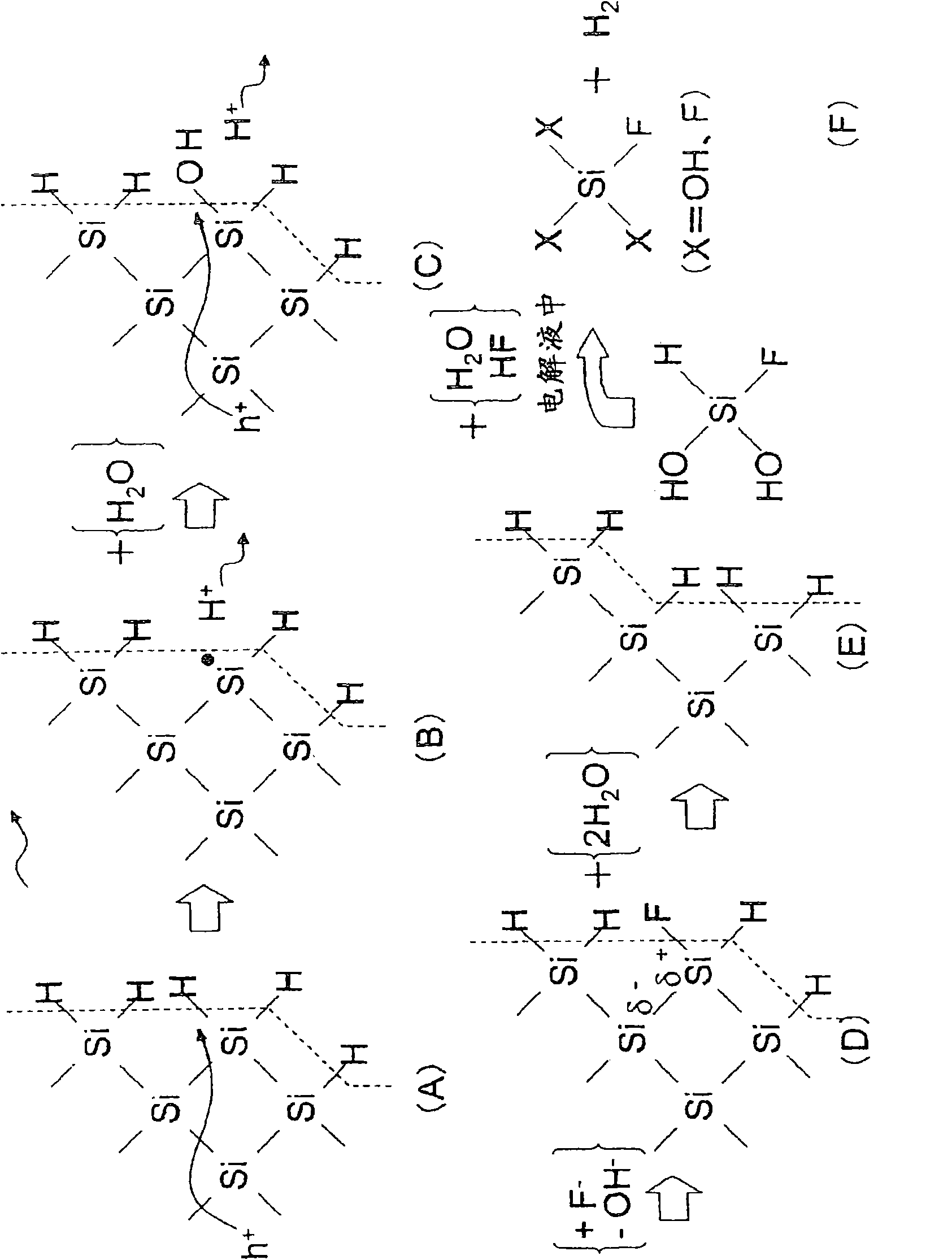
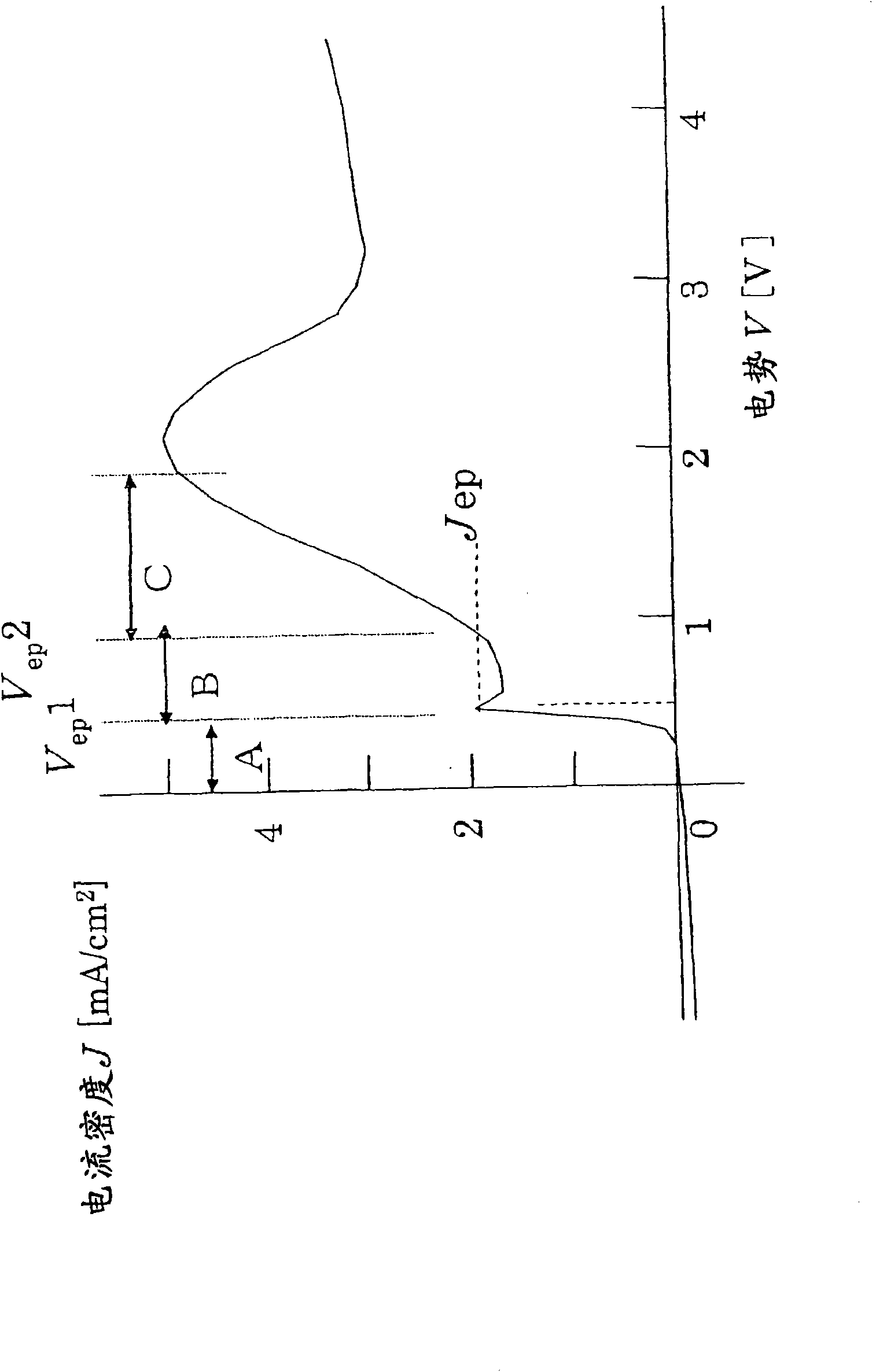
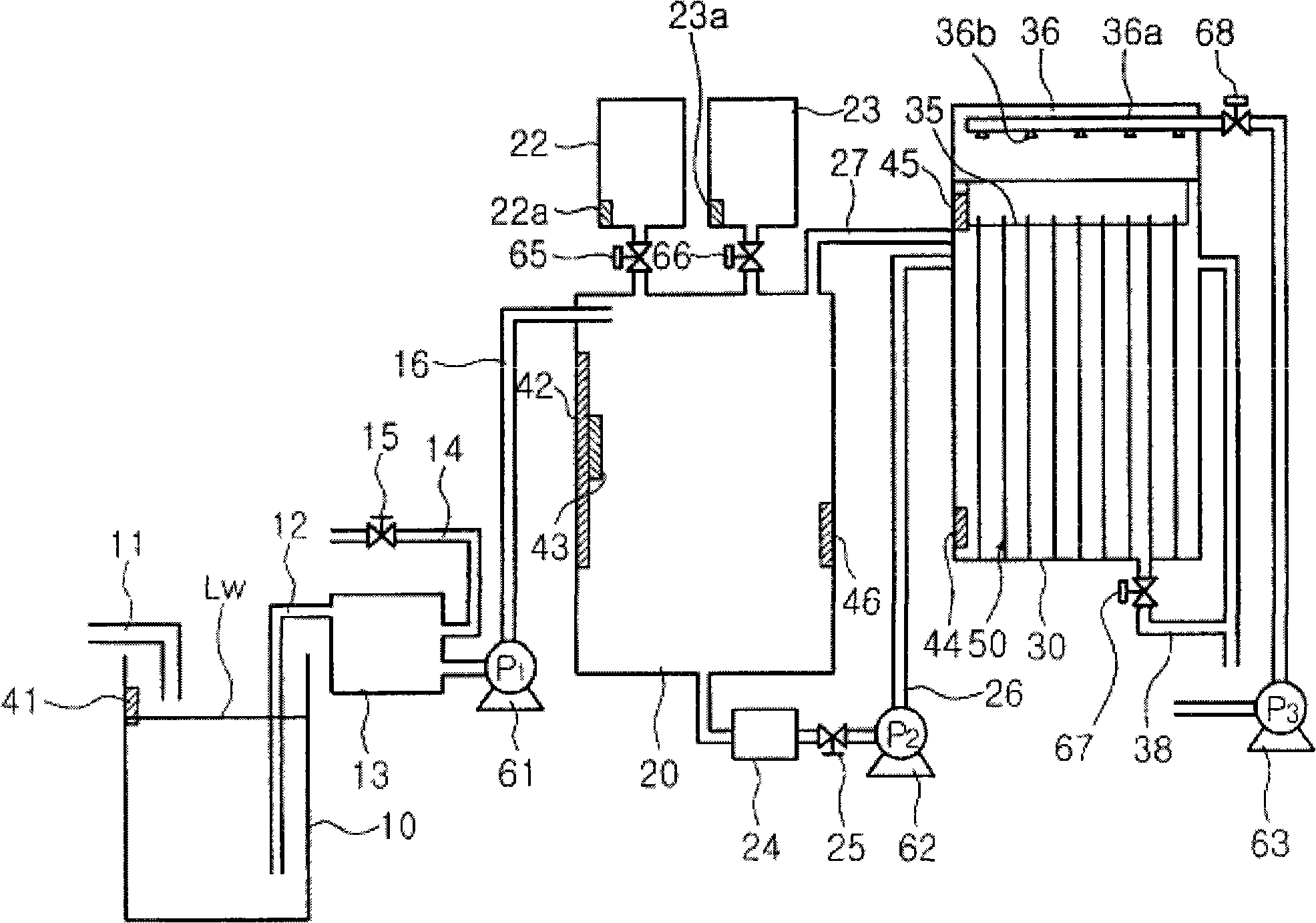
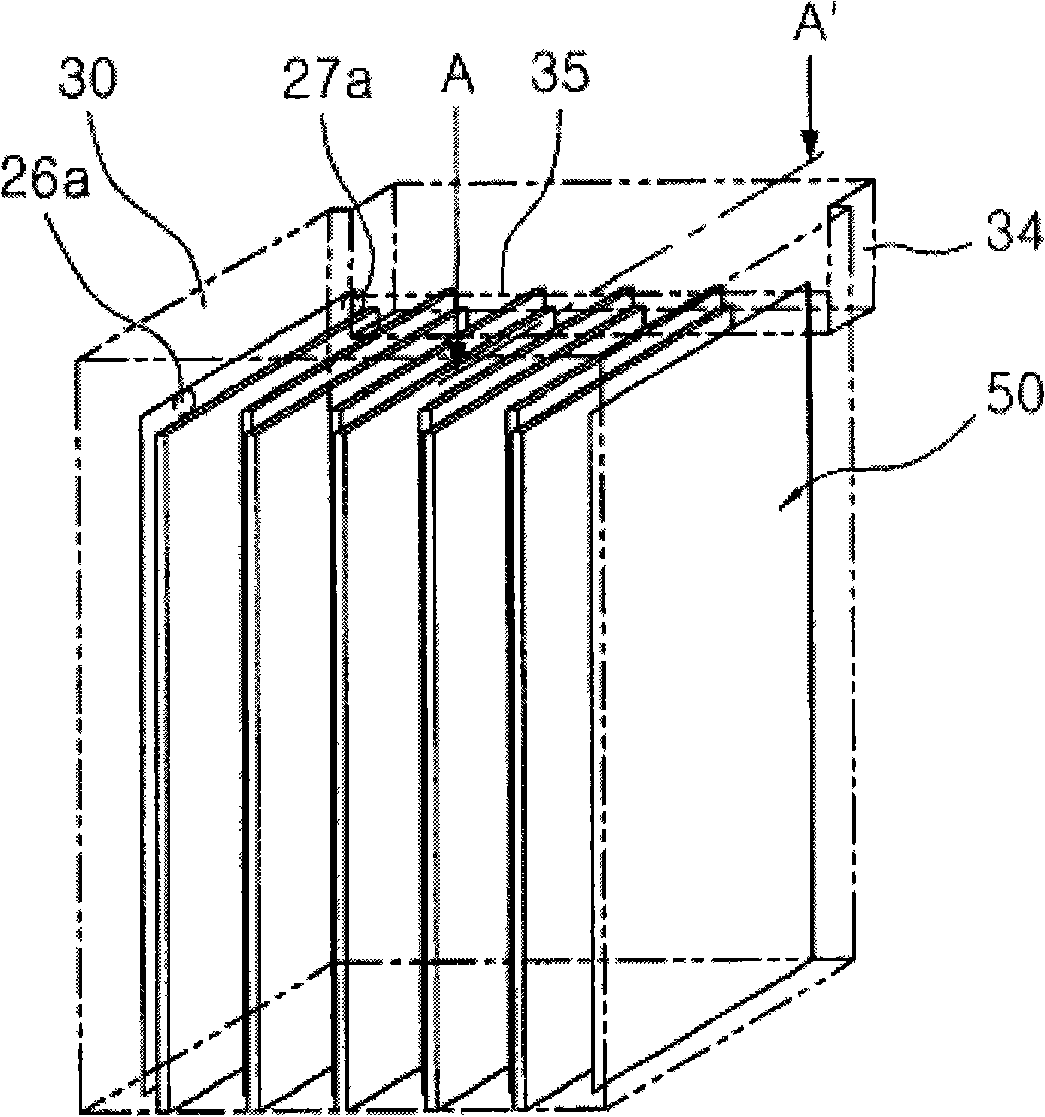
In a state where a silicon base material (1) is used as an anode, a fine platinum member (2) is used as a cathode, and an electrolyte solution (4) is arranged between the anode and the cathode, anodicoxidation is performed in constant current mode under the conditions where porous formation mode and electrolytic polishing mode coexist. The platinum member (2) is fitted in the silicon base material (1) with silicon elution, and processes such as hole making, cutting, single-side pressing are performed. Since the silicon base material can be processed at a room temperature with small energy, the crystal quality of the processing surface is not deteriorated. Thus, efficient and highly accurate processing can be performed without using a mechanical method, which consumes much material in conventional processes such as cutting of solar cell silicon base material, and without using laser whose energy unit cost is high, and furthermore, without leaving a crystal damage on a processed surface.
Owner:QUANTUM 14
Electro-chemical water processing apparatus and method thereof
InactiveCN101573299AEasy to separateLow costWater treatment parameter controlSpecific water treatment objectivesElectrolysisHydrogen-Ion Concentrations
The present invention provides an electro-chemical water treatment apparatus and method for removing total nitrogen ingredients of ammonia nitrogen, nitrous acid nitrogen, nitrate nitrogen etc., organic materials of BOD and COD induction ingredients, and cyanogen included in wastewater and dirty water. The apparatus includes: a wastewater collection reservoir that contains wastewater; a wastewaterstorage retention reservoir that controls a hydrogen ion concentration (pH), an electrical conductivity and an amount of flow of wastewater; an electrolyte tank which makes the electrical conductivity of the wastewater as an electrical conductivity at which an electrolysis can be achieved; a pH conditioner tank that supplies a pH conditioner for the wastewater; an electrolyzer including an anodeplate and a cathode plate, and a number of electrodes which are arranged as an electrification body between the anode plate and the cathode plate; and a controller which grasps state of wastewater andwhich is connected to the anode plate and the cathode plate to thereby control the electrolysis.
