Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
202 results about "Hydrazine derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
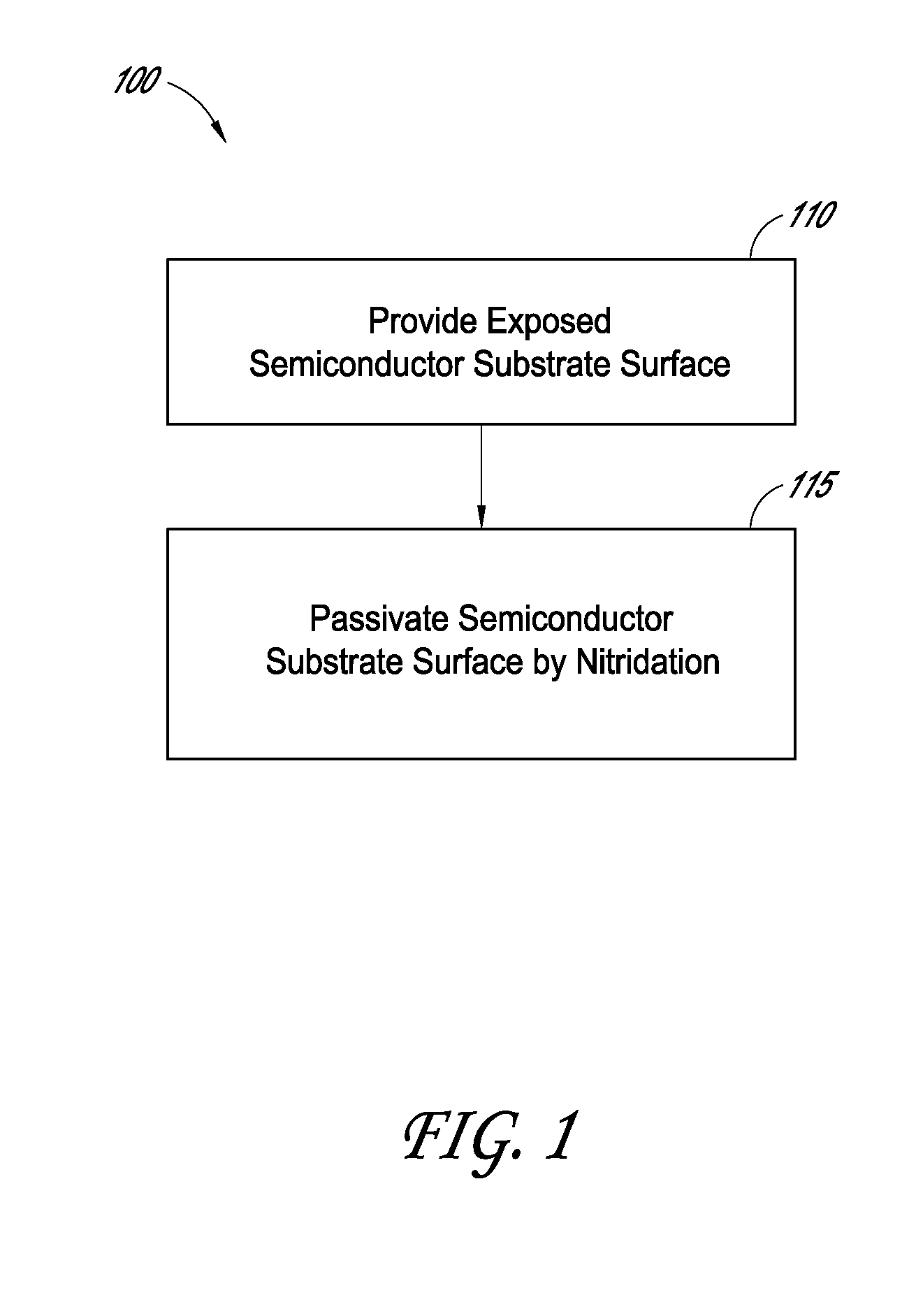
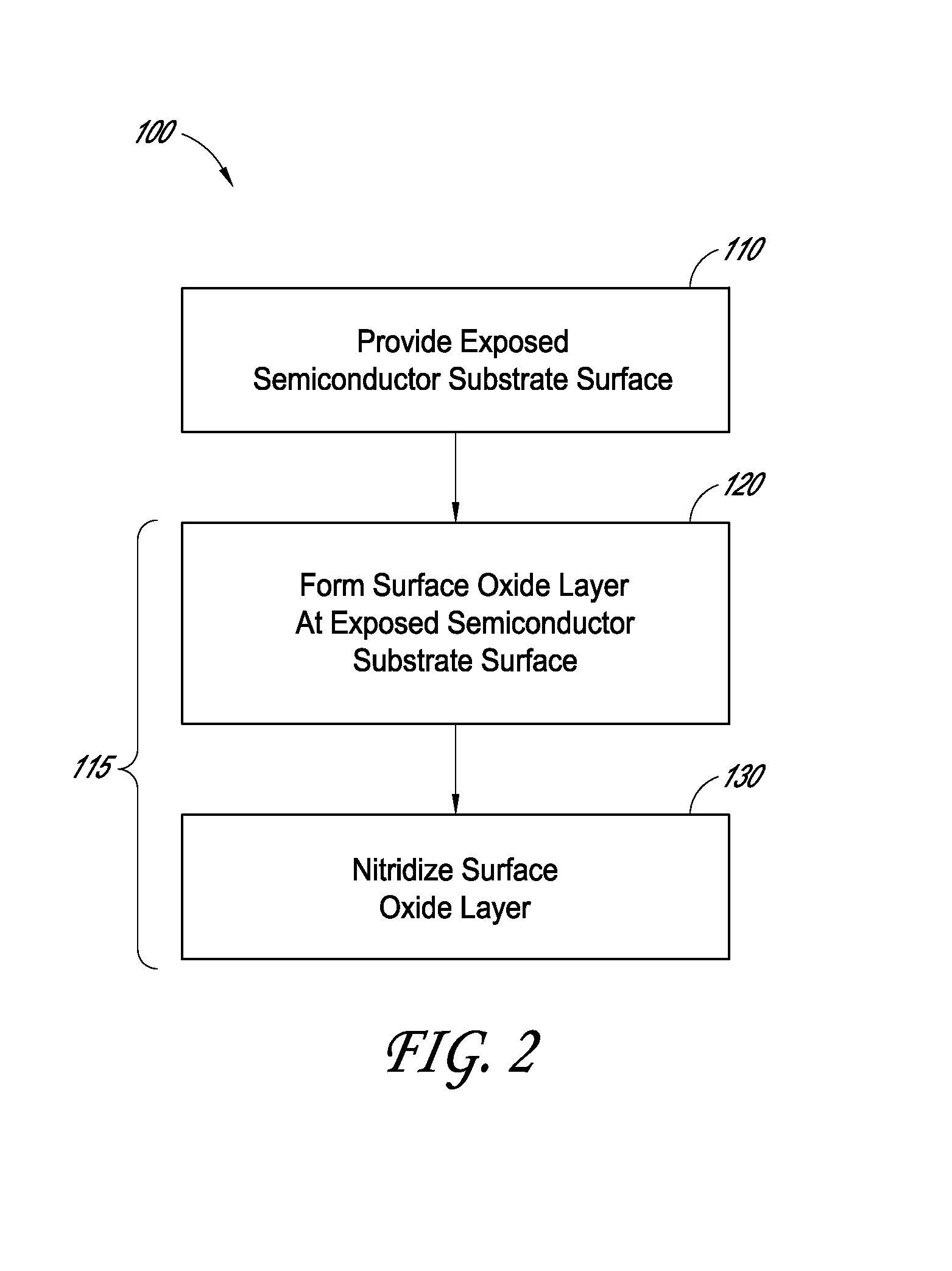
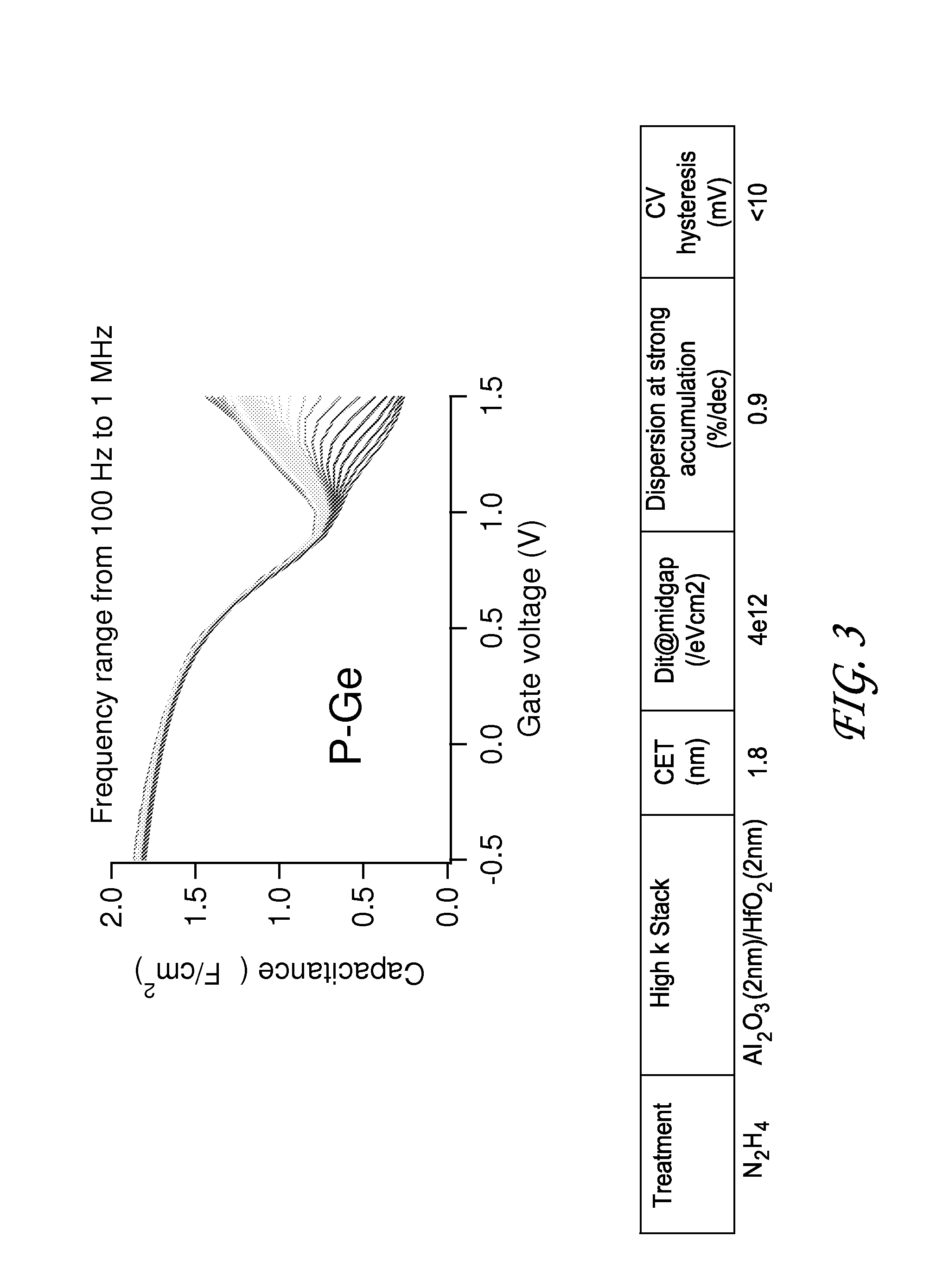
Methods for semiconductor passivation by nitridation
ActiveUS20160358772A1Improve mobilitySemiconductor/solid-state device detailsSolid-state devicesNitrogenAtomic layer deposition
In some embodiments, a semiconductor surface having a high mobility semiconductor may be effectively passivated by nitridation, preferably using hydrazine, a hydrazine derivative, or a combination thereof. The surface may be the semiconductor surface of a transistor channel region. In some embodiments, a semiconductor surface oxide layer is formed at the semiconductor surface and the passivation is accomplished by forming a semiconductor oxynitride layer at the surface, with the nitridation contributing nitrogen to the surface oxide to form the oxynitride layer. The semiconductor oxide layer may be deposited by atomic layer deposition (ALD) and the nitridation may also be conducted as part of the ALD.
