Nonaqueous electrolytic liquid and lithium secondary battery employing same
A technology of non-aqueous electrolyte and secondary lithium battery, applied in the field of non-aqueous electrolyte, which can solve the problems of reduced conductivity and achieve high battery stability, excellent battery charge and discharge characteristics, high conductivity and electrochemical stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
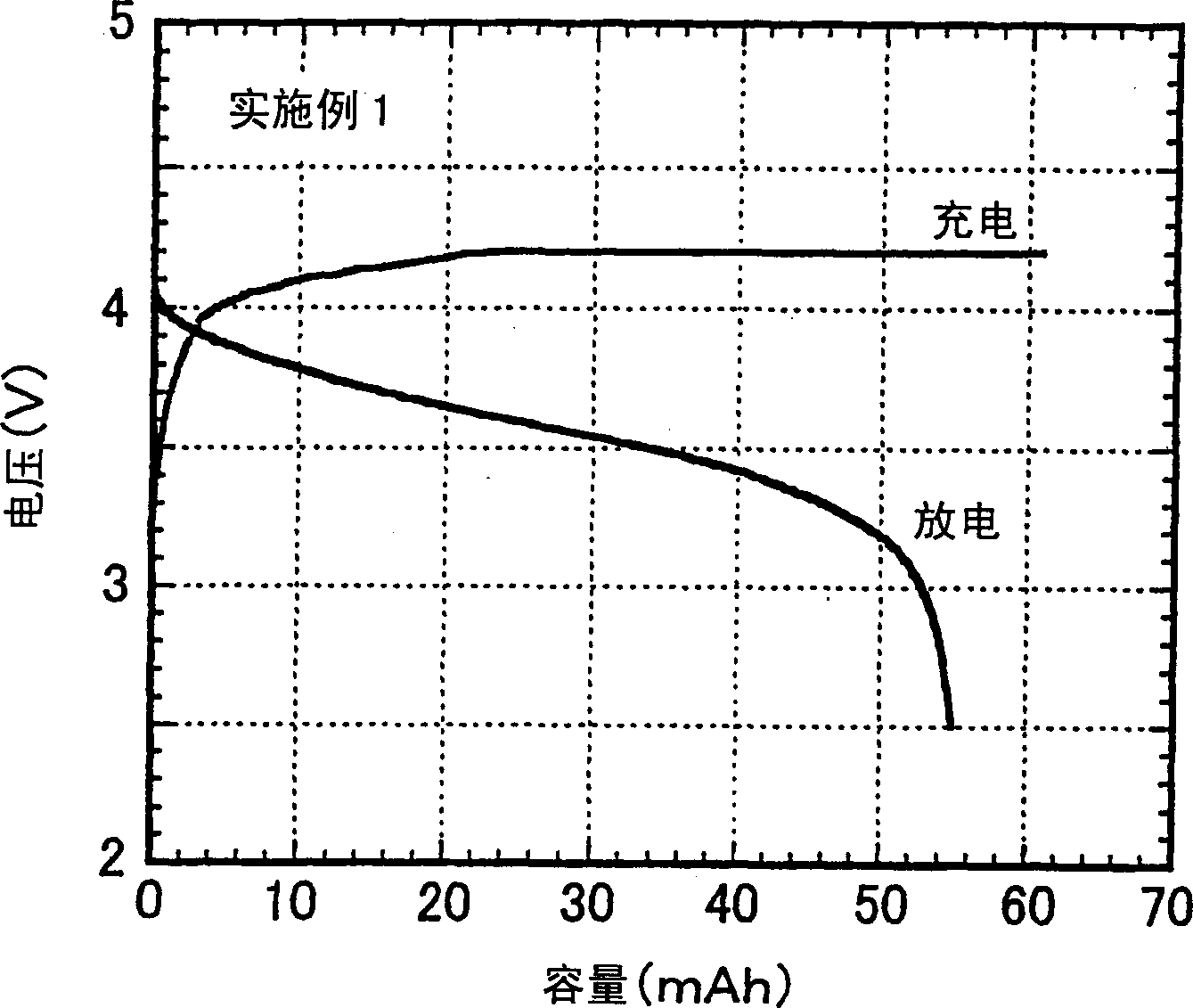
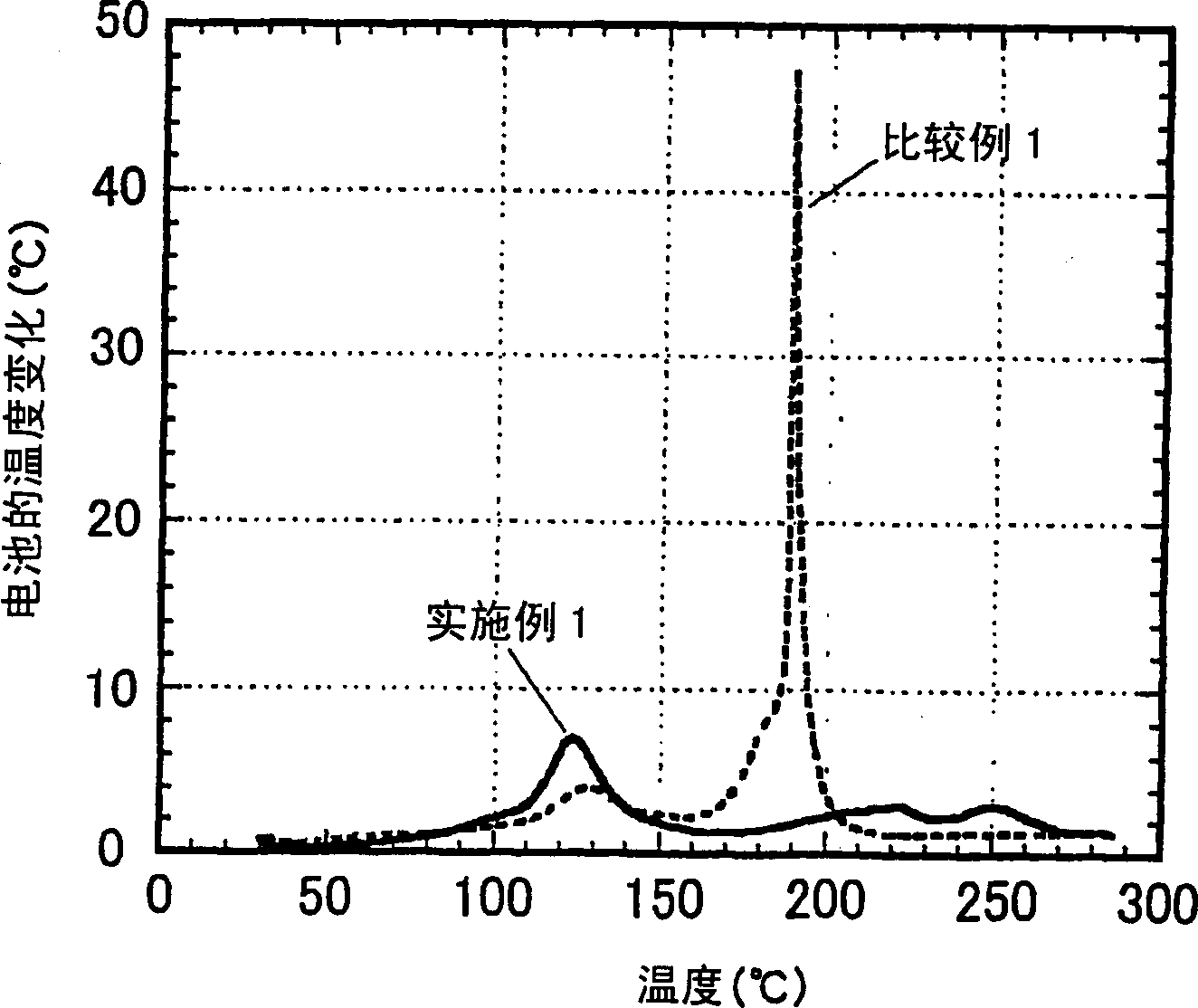
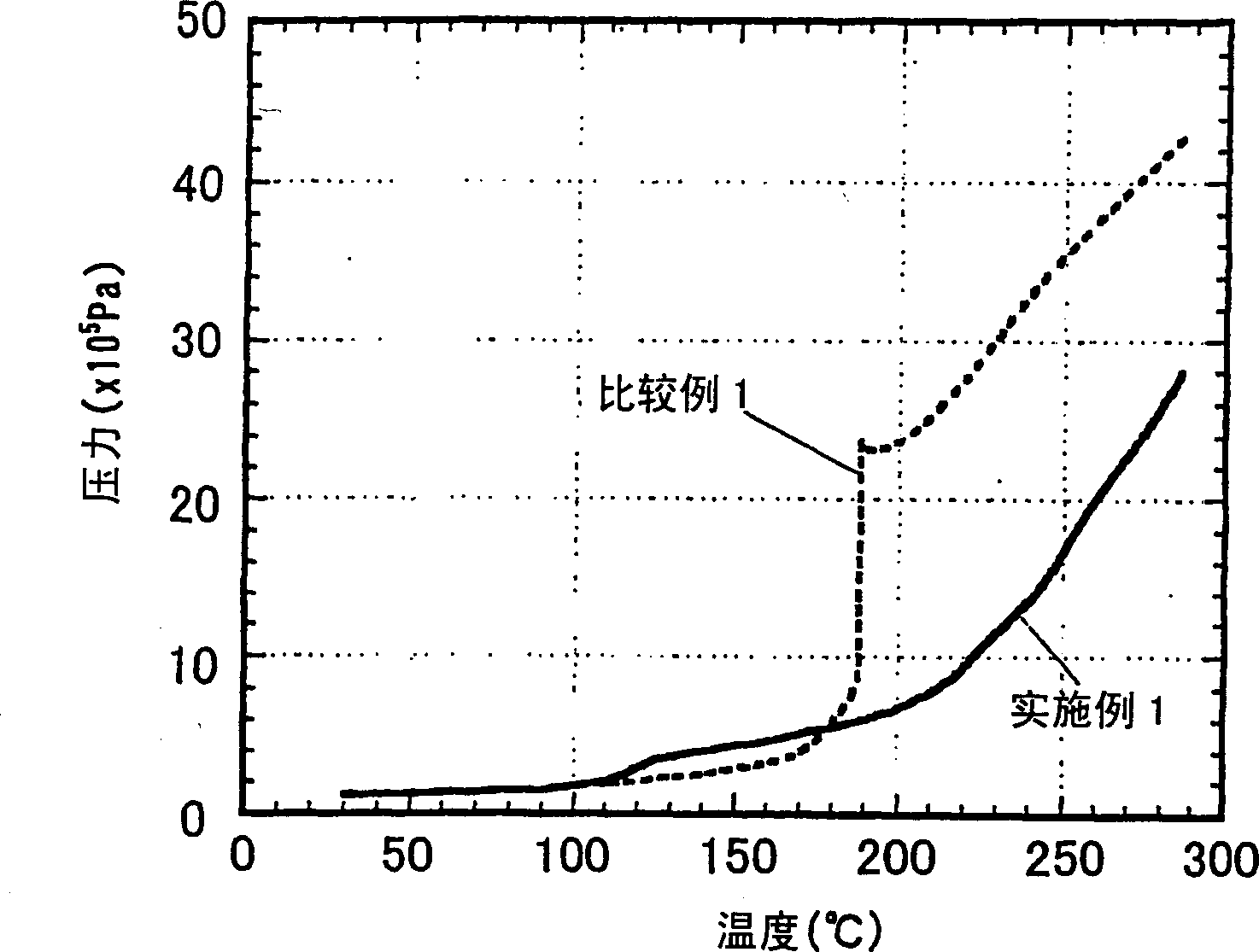
[0114] Hereinafter, the present invention will be explained in more detail with reference to examples of the non-aqueous electrolyte produced according to the present invention and the secondary battery produced therewith. As long as it does not exceed the scope of the present invention, the present invention is not restricted by these embodiments.
[0115] The performance of the non-aqueous electrolyte was evaluated according to the following methods.
[0116] (1) Self-extinguishing ability of electrolyte:
[0117] Immerse a long glass fiber filter paper with a width of 15 mm, a length of 300 mm and a thickness of 0.19 mm in a beaker containing the electrolyte for more than 10 minutes, so that the glass fiber filter paper is fully immersed in the electrolyte. Next, after removing the excess electrolyte adhering to the glass fiber filter paper with the edge of the beaker, clamp one end of the glass fiber filter paper with a clamp and hang it vertically. Use a flame like an igniter...
Embodiment 37-39
[0220] The nonaqueous electrolyte 2 or 4 of the present invention is used. That is, (a1) chain phosphate, and (B1) cyclic carboxylic acid ester and / or (B2) cyclic carbonate, in a non-aqueous solvent specified in the specified volume ratio shown in Table 7, the quantitative amount is dissolved. C1) Avinyl carbonate compound and / or (C2) vinylvinyl carbonate compound, and a solute lithium salt, modulated a solute concentration of 1 mol / dm 3 Example 37-39 of the nonaqueous electrolyte. The nonaqueous electrolyte of Comparative Examples 15, 16 is a nonaqueous electrolyte other than the present invention.
[0221] The negative electrode material of Example 37 was produced in the following method.
[0222] The average particle diameter D50 is 25 μm, the ABC / AB ratio of 0.25, and the petroleum bitumen (Mitsubishi Chemical System) is measured by the laser diffraction method, and the mixer is stirred in the atmosphere. Uniform. With a batch heating furnace, in an inert environment, ...
Embodiment 40-46
[0237] Non-aqueous electrolyte 3 is used. That is, using (a1) chain phosphate, and optionally (b1) cyclic carboxylic acid ester, and dissolving a predetermined amount of (c1) vinylene carbonate in a non-aqueous solvent with a predetermined volume ratio shown in Table 9 Compound and / or (c2) vinyl ethylene carbonate compound, and specified amount of (d1) cyclic amide compound, (d2) cyclic carbamate compound, (d3) cyclic heterocyclic compound, and solute lithium salt , Adjusted to a solute concentration of 1mol / dm 3 The non-aqueous electrolyte of Examples 40-46. The non-aqueous electrolyte solutions of Comparative Examples 16 and 17 are non-aqueous electrolyte solutions other than the present invention.
[0238] The anode materials of Examples 40 and 41 were prepared in the following method.
[0239] Graphite-based carbonaceous material (A) and petroleum-based pitch (manufactured by Mitsubishi Chemical Corporation) with an average particle size d50 of 25 μm and an ABC / AB ratio of 0.25 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com