Owner:田致重
Cross-lined polymethyl ethylene carbonate polymer electrolyte membrane and preparation method thereof
The present invention discloses cross-linked polymethacrylic ethylene carbonic acid ester polymer electrolyte membrane and the preparation method. The polymer electrolyte membrane consists of cross-linked polymethacrylic ethylene carbonic acid ester polymer and electrolyte solution including conductive lithium salt and non-protonic solvent. The preparation method adopts carbon dioxide, epoxy dimethylmethane and unsaturated acid anhydride which are synthesized into polymethacrylic ethylene carbonic acid ester trivalent copolymer with special functional group under the role of catalyst dyadic carboxylic acid zinc. Then the trivalent copolymer is under the cross-linking reaction so as to get the dry polymethacrylic ethylene carbonic acid ester polymer membrane. Afterwards, the dry membrane is immerged into the liquid electrolyte solution for activation, thus obtaining the cross-linked polymethacrylic ethylene carbonic acid ester polymer electrolyte membrane. The polymer electrolyte membrane prepared by the present invention has the advantages of high mechanical performance, good thermal performance and high ion electric conduction. The present invention with low cost and simple process is applicable to the industrialized production.
Owner:SUN YAT SEN UNIV
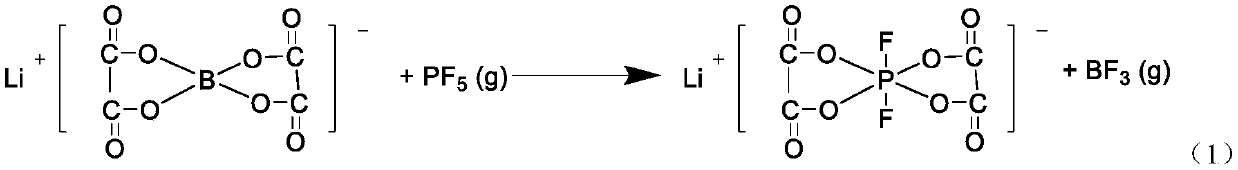
Lithium difluorobisoxalate phosphate preparation method, non-aqueous electrolyte and battery
ActiveCN109824726AImprove discharge rateLower requirementGroup 5/15 element organic compoundsLi-accumulatorsPhosphateDistillation
The invention relates to the technical field of energy storage batteries, and provides a lithium difluorobisoxalate phosphate preparation method which includes the steps: dissolving lithium difluoroborate by non-aqueous solvents to obtain first solution; heating the first solution to reach the temperature of 50-130 DEG C, leading phosphorus pentafluoride gas into the first solution and performingreaction to obtain lithium difluorobisoxalate phosphate solution; performing reduced pressure distillation on the lithium difluorobisoxalate phosphate solution and adding inert solvent crystals to obtain lithium difluorobisoxalate phosphate. Raw materials needed by the preparation method are low in cost and easy to obtain, and a reaction product is the lithium difluorobisoxalate phosphate, so thatthe prepared product is high in purify and yield. The technological process of the method is simple, requirements for equipment and environments are low, and the method is suitable for industrial production and application. The invention further provides non-aqueous electrolyte and a battery comprising the same. The non-aqueous electrolyte comprises the lithium difluorobisoxalate phosphate prepared by the method. The non-aqueous electrolyte is good in conductivity and electrochemically stable.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Composite LiFePO4/C cathode material for lithium ion battery and preparation method for composite LiFePO4/C cathode material
ActiveCN104319380ASimple processProduct quality is easy to controlCell electrodesHigh rateLithium-ion battery
The invention relates to a preparation method for a micron-sized composite LiFePO4 / C cathode material for a lithium ion battery. The method comprises the following steps of uniformly mixing an iron source, a phosphorus source and an organic carbon source, and pre-sintering the mixture to obtain a composite iron phosphate / carbon precursor; uniformly mixing the obtained composite precursor, a lithium source and an inorganic carbon source, performing compression molding, preserving heat for a certain time under 700 to 800 DEG C and certain inert gas pressure in a sintering furnace, and performing crushing to obtain the composite LiFePO4 / C cathode material after temperature reduction. The invention also relates to the micron-sized composite LiFePO4 / C cathode material obtained by the method and with a nano carbon conducting network. The material has the characteristics of high purity, high tap density, high consistency, high processability and high rate discharge performance.
Owner:四川浩普瑞新能源材料股份有限公司
Electrode for an Li ion battery having a polyether-siloxane copolymer as binder
InactiveCN103943818AElectrochemical stabilityChemically stableSecondary cellsNon-aqueous electrolyte accumulator electrodesPolyethylene glycolCopolymer
The object of the invention is an electrode for a Li-ion battery, which contains a crosslinked polyether-siloxane copolymer (V), which can be prepared by crosslinking of siloxane macromers (S) having the average general formula (1): HaR1bSiO(4-a-b) / 2 (1), where R1 is a monovalent, SiC-bonded C1-C18 hydrocarbon radical which is free of aliphatic carbon-carbon multiple bonds and a and b are nonnegative integers, with the proviso that 0.5<(a+b)<3.0 and 0<a<2, and that at least two silicon-bonded hydrogen atoms are present per molecule, by means of polyether macromers (P) containing at least two alkenyl groups per molecule and optionally further compounds (W) containing alkenyl groups, with polyethylene glycols functionalized by one allyl group being excepted from the compounds (W) as binder; and also a process for preparing a crosslinked polyether-siloxane copolymer (V) as binder for the electrode in a Li-ion battery in a crosslinking step.
Owner:WACKER CHEM GMBH
Imidazolium-based ionic liquid and preparation method and application thereof
PendingCN108586348AImprove electrochemical stabilityWide electrochemical stabilitySecondary cellsSulfonic acid amide preparationElectrolytic agentElectrical battery
The invention relates to an imidazolium-based ionic liquid and a preparation method and application thereof. The ionic liquid has a structural formula shown in the description, wherein n1 is 2 or 3 or4, n2 is 0 or 1, and Y- is BF4- or PF6- or TFSI- or N(CN)2- or (FSO2)2N-. By adopting triple substitution and introducing electron donating groups and electron withdrawing groups, the imidazolium-based ionic liquid has two different types of unsaturated bond structures of nitrile groups and alkenyl groups; in the preparation process, suitable parameters are selected to introduce nitrile groups. When serving as a lithium ion battery electrolyte, the imidazolium-based ionic liquid can improve the electrochemical window of the electrolyte; by applying the imidazolium-based ionic liquid to high-voltage lithium ion batteries with LiNi0.5Mn1.5O4 as the positive electrode material, the safety and electrochemical properties of the lithium ion batteries can be improved.
Owner:HEBEI UNIV OF TECH
Imidazole ionic liquid, ionic liquid electrolyte as well as preparation method and application thereof
InactiveCN108530363AImprove electrochemical stabilityImprove solubilityOrganic chemistrySecondary cellsEtherElectrochemical window
The invention provides imidazole ionic liquid. The structural formula of the ionic liquid is as follows: a formula is as shown in the description, wherein R1 is CH2(CH2)aO(CH2)bCH3; a is 0 or 1; b is0 or 1; R2 is CnH2n-1; n is 2 or 3; Y- is selected from one of BF4<->, PF6<->, TFSI<-> or (FSO2)2N<->. The imidazole ionic liquid is subjected to triple substitution; meanwhile, different types of electron contributing groups are introduced into the imidazole ionic liquid, so that a saturated ether group structure and an unsaturated alkenyl structure are arranged at a site N-1 and a site N-3 of the imidazole ring; the ether group is introduced by selecting suitable parameters in the preparation process; when the ionic liquid is used as lithium-ion battery electrolyte, the electrochemical window of the electrolyte can be improved; when the ionic liquid is applied to high-voltage lithium-ion batteries taking LiNi0.5Mn1.5O4 as positive electrode materials, the safety and the electrochemical performance of the lithium-ion batteries can be improved. (The formula is shown in the description.).
Owner:HEBEI UNIV OF TECH
Organic electroluminescent material and device
ActiveCN108191853AImprove efficiencyImprove mobilityOrganic chemistrySolid-state devicesSimple Organic CompoundsHydrogen
The invention provides an organic compound with a specific structure. The organic compound is characterized by being shown as a general formula (I): the formula (I) is shown in the description; in theformula (I), X1 to X8 are respectively independently selected from CR1 or N respectively, wherein at least one is an N atom; L is a single bond and is C5 to C12 substituted or unsubstituted arylene and heteroarylene; R1 is selected from hydrogen, C1 to C10 aryl or cycloalkyl, C6 to C15 aryl or C6 to C19 condensed ring aryl and at least one is azacarbazole; Ar is substituted or unsubstituted N heterophenyl. The invention discloses an inverse intersystem crossing constant and emission delayed fluorescence rule and the designed compound is used for an organic electroluminescent device, can be used for effectively improving the current efficiency and is an organic electroluminescent material with good performance. The invention also provides the organic electroluminescent device adopting thecompound shown as the general formula.