Owner:ASM IP HLDG BV
PVC foam wood/plastic composite material and manufacturing method thereof
The invention discloses a PVC foam wood / plastic composite material and relates to the technical field of composite materials. The PVC foam wood / plastic composite material is made from the following raw materials in parts by weight: 20-70 parts of PVC resin powder, 0-55 parts of wood flour, 5-40 parts of calcium carbonate powder, 0.1-0.5 part of sodium bicarbonate, 0.3-0.6 part of an azo-compound or hydrazine derivative, semicarbazide compound or nitroso-compound, 2-10 parts of a foaming regulator, 1.5-6 parts of a composite stabilizer, 0.7-2.1 parts of a lubricant, 0.5-1.2 part of soybean oil, 2.5-5.5 parts of an impact modifier, and 0.5-3 parts of a processing agent. The PVC foam wood / plastic composite material provided by the invention has the advantages of strong chemical stability, high strength, resistance to acid / alkaline corrosion, resistance to water seepage, flame retardancy and low cost.
Owner:山东宜群木塑科技有限公司
Composite of aluminum alloy and resin and production method therefor
InactiveUS20080127479A1Excellent mechanical propertiesGood physical propertiesMetal rolling stand detailsMetallic material coating processesMetal frameworkWater soluble
The present invention is useful for achieving a reduction in weight and for attaining increased strength in not only electronic devices and domestic electric devices but also various parts and structures. As a pretreatment, a rib (3) is dipped in an aqueous solution of ammonia, hydrazine, a hydrazine derivative, or a water-soluble amine compound. A metal frame (2) is inserted into an injection mold for forming ribs (3) by injection molding. A thermoplastic resin composition is injected to the surface of the metal frame (2) by injection molding to form ribs (3). In the housing of a casing cover (1) thus formed, the metal frame (2) and the ribs (3) made of the thermoplastic resin composition are integrally bonded together. Thus, the housing improves strength and external appearance. Moreover, a complicated configuration and structure can be formed in the housing.
Owner:TAISEI PLAS CO LTD
Surface-treated steel sheet excellent in resistance to white rust and method for production thereof
InactiveUS20050147832A1Improve corrosion resistanceEconomically and stably producingHot-dipping/immersion processesSynthetic resin layered productsEpoxyO-Phosphoric Acid
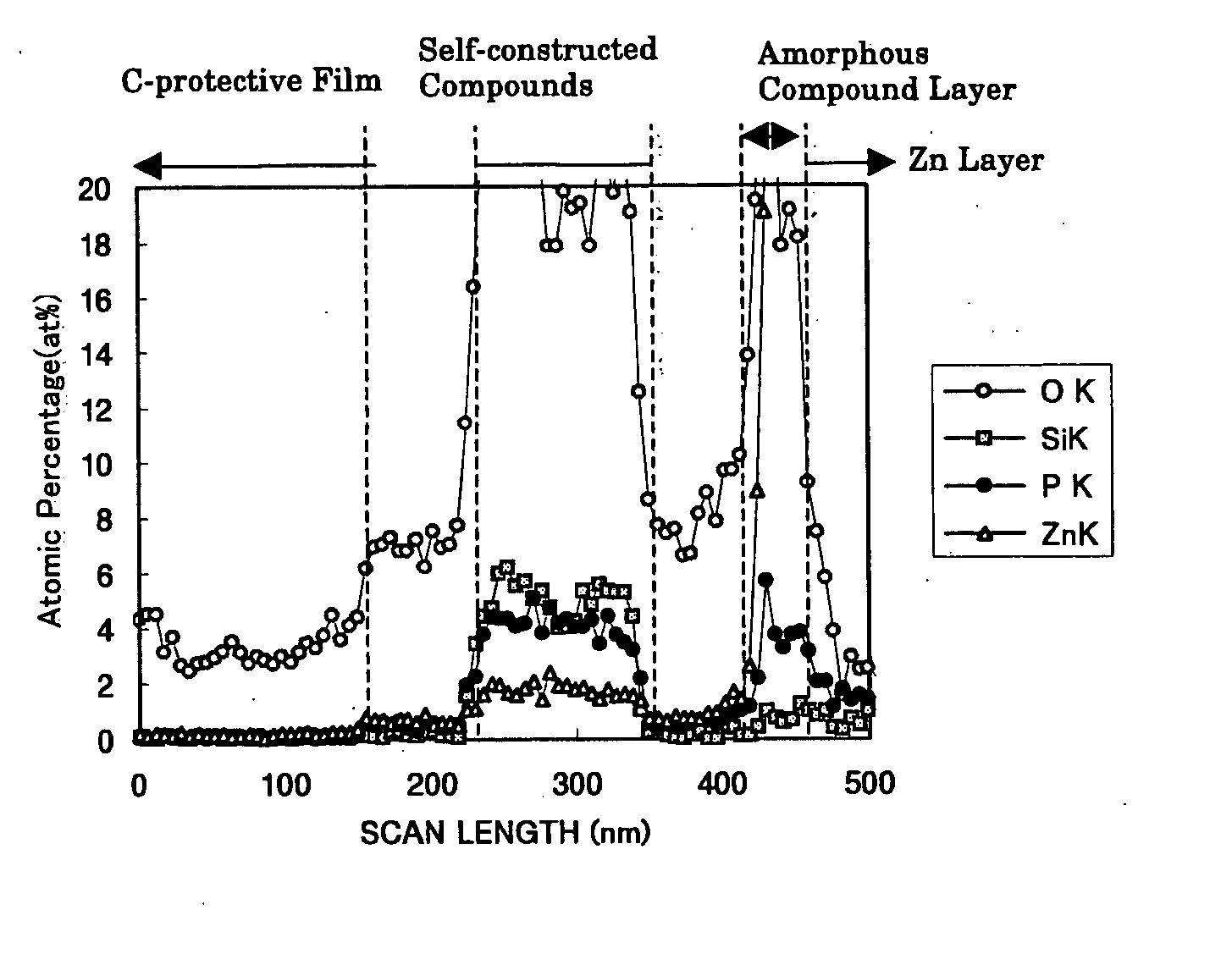
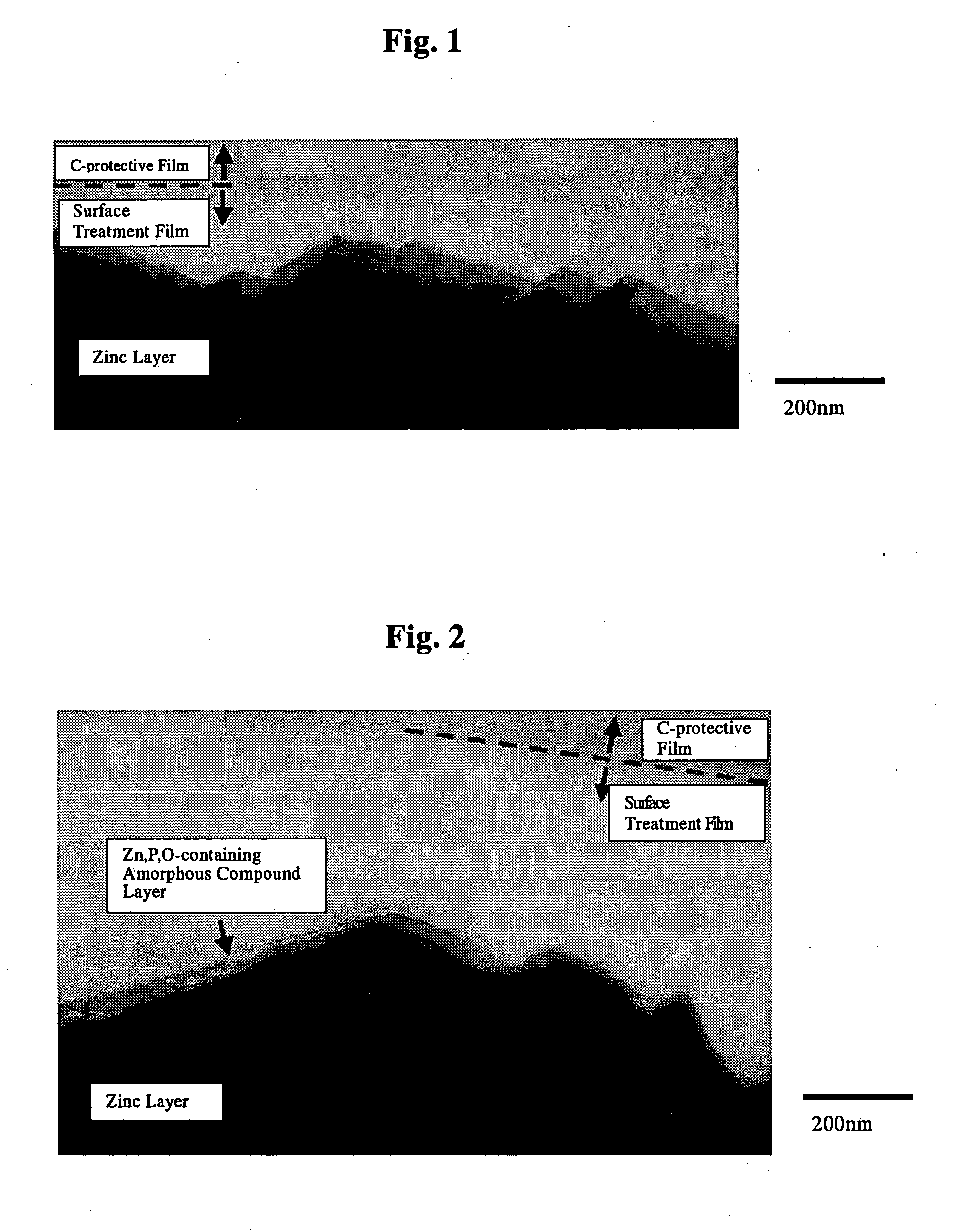
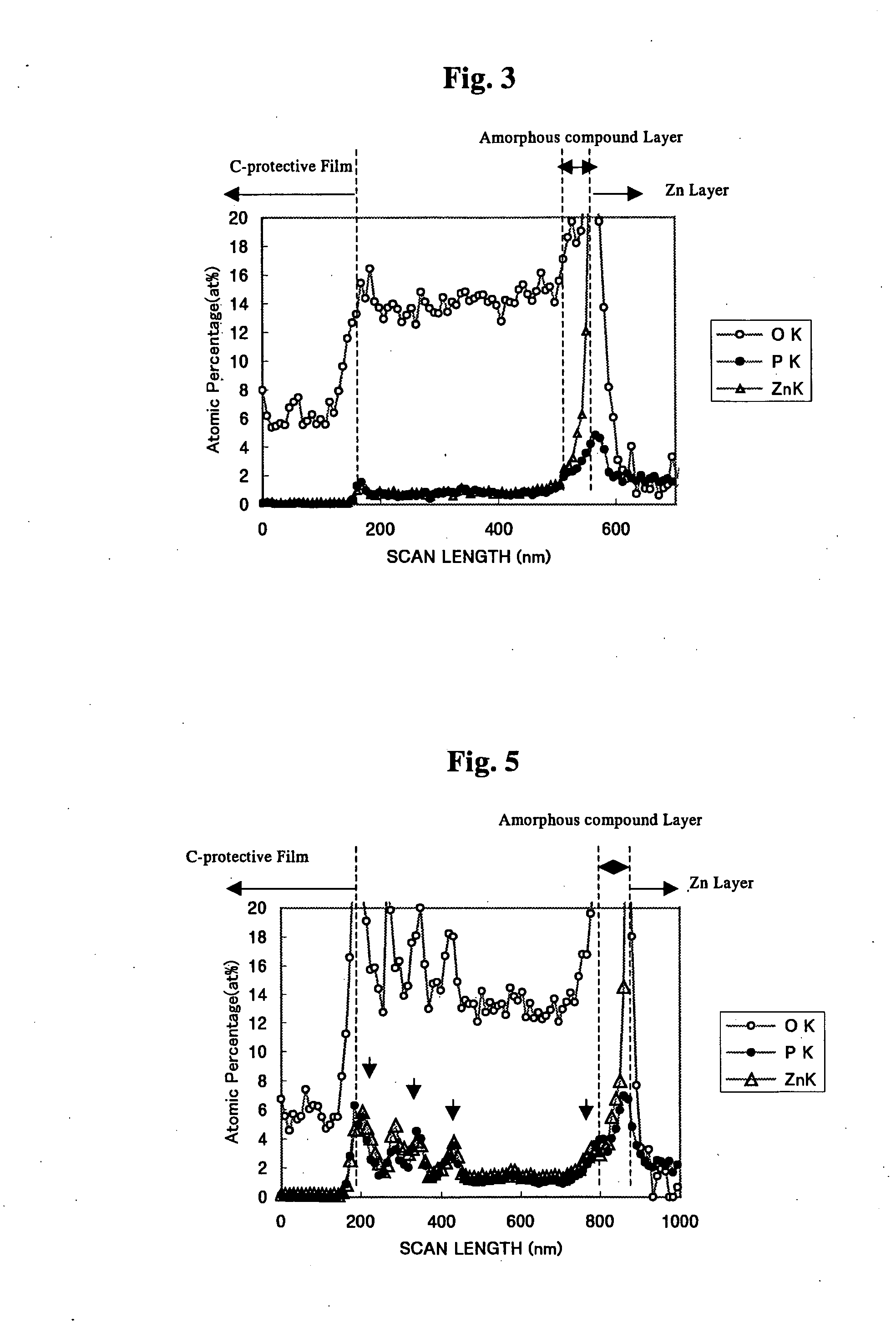
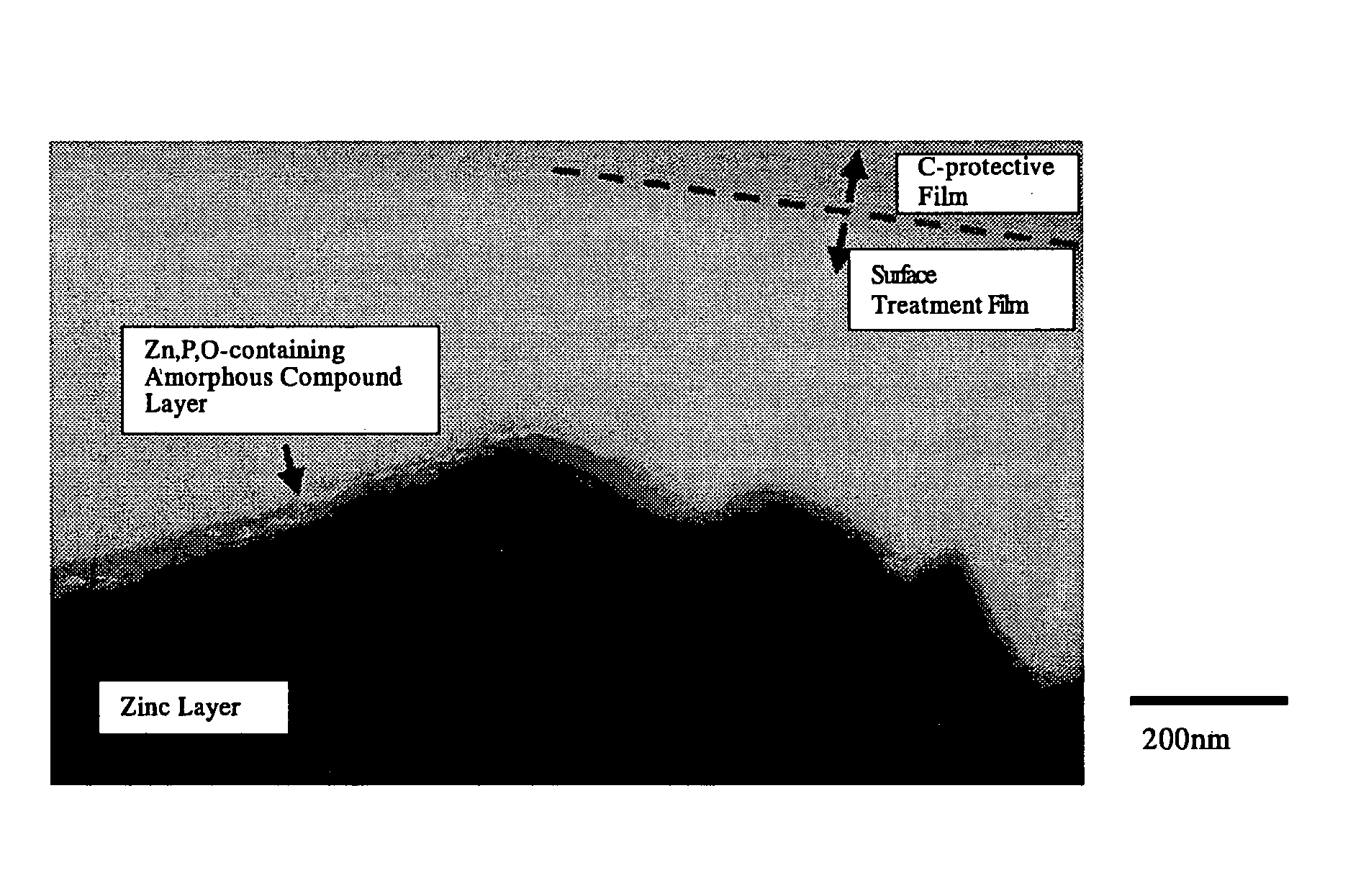
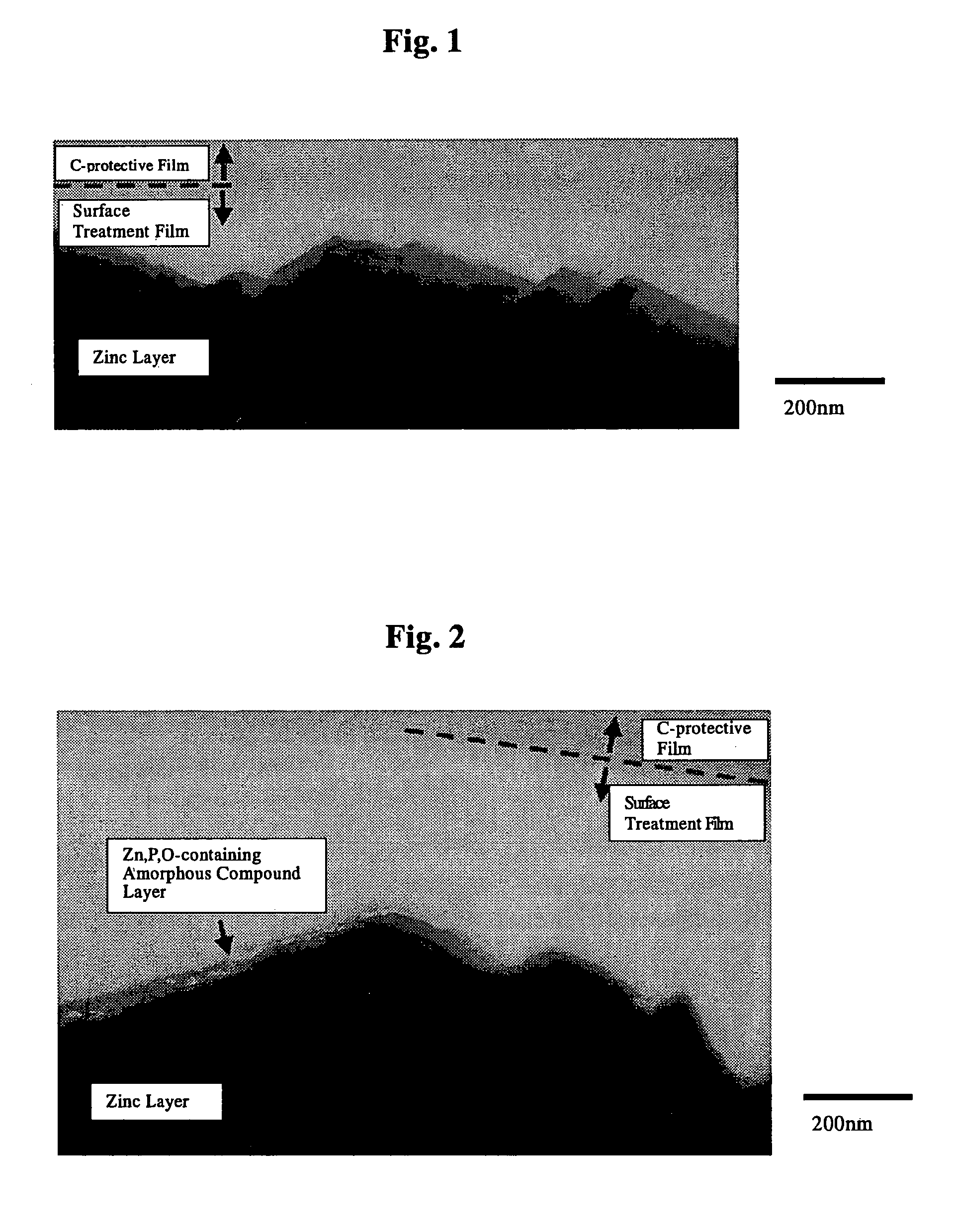
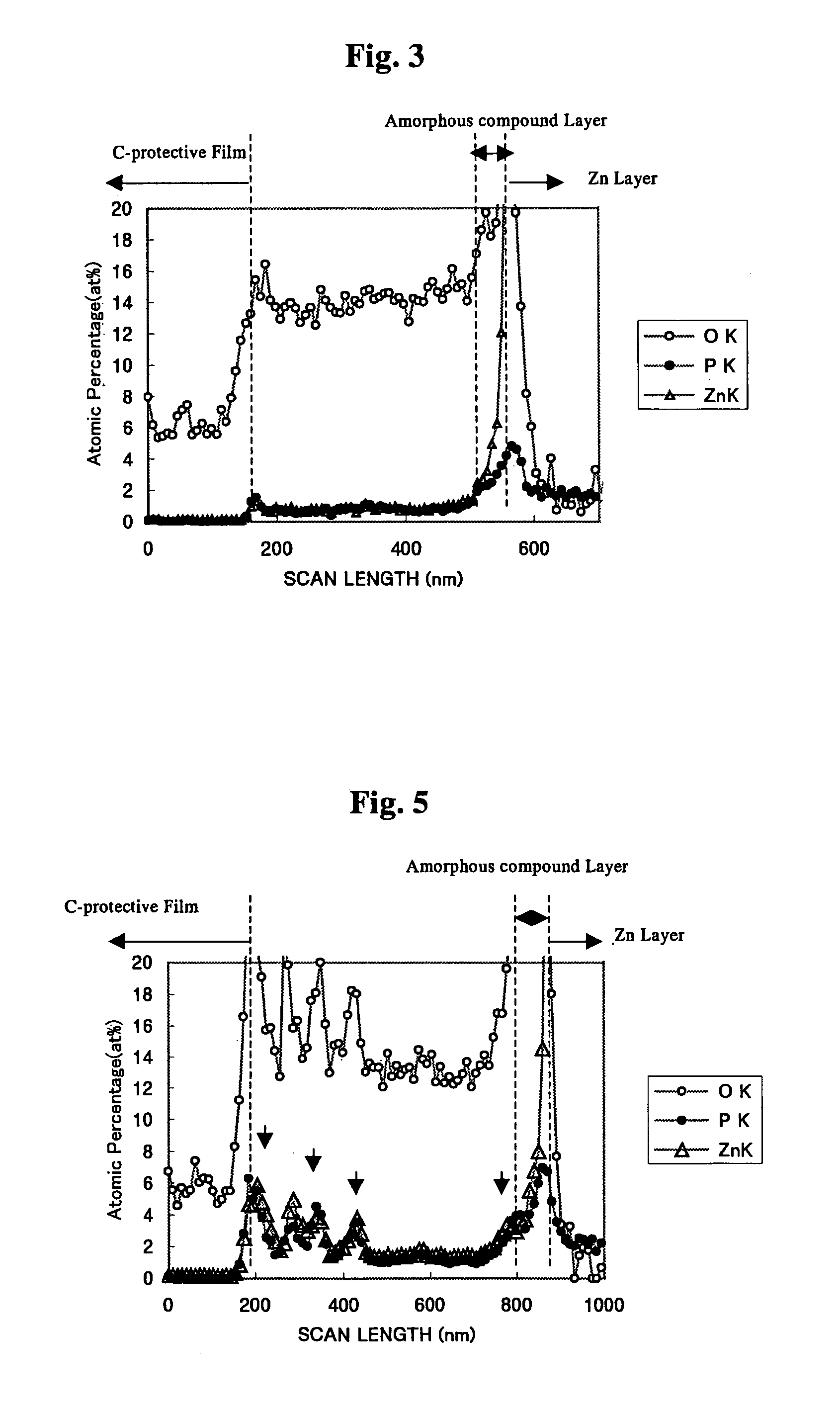