Owner:BEIJING ETERNAL MATERIAL TECH +1
High-voltage electrolyte and lithium ion battery containing electrolyte
InactiveCN105762412AElectrochemical and chemical stabilityInhibit surface activitySecondary cellsElectrolytesDouble bondLithium electrode
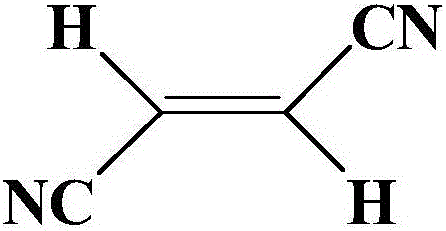
The invention belongs to the technical field of lithium ion battery materials, and discloses high-voltage electrolyte and a lithium ion battery containing the electrolyte. The high-voltage electrolyte is obtained by adding a functional additive to common electrolyte, wherein the mass of the functional additive is equivalent to 0.1-5% of that of common electrolyte, and the functional additive refers to an alkene dinitrile type compound. According to the high-voltage electrolyte and the lithium ion battery containing the electrolyte disclosed by the invention, the alkene dinitrile type compound containing nitrogen elements and double-bond functional groups is used as a high-voltage film forming additive of the lithium ion electrolyte, the additive is based on the oxidation or the reduction of double bonds, and a layer of compact passivating films can be formed on the surface of a positive pole and the surface of a negative pole in the process of first charge and discharge. Stable cyanogen bases are introduced, so that surface films are stabler in electrochemistry and chemistry. The cycle performance of the lithium ion battery containing the additive of the electrolyte under 3-4.5V is improved, the safety performance and the energy density of the lithium ion battery are improved, and the service life of the lithium ion battery is prolonged.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Method for preparing double-meso-pore ordered mesoporous carbon/ polyaniline nanometer line composite materials and application thereof
InactiveCN102924715AStable contentThe content is easy to controlElectrolytic capacitorsElectrolytic agentCapacitance
The invention relates to a method for preparing double-meso-pore ordered mesoporous carbon / polyaniline nanometer line composite materials and an application thereof. The method includes: using the ordered mesoporous carbon with silica nanometer particles embedded on carbon walls as a carrier, enabling a polyaniline nanometer line array to grow from the inside of a carbon duct to the outside in a chemical oxidative polymerization method, removing silica through hydrofluoric acid to obtain the classified composite materials of double-meso-pore ordered mesoporous carbon / polyaniline nanometer line composite materials. Small meso pores on a carbon wall of mesoporous carbon can be effectively retained through the method which performs compounding and then removes the silica, specific surface area of the composite materials is improved, migration and diffusion between adjacent ducts of electrolyte ions are facilitated, and the electrochemical performance of the materials is further improved. The method has the advantages of being simple in preparing process, low in cost, capable of controlling the content of polyaniline in the composite materials and capable of being produced in scale mode, and the prepared electrode materials are high in specific capacitance, circulation stability and rate capability.
Owner:EAST CHINA UNIV OF SCI & TECH
Composite conductive agent for lithium ion battery, composite conductive liquid for lithium ion battery, preparation method of conductive agent and conductive liquid and lithium ion battery