The invention is to provide a surface-treated steel sheet of good white rust resistance with no chromium in its surface treatment film, and it has realized a surface-treated steel sheet of higher-level corrosion resistance by forming a reaction layer of plating metal with a barrier layer effective for anticorrosion not in a two-layered film but in a single-layered film. The surface-treated steel sheet is fabricated by applying a surface-treating composition that contains: (a) a water-dispersible resin and / or a water-soluble resin obtained through reaction of an epoxy group-containing resin (A) with an active hydrogen-containing compound (B) a part or all of which is an active hydrogen-containing hydrazine derivative and preferably with a hydrophilic organic component (C), (b) a silane coupling agent, (c) a phosphoric acid and / or a hexafluoro-metal acid, and (d) preferably an aqueous dispersion of an urethane resin, to a zinc-plated or aluminium-plated steel sheet followed by drying it to form thereon a surface treatment film having a thickness of from 0.02 to 5 μm. Regarding its film structure, the surface treatment film comprises an amorphous compound layer containing P, Zn and / or Al, and O, and an overlying organic resin matrix layer where the matrix is a hydrazine derivative-modified epoxy group-containing resin or the epoxy group-containing resin and an urethane resin.
Owner:JFE STEEL CORP +1
Selective barrier slurry for chemical mechanical polishing
ActiveUS20060068589A1Remove obstaclesOther chemical processesDecorative surface effectsNonferrous metalCompound (substance)
The polishing solution is useful for removing a barrier from a semiconductor substrate. The solution contains by weight percent 0.001 to 25 oxidizer, 0.0001 to 5 anionic surfactant, 0 to 15 inhibitor for a nonferrous metal, 0 to 40 abrasive, 0 to 20 complexing agent for the nonferrous metal, 0.01 to 12 barrier removal agent selected from imine derivative compounds, hydrazine derivative compounds and mixtures thereof, and water; and the solution has an acidic pH.
Owner:ROHM & HAAS ELECTRONICS MATERIALS CMP HLDG INC
Aqueous resin composition having gas barrier properties and laminated film using the same
InactiveUS20070031679A1Excellent gas barrier performanceSynthetic resin layered productsPolyurea/polyurethane coatingsAlkanePolymer science
An aqueous resin composition with gas barrier properties contains (i) polyurethane resin having aurethane group and a urea group in a total concentration of 25 to 60% by weight and having a acid value of 5 to 100 mgKOH / g, (ii) a swelling inorganic layered compound (e.g., a water-swelling mica, and a montmorillonite), and (iii) a polyamine compound having an amine value of 100 to 1900 mgKOH / g. The polyurethane