ActiveCN106099115ALower impedanceImprove high temperature cycle performanceCell electrodesSecondary cellsTrimethylsilylDissolution
The invention discloses a composite conductive agent for a lithium ion battery, a composite conductive liquid for the lithium ion battery, a preparation method of the conductive agent and the conductive liquid and the lithium ion battery, and belongs to the technical field of lithium ion batteries. The composite conductive agent for the lithium ion battery is prepared from, by weight, 0.5-2 parts of 3-(trimethylsilyl) borate and 1-5 parts of conductive agent. In the composite conductive agent for the lithium ion battery, 3-(trimethylsilyl) borate is added, the conductivity of lithium ions in the charging and discharging circulation process is benefited, impedance of the lithium ion battery is reduced easily, and accordingly it is guaranteed that circulation stability is excellent. Meanwhile, dissolution of Fe2+ in electrolyte can be well inhibited, SEI quality is improved, accordingly high-temperature circulating performance of the lithium ion battery can be improved, and application prospects in the lithium ion battery field are good.
Owner:LUOYANG LIRONG NEW ENERGY TECH
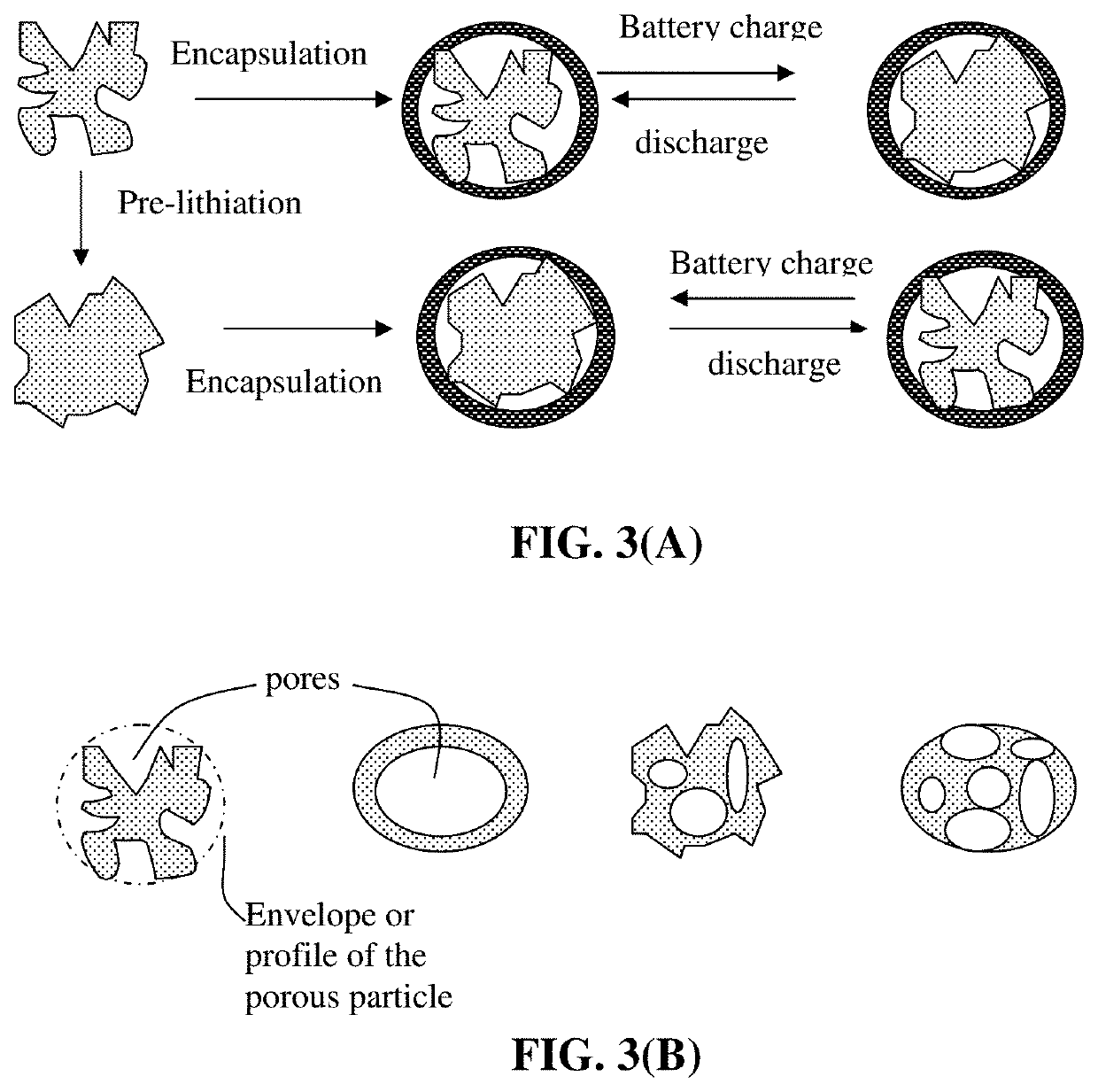
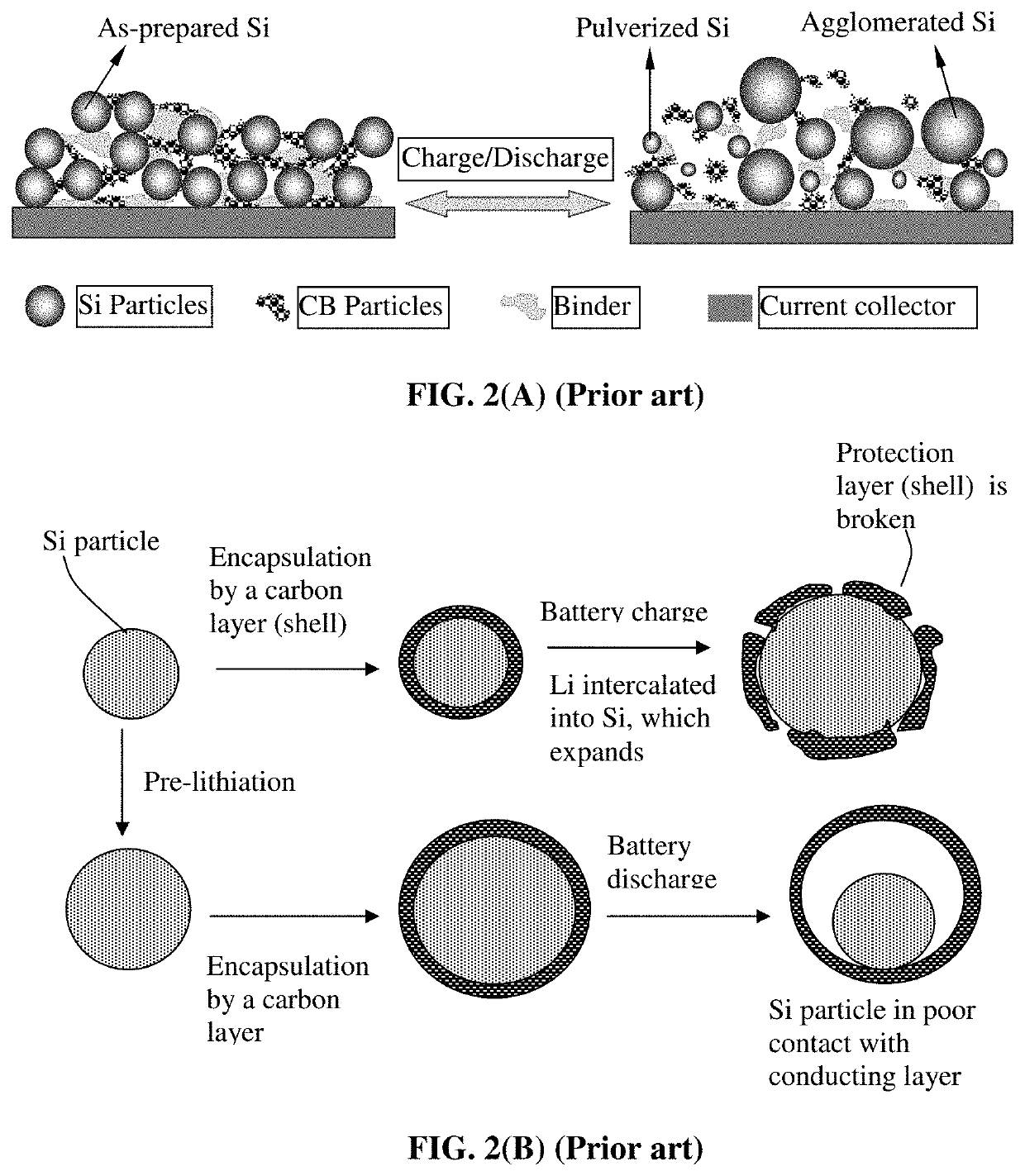
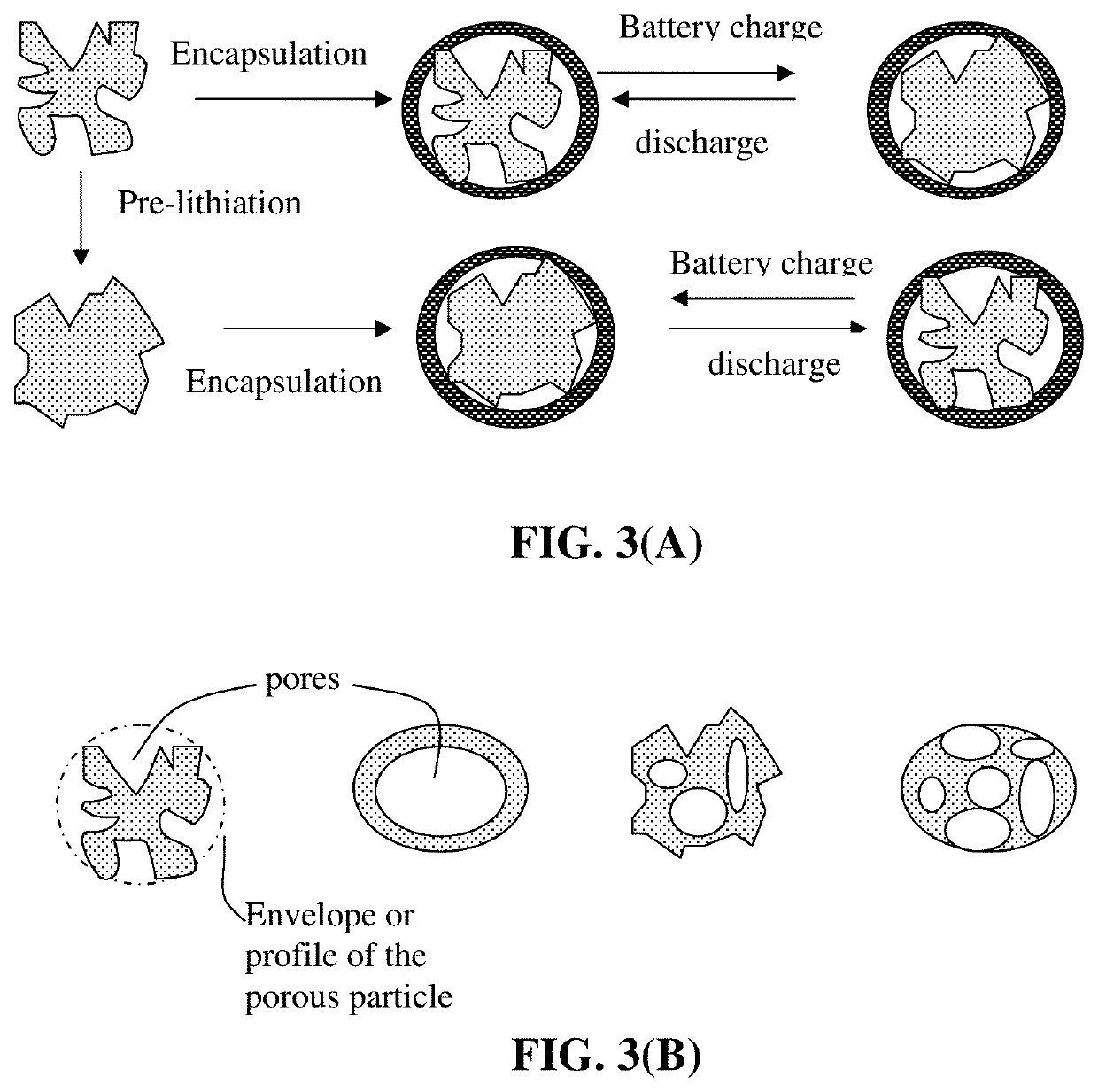
Production method for electrochemically stable anode particulates for lithium secondary batteries
ActiveUS20200119338A1Electrochemical stabilityReduce and eliminate volume expansionFinal product manufactureNegative electrodesLiquid mediumElectrical battery
Provided is a method of producing multiple anode particulates, comprising: a) dispersing an electrically conducting material, primary particles of an anode active material, an optional electron-conducting material, and a sacrificial material in a liquid medium to form a precursor mixture; b) forming the precursor mixture into droplets and drying the droplets; and c) removing the sacrificial material or thermally converting the sacrificial material into a carbon material to obtain multiple particulates, wherein a particulate comprises one or a plurality of anode active material particles having a volume Va, an electron-conducting material, and pores having a volume Vp which are encapsulated by a thin encapsulating layer having a thickness from 1 nm to 10 μm and a lithium ion conductivity from 10−8 S / cm to 5×10−2 S / cm and the volume ratio Vp / Va in the particulate is from 0.3 / 1.0 to 5.0 / 1.0.
Owner:GLOBAL GRAPHENE GRP INC
Porous membrane for a secondary battery and a secondary battery
ActiveUS8852788B2High strengthImprove lithium ion conductivityFinal product manufactureActive material electrodesEpoxyLithium
Disclosed is a porous membrane used for a secondary battery, such as lithium-ion secondary battery, wherein strength is improved and break is difficult to occur while maintaining lithium-ion conductivity. The porous membrane for a secondary battery comprising a binder for the porous membrane and a nonconductive particle, wherein the binder for the porous membrane is a polymer particle having a hetero phase structure, in which internal layer is a polymer wherein vinyl monomer components are polymerized and outer layer is a polymer wherein monomer components containing a hydrophilic functional group are polymerized. The hydrophilic functional group is at least one selected from the group consisting sulfonic acid group, carboxyl group, hydroxyl group and epoxy group.