resin (i) is obtained by a reaction of (A) an aromatic, araliphatic or alicyclic polyisocyanate, (B) a polyhydroxyalkanecarboxylic acid, and at least one component selected from (C) a C2-8alkylene glycol and (D) a chain-extension agent (e.g., diamine, hydrazine and a hydrazine derivative), and neutralized with a neutralizing agent. The proportion of the acid group of the polyurethane resin (i) relative to the basic nitrogen atom of the polyamine compound (iii) is 10 / 1 to 1 / 5 as the equivalent ratio. A laminated film with high gas barrier properties is obtainable by coating a base film with the aqueous resin composition. The present invention provides an aqueous resin composition with excellent gas barrier properties, and a laminated film using the same.
Owner:MITSUI CHEM INC
High-rate barrier polishing composition
InactiveUS7300480B2Pigmenting treatmentSoap detergents with inorganic compounding agentsHigh rateNonferrous metal
The solution is useful for removing a barrier material from a semiconductor substrate. The solution comprises, by weight percent, 0.01 to 25 oxidizer, 0 to 15 inhibitor for a nonferrous metal, 0 to 15 abrasive, 0 to 20 complexing agent for the nonferrous metal, 0.01 to 12 barrier removal agent and balance water. The barrier removal agent is selected from the group comprising imine derivative compounds, hydrazine derivative compounds and mixtures thereof.
Owner:ROHM & HAAS ELECTRONICS MATERIALS CMP HLDG INC
Arylsalicylaldehyde-diphenyl-azine hydrazine compound as well as preparation and application
ActiveCN105541660ASmall steric hindranceEasy to see throughCarboxylic acid nitrile preparationOrganic compound preparationArylHydrogen
The invention belongs to the technical field of analysis and detection materials, and discloses an arylsalicylaldehyde-diphenyl-azine hydrazine compound as well as preparation and application. The compound has a structural general formula of formula (1) shown in the description, wherein Ar represents an aryl group or a derivative structure thereof, and substituent groups R1 to R10 are respectively selected from hydrogen, alkyl, alkoxy, aryl group and relative derivative structures thereof. The preparation method of the compound comprises the following steps: heating diphenyl hydrazine derivative and arylsalicylaldehyde to be 30 to 90 DEG C in a solvent for 6 to 12 hours' reaction, and separating and purifying reaction products, so as to obtain the arylsalicylaldehyde-diphenyl-azine hydrazine compound. The compound provided by the invention shows strong characters of a fluorescence probe, and also shows stronger selective recognition capability to substructures and metal ions in a cell, thereby achieving great high development value.