Owner:ZEON CORP
Electrochemically stable anode particulates for lithium secondary batteries and method of production
PendingUS20200119337A1Electrochemical stabilityReduce and eliminate volume expansionFuel and secondary cellsNegative electrodesElectro conductivityElectrically conductive
Provided is a lithium battery anode electrode comprising multiple particulates of an anode active material, wherein at least a particulate comprises one or a plurality of particles of said anode active material having a volume Va, an electron-conducting material as a matrix, binder or filler material, and pores having a volume Vp which are encapsulated by a thin encapsulating layer of an electrically conducting material, wherein the thin encapsulating layer has a thickness from 1 nm to 10 μm, an electric conductivity from 10−6 S / cm to 20,000 S / cm and a lithium ion conductivity from 10−8 S / cm to 5×10−2 S / cm and the volume ratio Vp / Va in the particulate is from 0.3 / 1.0 to 5.0 / 1.0. If a single primary particle is encapsulated, the single primary particle is itself porous having a free space to expand into without straining the thin encapsulating layer when the lithium battery is charged.
Owner:GLOBAL GRAPHENE GRP INC
Ionic plastic crystal-polymer-inorganic composite electrolyte membrane and preparation method and application thereof
ActiveCN111261932AHigh mechanical strengthImprove conductivitySecondary cellsComposite electrolytePlastic crystal
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Cross-lined polymethyl ethylene carbonate polymer electrolyte membrane and preparation method thereof
The present invention discloses cross-linked polymethacrylic ethylene carbonic acid ester polymer electrolyte membrane and the preparation method. The polymer electrolyte membrane consists of cross-linked polymethacrylic ethylene carbonic acid ester polymer and electrolyte solution including conductive lithium salt and non-protonic solvent. The preparation method adopts carbon dioxide, epoxy dimethylmethane and unsaturated acid anhydride which are synthesized into polymethacrylic ethylene carbonic acid ester trivalent copolymer with special functional group under the role of catalyst dyadic carboxylic acid zinc. Then the trivalent copolymer is under the cross-linking reaction so as to get the dry polymethacrylic ethylene carbonic acid ester polymer membrane. Afterwards, the dry membrane is immerged into the liquid electrolyte solution for activation, thus obtaining the cross-linked polymethacrylic ethylene carbonic acid ester polymer electrolyte membrane. The polymer electrolyte membrane prepared by the present invention has the advantages of high mechanical performance, good thermal performance and high ion electric conduction. The present invention with low cost and simple process is applicable to the industrialized production.
Owner:SUN YAT SEN UNIV
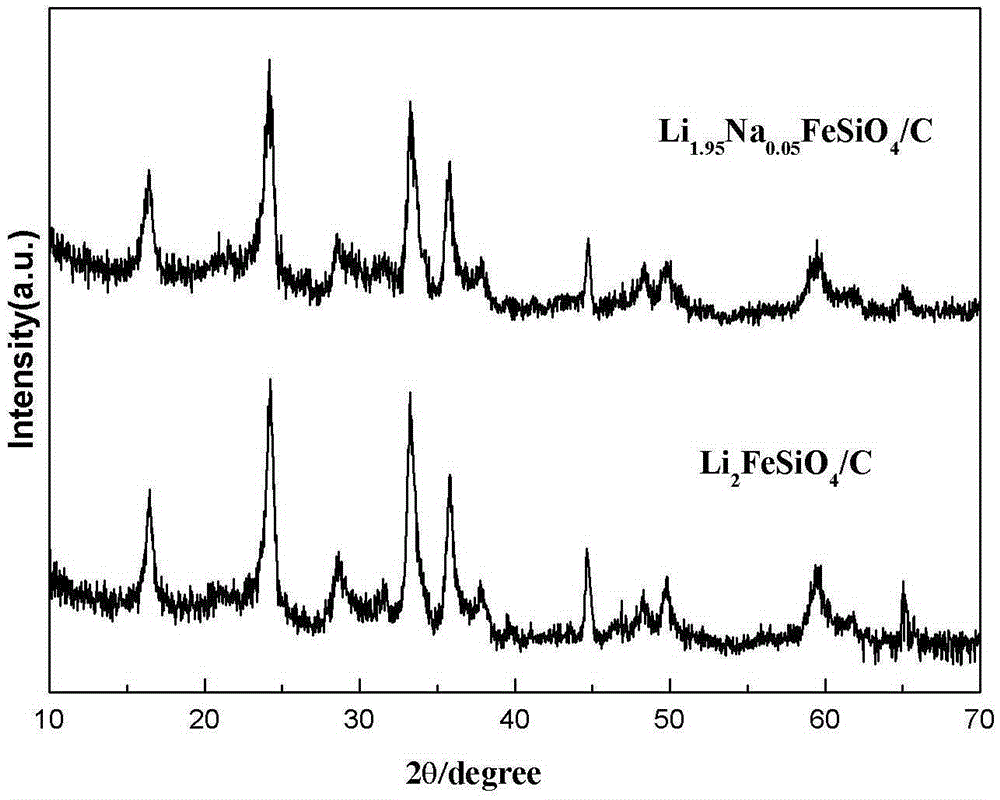
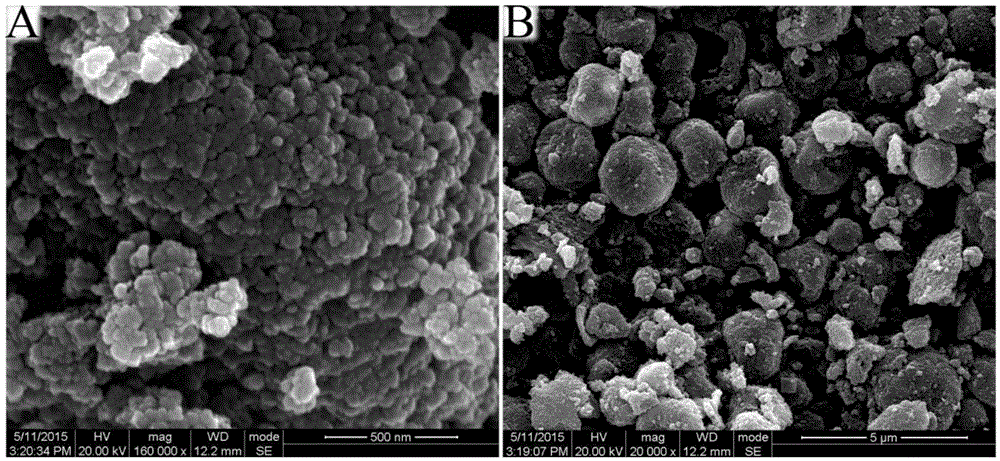
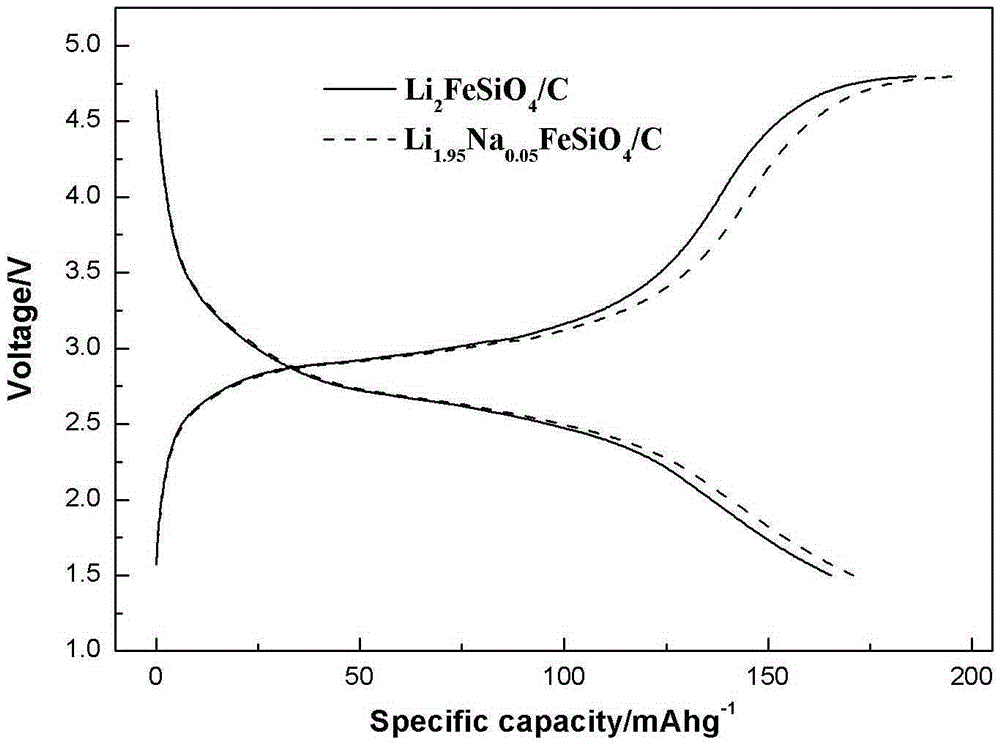
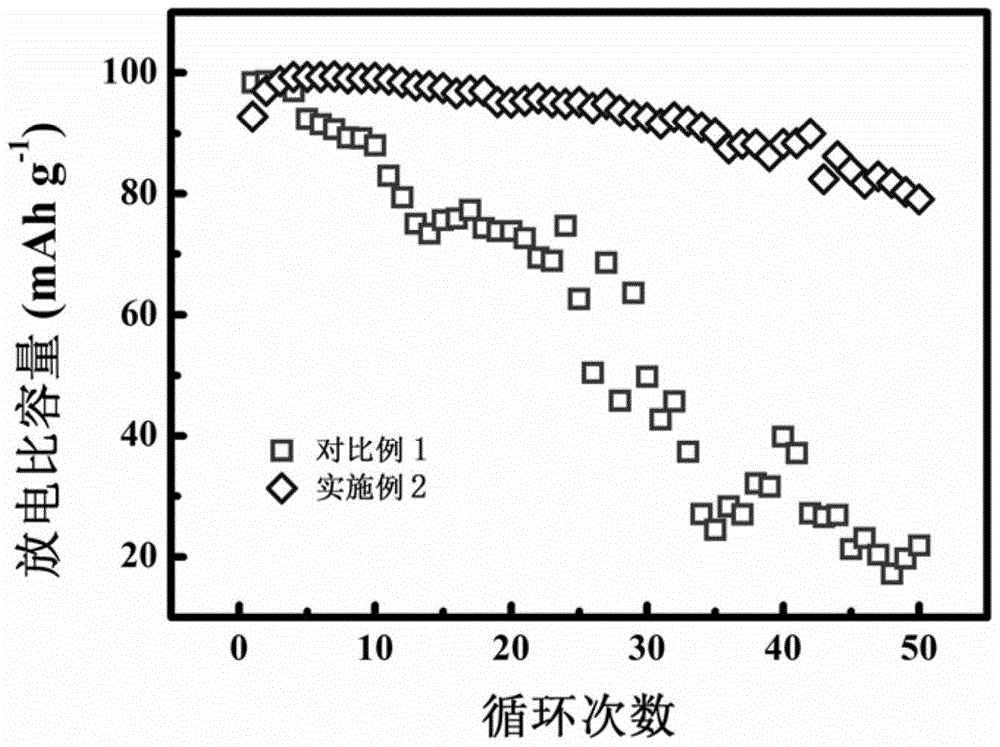
Method for preparing sodium-doped lithium ferrous silicate/carbon nano-micro structure composite cathode material
InactiveCN105375013AImprove Li+ migrationImprove electrochemical performanceCell electrodesSecondary cellsComposite cathodeLithium electrode
The invention relates to the technical field of lithium ion battery manufacturing, and especially a method for preparing a sodium-doped lithium ferrous silicate / carbon nano-micro structure composite cathode material. The method comprises the following steps: adding citric acid, lithium hydroxide, sodium hydroxide and ferrous oxalate into water, stirring and dissolving, performing oil bath and forming a deep green solution; and adding nanosilicon dioxide to obtain a sol, spray drying to obtain a precursor of the sodium-doped lithium ferrous silicate / carbon nano-micro structure composite cathode material, and calcinating in argon to obtain the sodium-doped lithium ferrous silicate / carbon nano-micro structure composite cathode material. The method for preparing the sodium-doped lithium ferrous silicate / carbon nano-micro structure composite cathode material provided by the invention is simple and safe in process, low in cost, and the obtained sodium-doped lithium ferrous silicate / carbon nano-micro structure composite cathode material is uniform in particle distribution and excellent in electrochemical performance.
Owner:CHANGZHOU UNIV
A kind of electrolyte solution and lithium ion secondary battery
ActiveCN103779607BImprove thermal stabilityIncreased high temperature capacity retentionSecondary cellsOrganic solventDecomposition
The invention discloses an electrolyte solution and a lithium-ion secondary battery. The electrolyte solution comprises an aprotic organic solvent, electrolyte lithium salt and an additive, wherein the additive is cyclic phosphoric anhydride; and the mass of the additive accounts for 0.01-10 percent of the total mass of the electrolyte solution. The lithium-ion secondary battery comprising the electrolyte solution can be subjected to electrochemical oxidation polymerization under the voltage of over 4.4V (vs.Li / Li+). By forming a polymer on the surface of a positive pole material, a good barrier is formed to cover strongly-oxidative active points of the positive pole material, and decomposition of a main solvent of the lithium-ion secondary battery is inhibited, so that the stability of the electrolyte solution under a high-voltage state and the high-voltage cycle and storage performances of a lithium-ion battery are improved.
Owner:CENT SOUTH UNIV
Carbon-coated mesoporous transition metal sulfide negative electrode material as well as preparation method and application thereof
InactiveCN112768656AStable structureElectrochemical stabilityCell electrodesSecondary cellsPyrrolidinonesMetallic sulfide
The invention provides a carbon-coated mesoporous transition metal sulfide negative electrode material as well as a preparation method and application thereof. The preparation method comprises the following steps of: mixing polyvinylpyrrolidone, a transition metal salt solution and a sulfur source, and performing heating until the reaction is finished to obtain a transition metal sulfide-containing solution; uniformly mixing the transition metal sulfide-containing solution with a carbon source, and performing a reaction to obtain a coke-coated transition metal sulfide; centrifuging the coke-coated transition metal sulfide, preforming cleaning, and drying to obtain solid transition metal sulfide powder; and calcining the solid transition metal sulfide powder in an inert atmosphere to obtain the carbon-coated mesoporous transition metal sulfide negative electrode material. The negative electrode material is formed by stacking a plurality of nanoscale primary particles, each primary particle is composed of an inner transition metal sulfide and an amorphous carbon coating layer coating the surface of the transition metal sulfide, and a mesoporous structure is formed among the primary particles. The material has high structural stability; and the preparation method is simple.
Owner:KUNMING UNIV OF SCI & TECH
Method for preparing aromatic monomer products through degradation of lignin
InactiveCN104892407AImprove thermal stabilityElectrochemical stabilityOrganic compound preparationCarbonyl compound preparationOrganic solventSulfate
The invention discloses a method for preparing aromatic monomer products through degradation of lignin; the method takes sulfate pulp lignin as a raw material, an ionic liquid as a reaction medium and oxygen as an oxidant, and adopts the following product processes: dissolving the lignin in the ionic liquid, carrying out oxygen oxidation degrading, extracting with an organic solvent, and concentrating to obtain the aromatic monomer products. The oxidation products are aromatic compound monomers mainly containing aromatic aldehydes, acids and ketones. Compared with a traditional water medium oxidation system strong alkali, the aromatic method not only overcomes pollution problems to the environment, but also has the product concentration significantly higher than that of a traditional strong alkali oxidation system. The yield of the monomer aromatic products adopting the reaction system is more than 3 times that of traditional strong alkali oxidation products.
Owner:SOUTH CHINA UNIV OF TECH
Silicon-containing sulfuric acid ester salt
ActiveCN109071570AElectrochemical stabilityReduce loadSilicon organic compoundsHybrid capacitor electrolytesMedicinal chemistryOrganosulfate
Provided is a silicon-containing sulfuric acid ester salt comprising a silicon-containing sulfuric acid ester anion represented by formula (1) and a cation selected from cations respectively represented by formulae (2) to (5).