Owner:SOUTH CHINA UNIV OF TECH
Ectoparasitic insect pest controllers for animals and their usage
InactiveUS6903237B2Eliminate the effects ofBiocideOrganic chemistryInsect pestBULK ACTIVE INGREDIENT
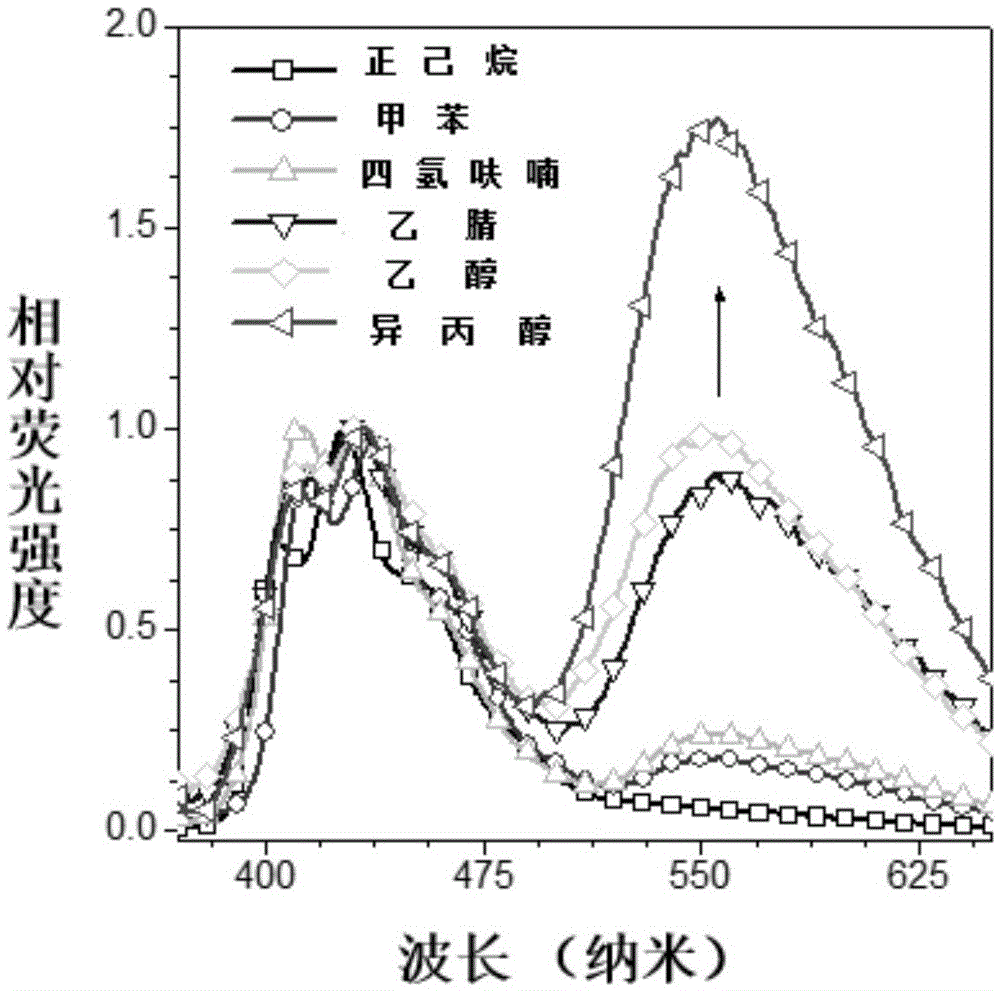
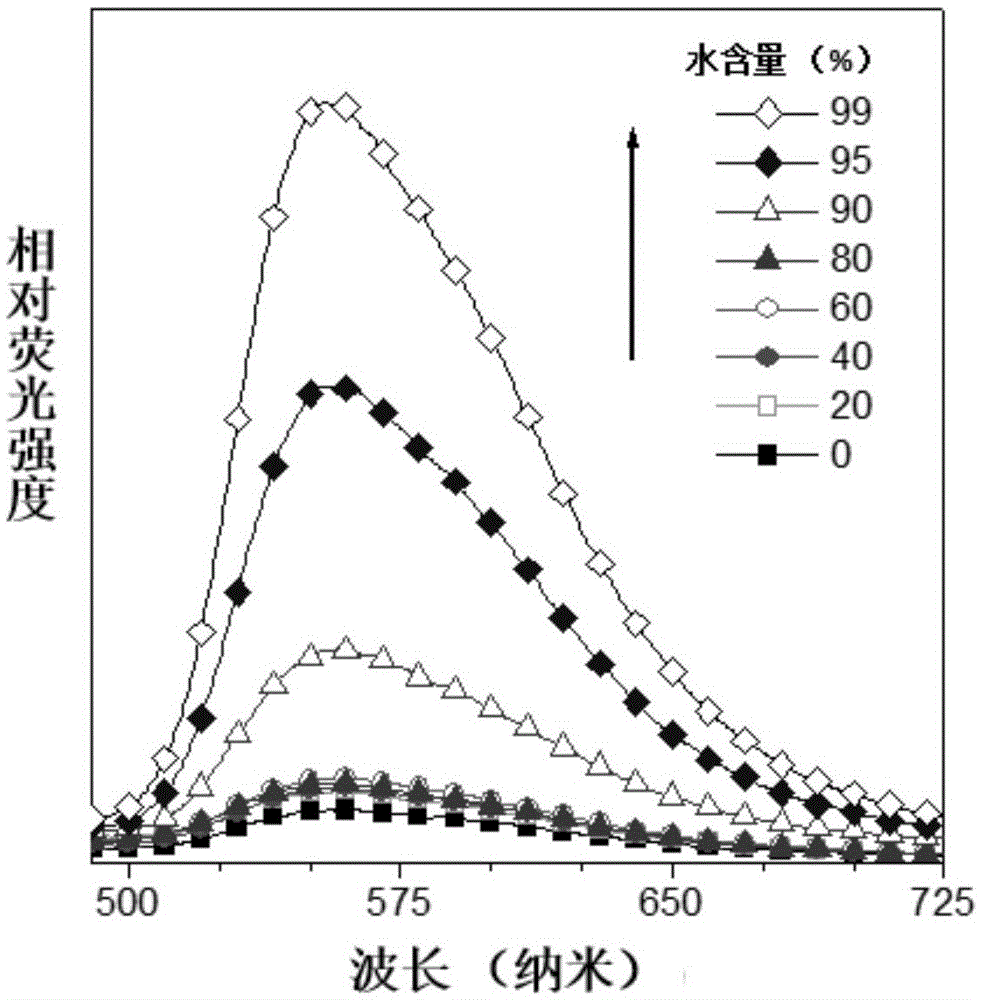
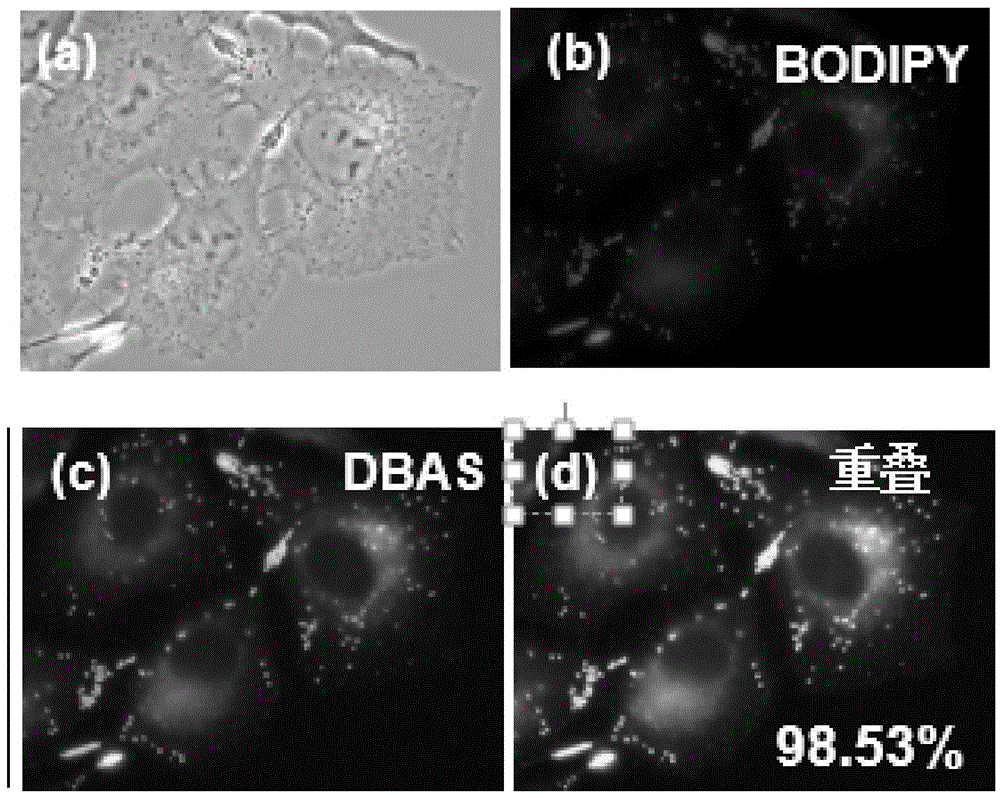
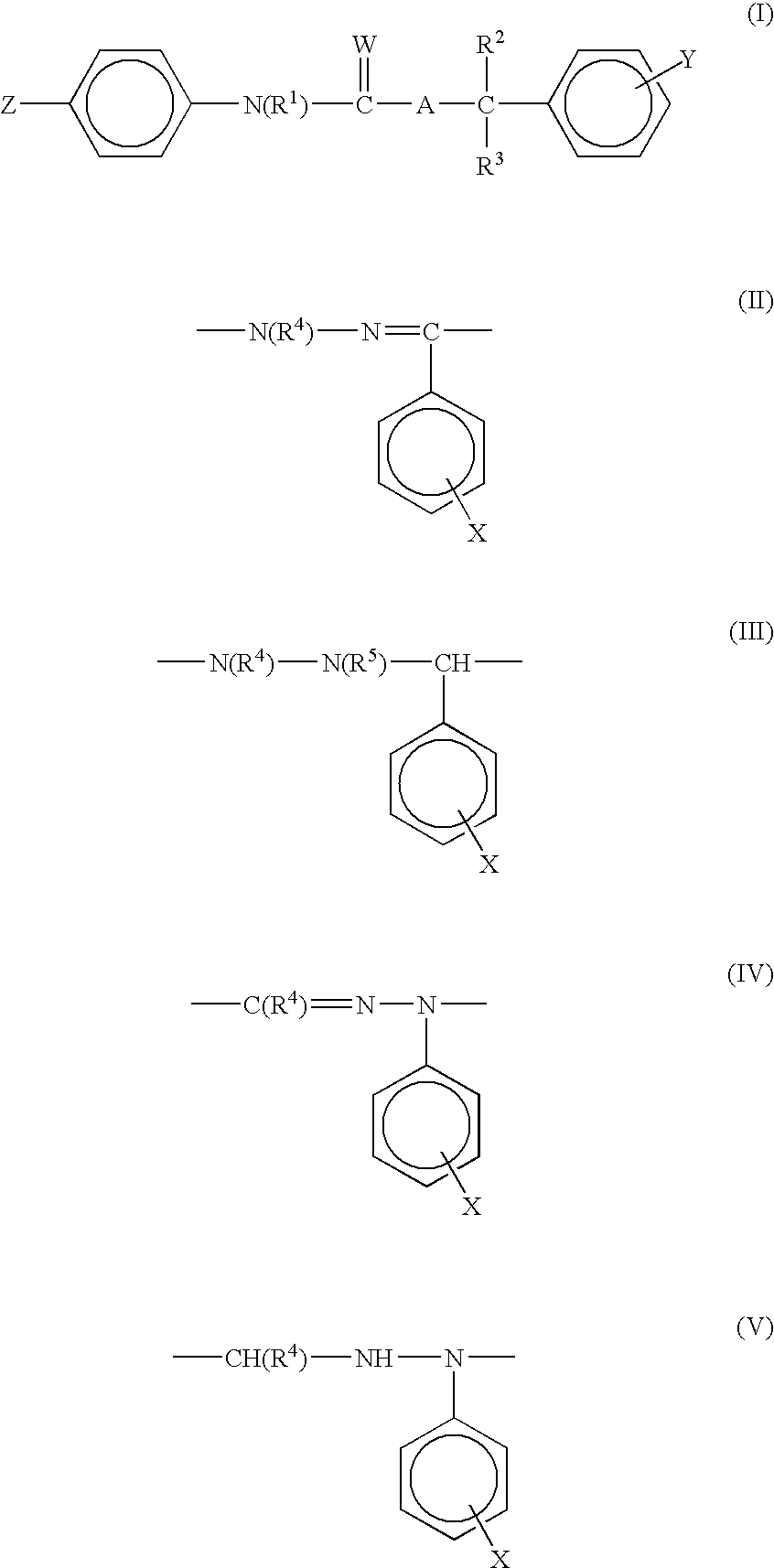
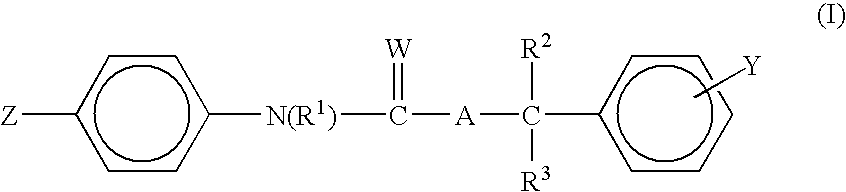
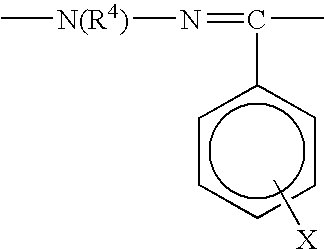
Ectoparasitic insect pest controllers for animals, containing hydrazine derivatives of the general formula (I) as the active ingredient, and methods for application of the same: (I) [wherein A is (II), (III), (IV), (V) (wherein R4 and R5 are each H, C1-6 alkyl, or the like; and X is H, or one to five substituents selected from among halogeno and optionally halogenated C1-6 alkyl groups); R1 is H or C1-6 alkyl; R2 and R3 are each H, OH, C1-6 alkyl, phenylcarbonyl, or the like; Y is H, or one to five substituents selected from among halogeno, nitro, and cyano; Z is halogeno, cyano, C1-6 alkyl, or the like; and W is O or S]. The insect pest controllers exert remarkable controlling effects on parasitic insect pests harmful to domestic or pet animals, e.g. fleas, lice, ticks.