Owner:NISSHINBO IND INC
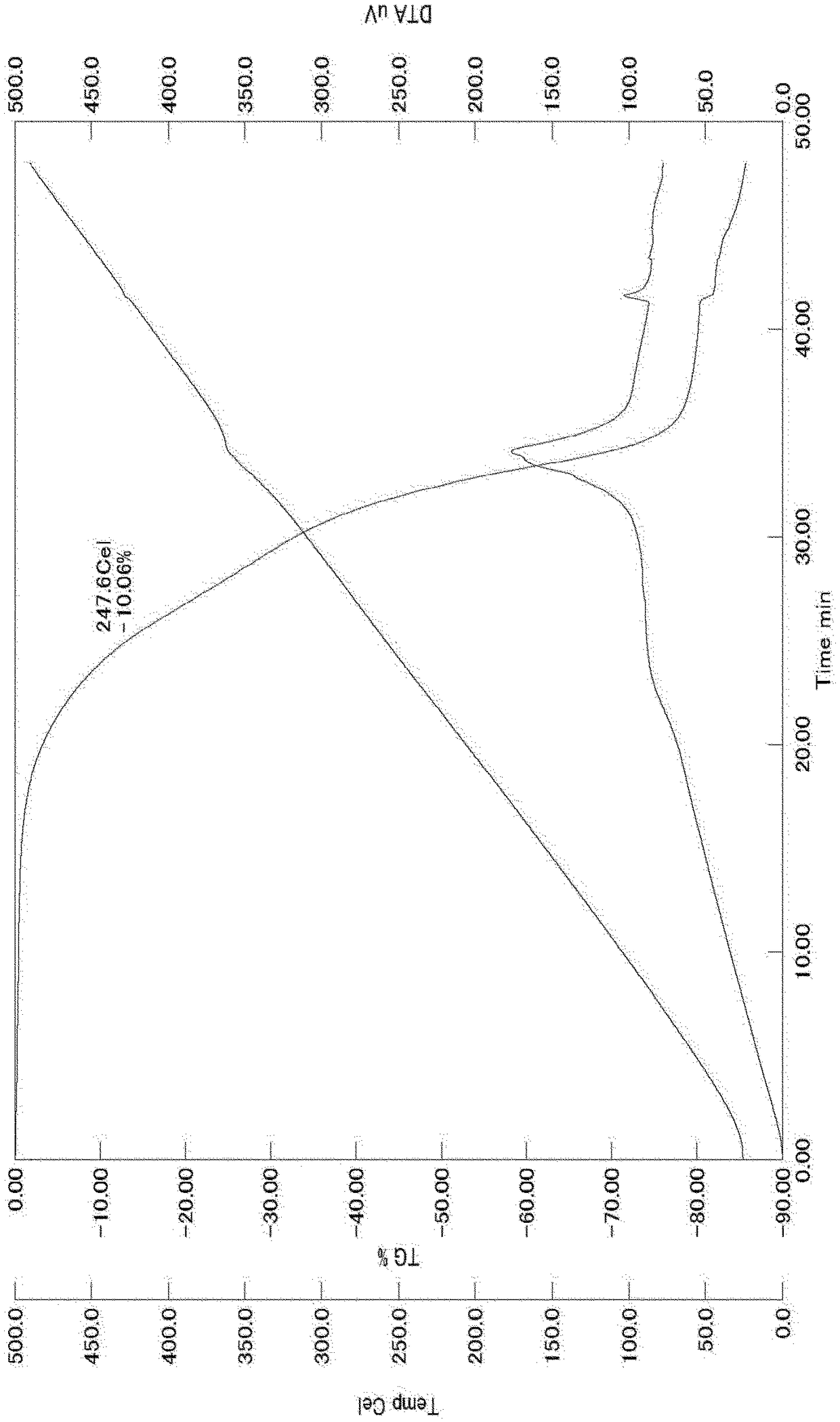
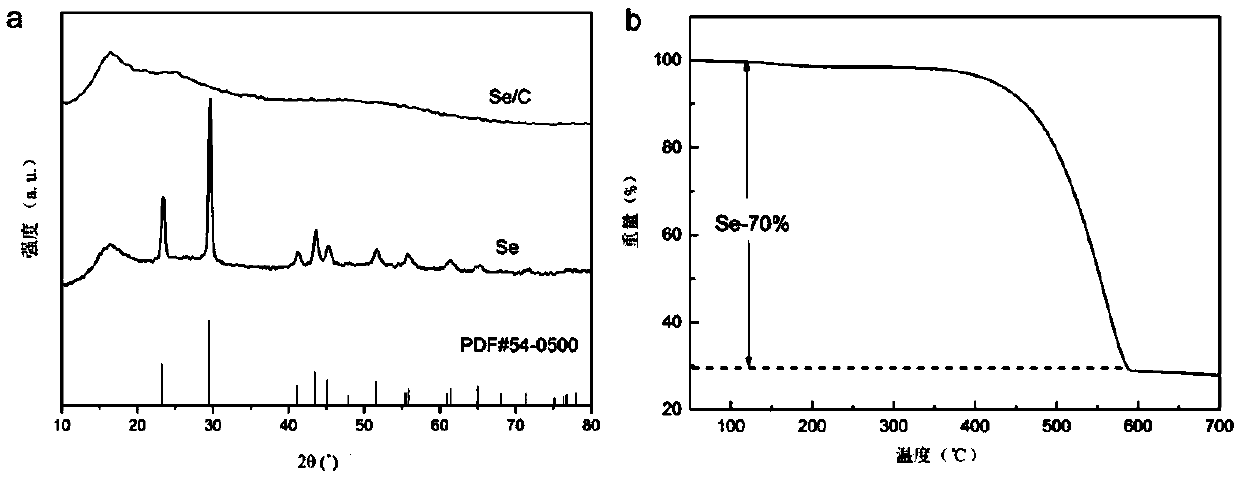
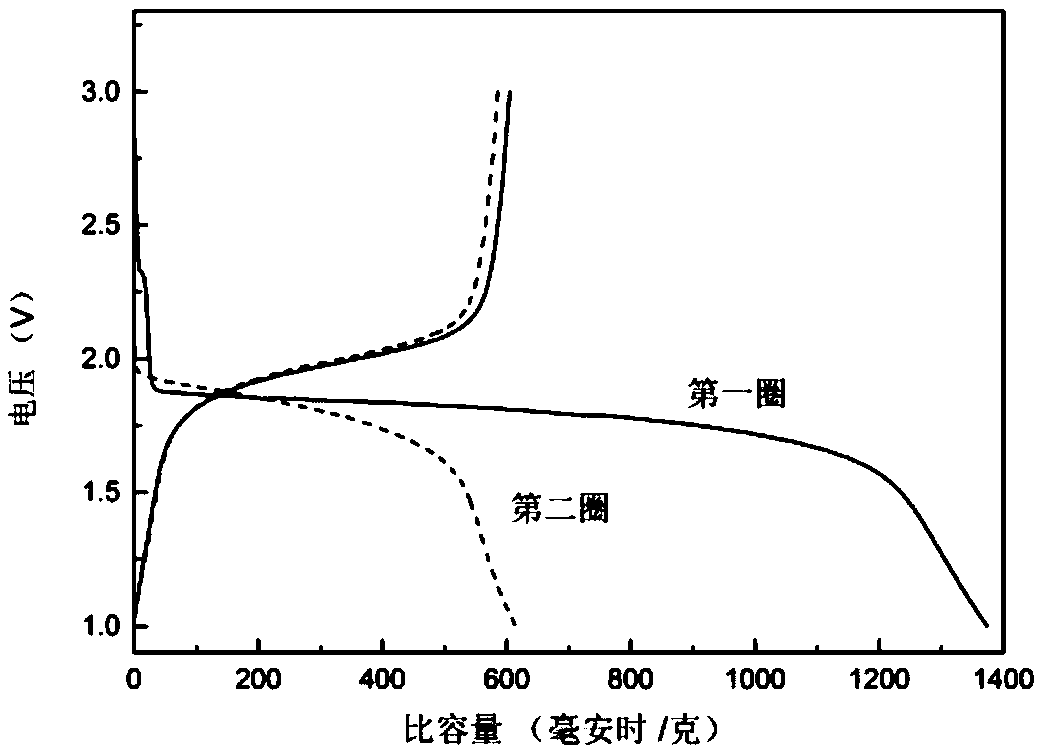
Carbon-selenium composite material, preparation method thereof, and application thereof in lithium-selenium battery
InactiveCN111384368AImprove electrochemical performanceEasy to preparePositive electrodesLi-accumulatorsElectrical batteryGraphite
The invention provides a carbon-selenium composite material, a preparation method thereof, and an application of the same in a lithium-selenium battery. Compared with the prior art, in the preparationmethod of the carbon-selenium composite material, the two-dimensional carbon material is wide in raw material source, simple and easy to obtain, so that the preparation method is simple, the practical degree is high, and the obtained carbon-selenium composite material shows excellent electrochemical performance. The two-dimensional carbon material is prepared by adopting a one-step hydrothermal reaction; the two-dimensional carbon material contains rich micro-mesoporous structures and has a high graphitization degree, so that when the two-dimensional carbon material and selenium are subjectedto low-temperature compounding and activation to obtain the carbon-selenium composite material, elemental selenium in the carbon-selenium composite material is uniformly loaded in micro-mesopores inthe surface of the two-dimensional carbon material, and the loading rate reaches up to 80%; as a result, the carbon-selenium composite material is used as a positive electrode material to be assembledto obtain the lithium-selenium battery with electrochemical stability.
Owner:HUNAN AGRICULTURAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com