Owner:NIHON NOHYAKU CO LTD
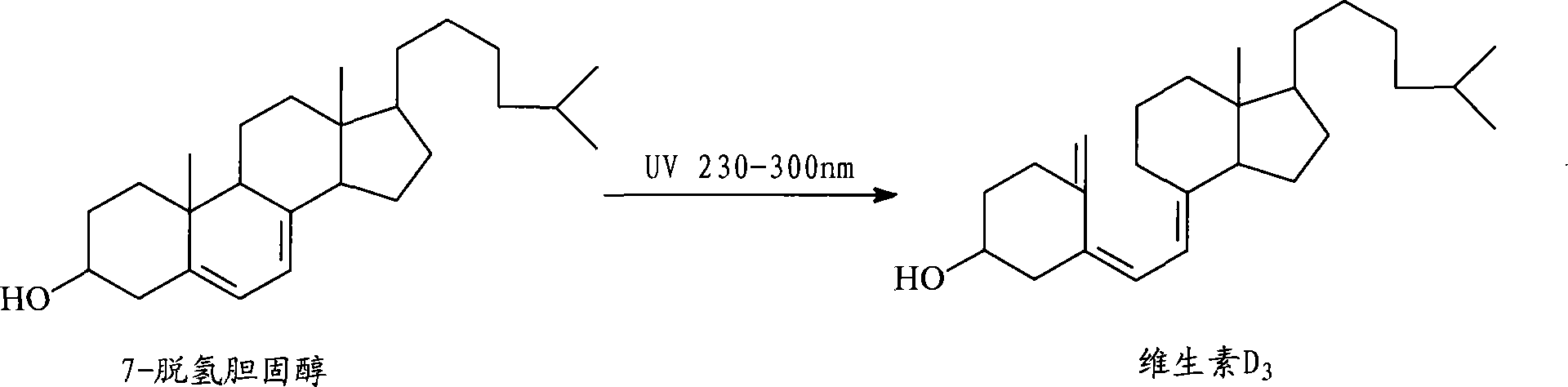
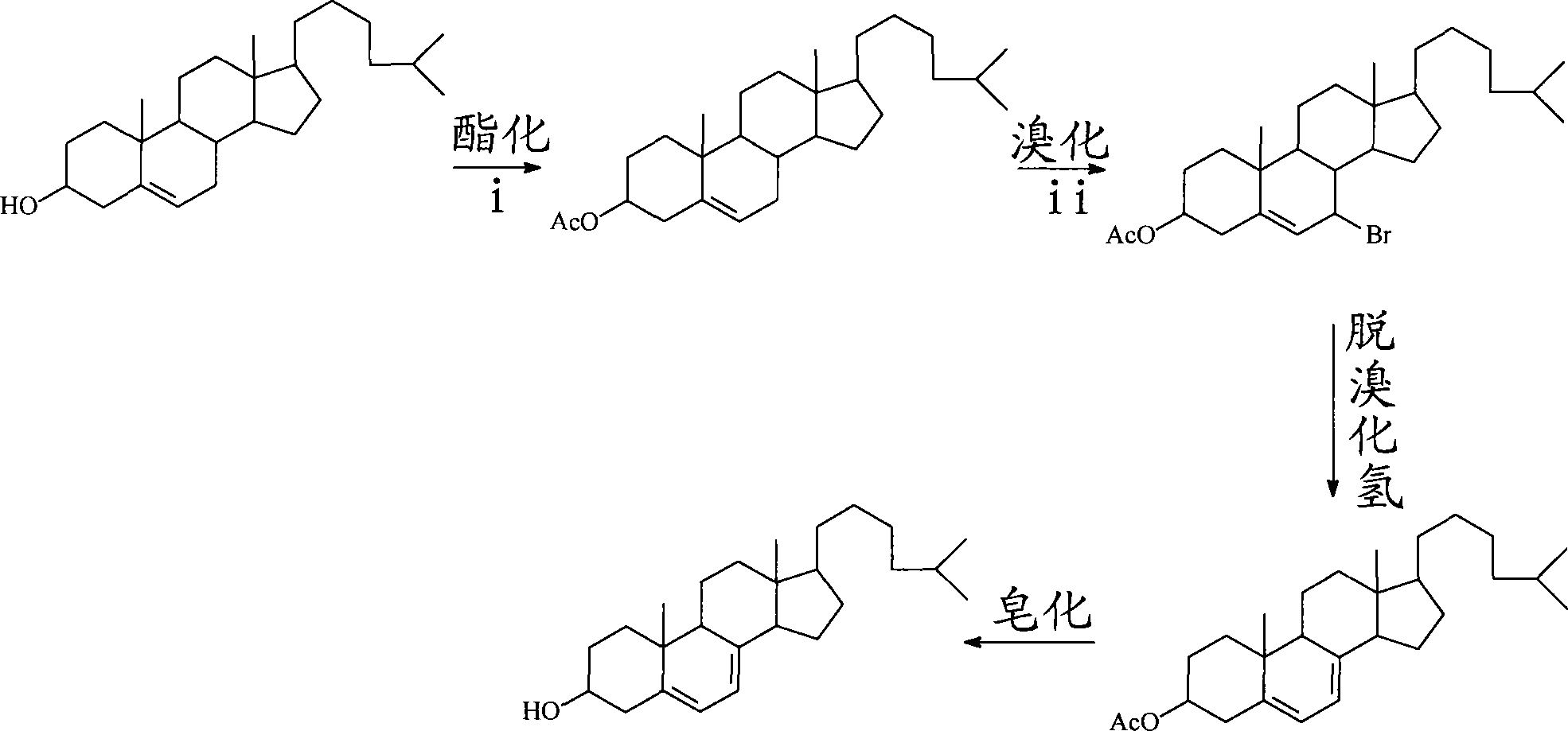
Preparation method for 7-dehydrochol esterol
The invention discloses a preparation method of a 7-dehydrocholesterol, which comprises the following steps: a) protecting a hydroxyl on the 3 position of a cholesterol; b) oxidizing a carbon on the 7 position of the product made in step a) into a carbonyl; c) carrying out hydrazone reaction to the product made in step b) with hydrazine derivatives, the structure of the hydrazine derivatives being at most one H to be substituted by the following hydrazine; d) reacting the product made in step c) with a strong basic reagent having stronger alkaline than a sodium hydroxide; e) reacting the product made in step d) with water or an acid. In the preparation method, no halogen group elements such as Br element are participated, the side effects are less and the yield is high, and the prepared product is safe and innocuous.
Owner:BEIJING UNIV OF CHEM TECH
Method for manufacturing metal nanometer particle
A method for manufacturing metal nanometer replaces two stages (firstly producing cuprous oxide, then producing copper particles by a reducing agent) of a metal particle producing process by one stage, such that it is capable of simplifying the working process obviously, obtaining metal copper particles easily by a short-time reaction at a low temperature (15-60 DEG C), omitting a complicated rinshing process of removing metal salts by secondary rinshing, and particle size dispersion is uniform and there is non requirement of a hierarchical process, thus the method is suitable for lot production. The method includes a first stage of dissolving metal precursor in a dissolvent containing a glycols dissolvent; a second stage of adding organic amine to the prepared solution, stirring until the color of the solution does not change; and a third stage of slowly adding more than one compounds selected from hydrazine derivatives, sodium hypophosphate, hydroxyl amine and sodium borohydride to the solution containing the organic amine of the second stage, so as to deoxidize and precipitate the metal(s).
Owner:DONGJIN SEMICHEM CO LTD
STAT3 small-molecule inhibitor and its application
ActiveCN103880707AOrganic compound preparationSulfonic acid amide preparationFluorescenceStat3 inhibitor
The invention discloses a STAT3 small-molecule inhibitor and its application, and the STAT3 inhibitor takes an N'-(1-(2,4 dihydroxy phenyl)ethylidene) benzoyl hydrazine derivative or its pharmacologically acceptable salt shown in a formula (1) or a formula (1a) as an effective component. In the formula, R1 and R2 on A ring can be hydroxyl, methoxy carboxyl and ester, and B can be an annular or a chain state. The STAT3 small-molecule inhibitor has selectivity and high activity, According to a three-grade structural design, biosynthesis and reconstructing optimization of STAT3, then a fluorescence polarization method and a CCK-8 method are employed for detecting activity and researching the mechanism on inhibition of tumor cells growth and promotion of differentiation apoptosis, so that effect of the STAT3 small-molecule inhibitor on prevention and control of tumor formation and tumor treatment can be described.
Owner:上海宇道生物技术有限公司
Hydrogels for the controlled release of bioactive materials
The present invention relates to the formation of hydrogels based on guanosine hydrazide derivatives in the presence of cations. The hydrogels can be used as a carrier / delivery system for biologically active substances such as flavors, fragrances, insect attractants or repellents, bactericides, fungicides, pharmaceuticals or agrochemicals.
Owner:UNIV LOUIS PASTEUR ULP +2
Preparation method of aluminum alloy-resin composite and aluminum alloy-resin composite prepared by using same
ActiveCN103287009AUnique structureUnique performanceAnodisationSynthetic resin layered productsAlloy substratePerylene derivatives
The invention provides a preparation method of an aluminum alloy-resin composite and an aluminum alloy-resin composite prepared by using the same. The preparation method comprises the following steps: S1. anodizing a pretreated aluminum alloy substrate to obtain an aluminum alloy of which the surface contains a nanoporous oxide film layer; S2. contacting the aluminum alloy containing the nanoporous oxide film layer obtained in the step S1 with a corrosive substance to form corrosion pores on the outer surface layer of the oxide film layer, thereby obtaining the surface-treated aluminum alloy substrate, wherein the corrosive substance is selected from at least one of ammonia, hydrazines, hydrazine derivatives and water-soluble amine compounds; and S3. putting the surface-treated aluminum alloy substrate in a mold, injecting a resin composition into the mold to be combined with the surface-treated aluminum alloy substrate, and forming to obtain the aluminum alloy-resin composite. A unique double-layer stereoscopic porous structure can be formed on the surface of the aluminum alloy to enhance the binding force between the resin and the aluminum alloy; and the invention has the advantages of easier formation, basically no influence on the appearance of the aluminum alloy, wider application range and no environment pollution, and is more suitable for large-scale production.
Owner:BYD CO LTD
Surface-treated steel sheets of good white rust resistance, and method for producing them
InactiveUS7291402B2Improve corrosion resistanceEconomically and stably producingHot-dipping/immersion processesSynthetic resin layered productsEpoxyPolymer science
The invention is to provide a surface-treated steel sheet of good white rust resistance with no chromium in its surface treatment film, and it has realized a surface-treated steel sheet of higher-level corrosion resistance by forming a reaction layer of plating metal with a barrier layer effective for anticorrosion not in a two-layered film but in a single-layered film. The surface-treated steel sheet is fabricated by applying a surface-treating composition that contains:(a) a water-dispersible resin and / or a water-soluble resin obtained through reaction of an epoxy group-containing resin (A) with an active hydrogen-containing compound (B) a part or all of which is an active hydrogen-containing hydrazine derivative and preferably with a hydrophilic organic component (C),(b) a silane coupling agent,(c) a phosphoric acid and / or a hexafluoro-metal acid, and(d) preferably an aqueous dispersion of an urethane resin, to a zinc-plated or aluminium-plated steel sheet followed by drying it to form thereon a surface treatment film having a thickness of from 0.02 to 5 μm. Regarding its film structure, the surface treatment film comprises an amorphous compound layer containing P, Zn and / or Al, and O, and an overlying organic resin matrix layer where the matrix is a hydrazine derivative-modified epoxy group-containing resin or the epoxy group-containing resin and an urethane resin.
Owner:JFE STEEL CORP +1
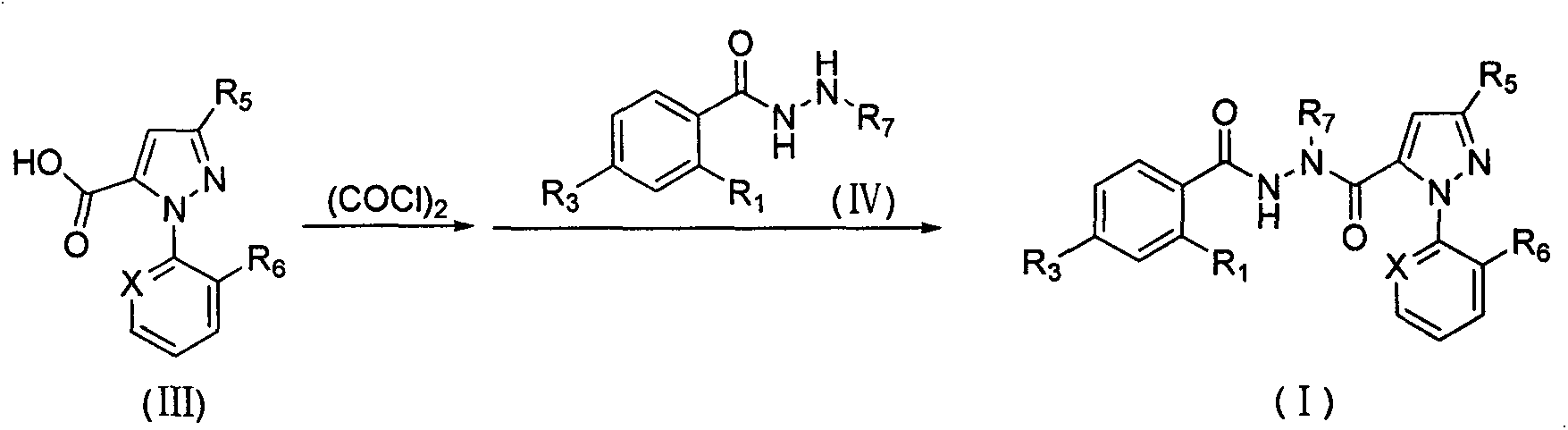
Synthesis of hydrazine derivatives of pyridazine
The present invention relates to both a novel method of preparing hydralazine hydrochloride and to a novel method of preparing hydrazine derivatives of compounds containing a pyridazine ring, including, for example, pyridazines, phthalazines and other compounds containing the pyridazine ring.
Owner:BIONICHE TEORANTA
Surface treated steel plate excellent in corrosion resitance electroconductivity and appearance of coating film
PROBLEM TO BE SOLVED: To provide a surface-treated steel sheet having superior corrosion resistance in spite of containing no hexavalent chromium in a coating, and besides, having superior conductivity and coating film appearance.SOLUTION: The surface-treated steel sheet has a composite oxide coating on the surface of a galvanized or aluminized steel sheet, as the first layer, and an organic coating thereon as the second layer. The composite oxide coating includes (α) silica, (β) phosphoric acid and / or a phosphate compound, (γ) one or more metals selected among Mg, Mn and Al and (σ) a tetravalent vanadium compound, each in a predetermined amount. The organic coating includes a reaction product (X) of a film-forming organic resin (A) and an active-hydrogen-containing compound (B) formed of a hydrazine derivative (C), of which one part or all parts have active hydrogen; and a particular corrosion-preventing component (Y).
Owner:JFE STEEL CORP
Preparation method for middle distillate type hydrocracking catalyst containing metal nitride
ActiveCN103785440AHigh activityImprove nitrogen toleranceMolecular sieve catalystsHydrocarbon oil crackingMolecular sieveBiological activation
The invention discloses a preparation method for a middle distillate type hydrocracking catalyst containing metal nitride. The preparation method comprises the following steps: (1) preparing a catalyst carrier containing a molecular sieve and inorganic refractory oxide; (2) preparing a hydrazine derivative solution, dipping the catalyst carrier prepared in the step (1) in the hydrazine derivative solution and drying the catalyst carrier; (3) preparing a dipping solution containing a group VI-B metal compound and a group VIII metal compound; (4) dipping a material obtained in the step (2) in the dipping solution prepared in the step (3) and drying the material; and 5) subjecting a material obtained in the step (4) to hydrogen activation so as to obtain the hydrocracking catalyst used for a hydrocracking reaction. The hydrocracking catalyst provided by the invention is used for treating heavy hydrocarbon materials and can realize maximum production of intermediate distillate oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
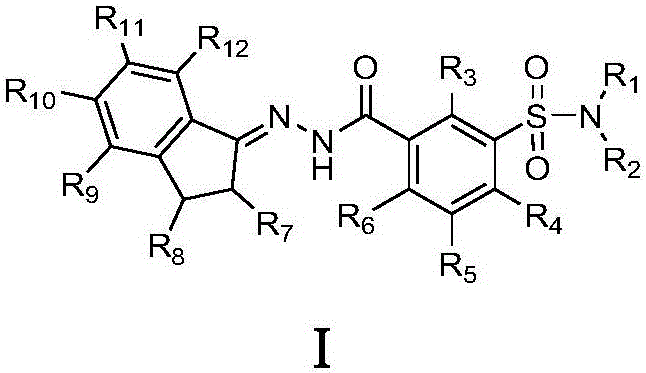
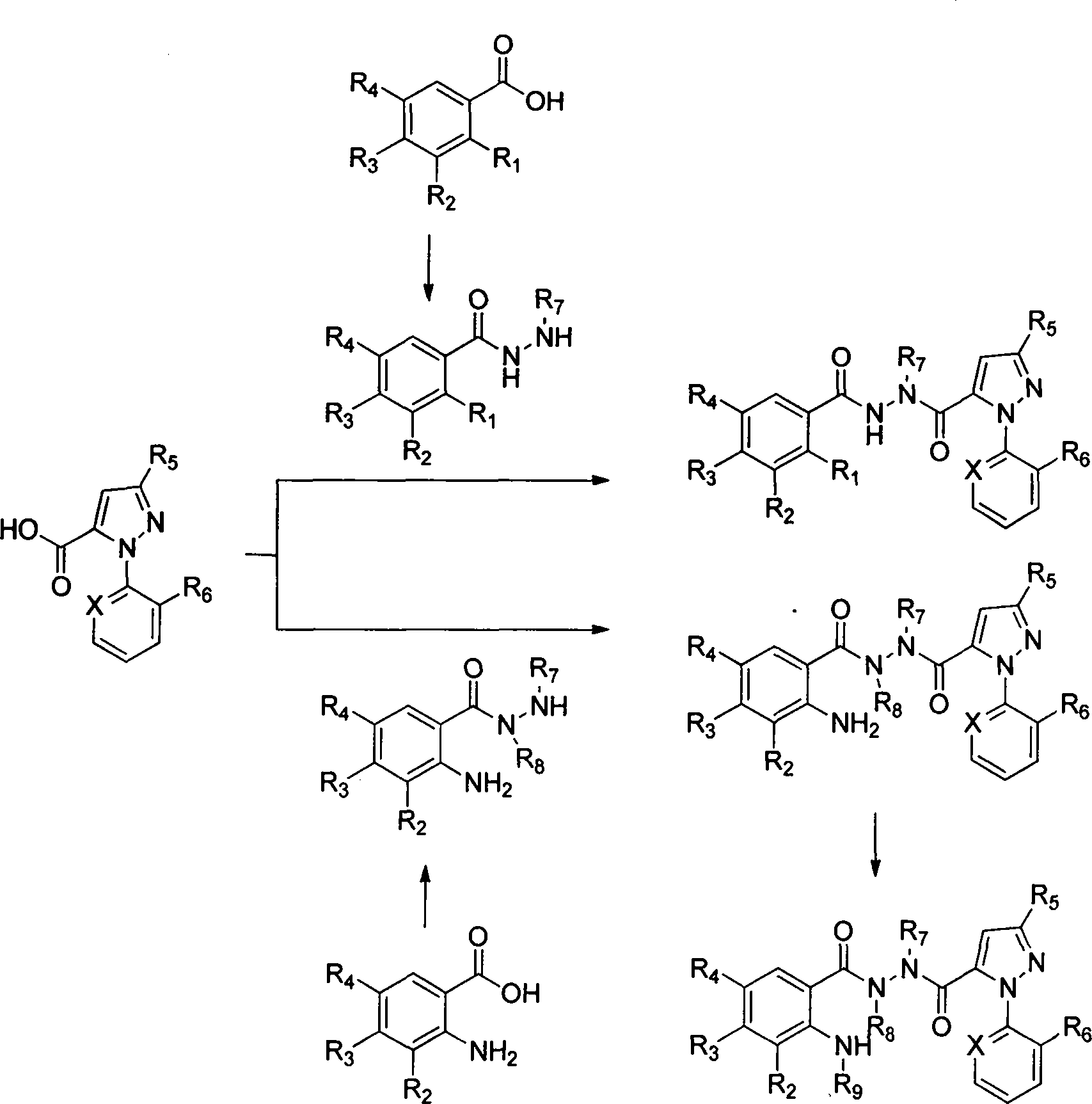
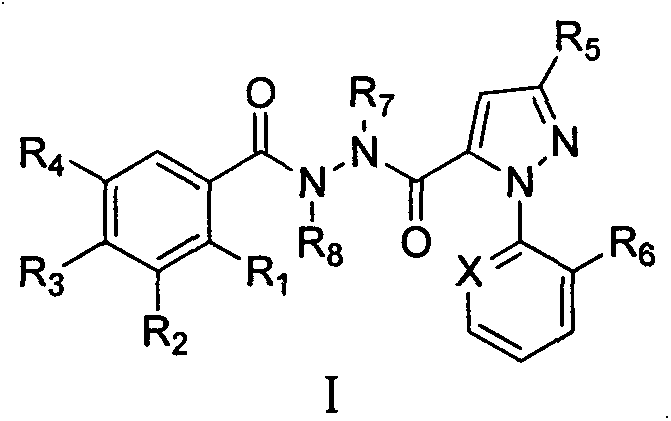
Indene-1-subunit sulfonyl benzoyl hydrazine derivative as well as preparation method and application thereof
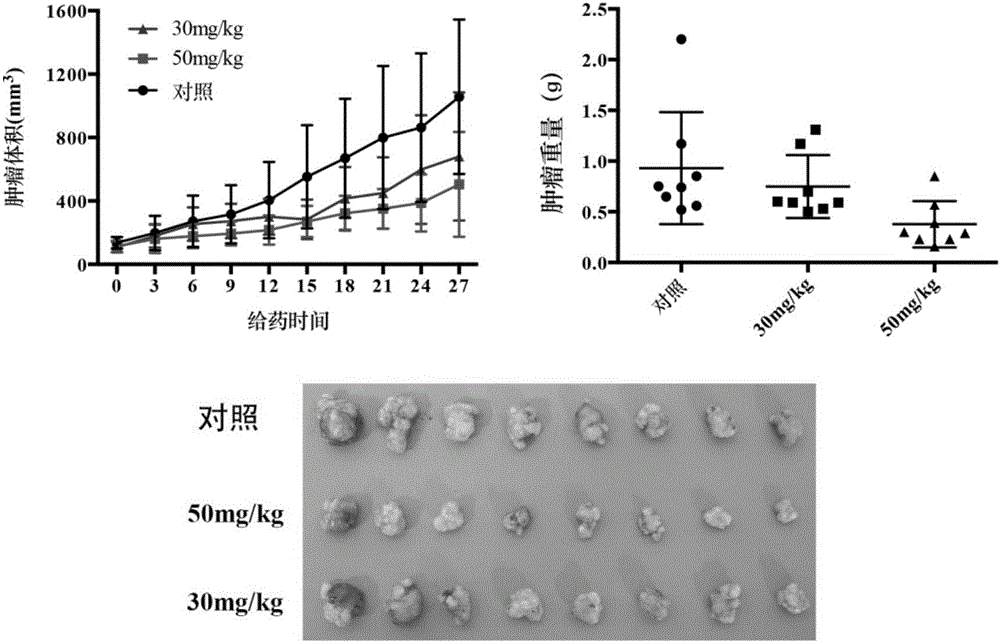
InactiveCN105985265AEnhanced inhibitory effectSulfonic acid amide preparationAmide active ingredientsInhibitory effectTumor cells
The invention belongs to the field of chemistry and medicines and particularly relates to an indene-1-subunit sulfonyl benzoyl hydrazine derivative as well as a preparation method and application thereof. The invention provides an indene-1-subunit sulfonyl benzoyl hydrazine derivative, wherein the structure is shown by formula I. The invention also provides a preparation method and application of the indene-1-subunit sulfonyl benzoyl hydrazine derivative shown by the formula I. The indene-1-subunit sulfonyl benzoyl hydrazine derivative provided by the invention has a relatively good inhibitory effect on the proliferation of tumor cells and provides a new option for the field of anti-tumor drug preparation.
Owner:SICHUAN UNIV
Novel hydrazide derivatives as well as preparation method and application thereof
InactiveCN103483313AHigh insecticidal activityIncreased drug resistanceBiocideOrganic chemistryOrder LepidopteraChemical compound
The invention relates to novel hydrazide derivatives as well as a preparation method and application thereof. The novel hydrazide derivatives have a general formula as shown in the specification, wherein each group is as shown in the claim 1. The novel hydrazide derivatives provided by the invention are improved in insecticidal activity while the previous compound lipid solubility thereof is improved; the derivatives have excellent insecticidal activity; instantly after the derivatives are applied, insects can lose control on muscle and stop eating, and are obviously shrank and paralyzed; at last, the insects can be caused to die, and in the meantime, abnormal ecdysis of insects also can be induced; the novel hydrazide derivatives are very effective especially for lepidoptera pests such as mythimna separata, plutella xylostella and asparagus caterpillar, and thus are pesticides having wide application prospect.
Owner:NANKAI UNIV
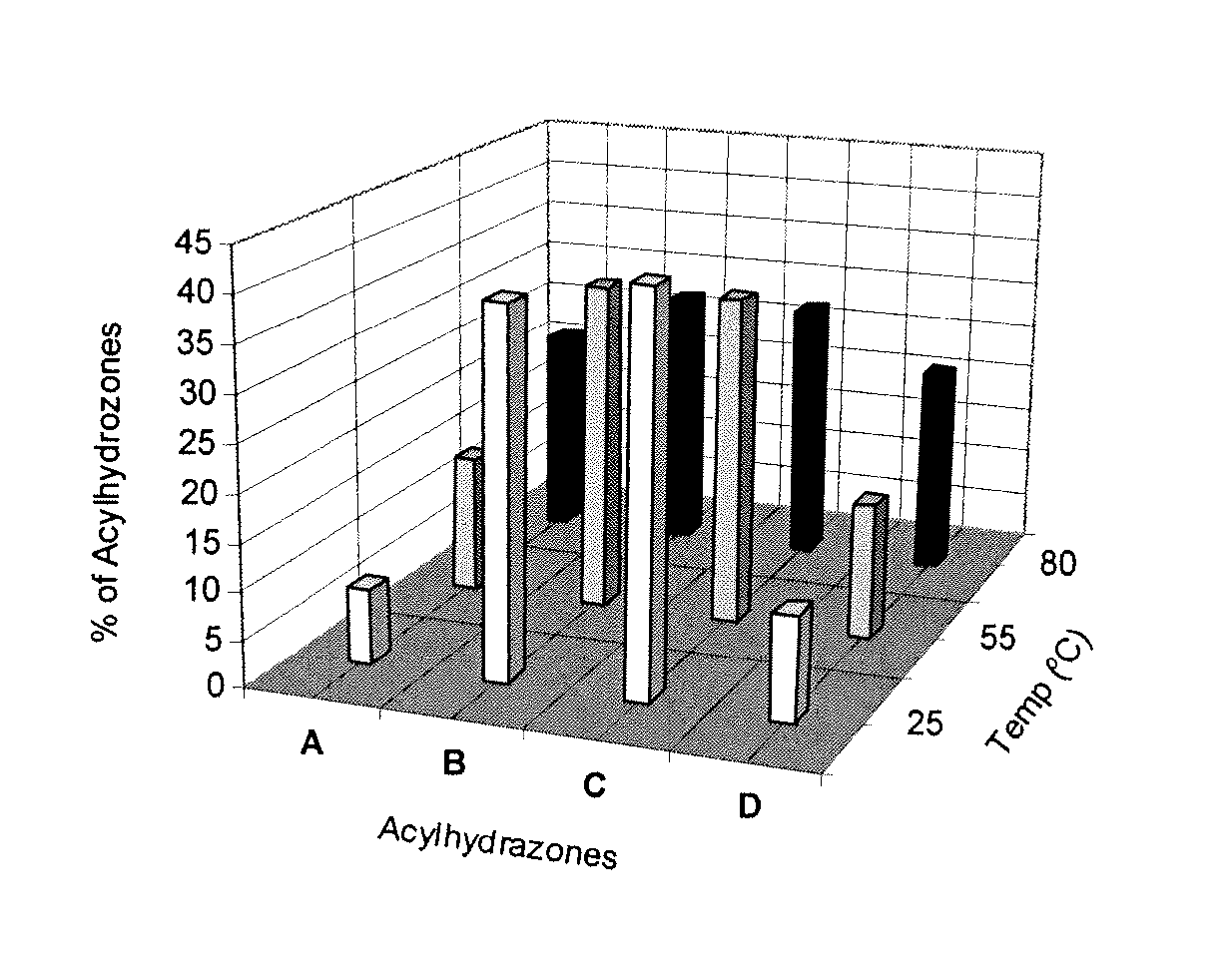
Preparation method and use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine
InactiveCN102659795AAdd structural unitEnhanced interactionOrganic chemistryAzo dyesFluoProbesPhenacyl
The invention discloses a preparation method and a use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine. N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine is shown in the structural formula I. A rhodamine dye is an excellent optical fluorescent probe matrix. The preparation method realizes the preparation of a double (rhodamine B) acylhydrazine derivative having a 1-naphthoyl substituent group and a spiro structure first. Under acidic conditions, the spiro structure of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine is transformed into an open state from a closed state and simultaneously, the change from an achromatic color to a pink color is produced in a molecular spectrum of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine; and under the action of exciting light having a certain wavelength, N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine produces fluorescent light. After being adsorbed on a silica gel plate, N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine shown in the structural formula I can produce visible light and a fluorescent or chroma response under the action of volatile acid gas. N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine shown in the structural formula I can be used for naked eye chromogenic identification and fluorescent identification probes of acid mist.
Owner:TIANJIN NORMAL UNIVERSITY
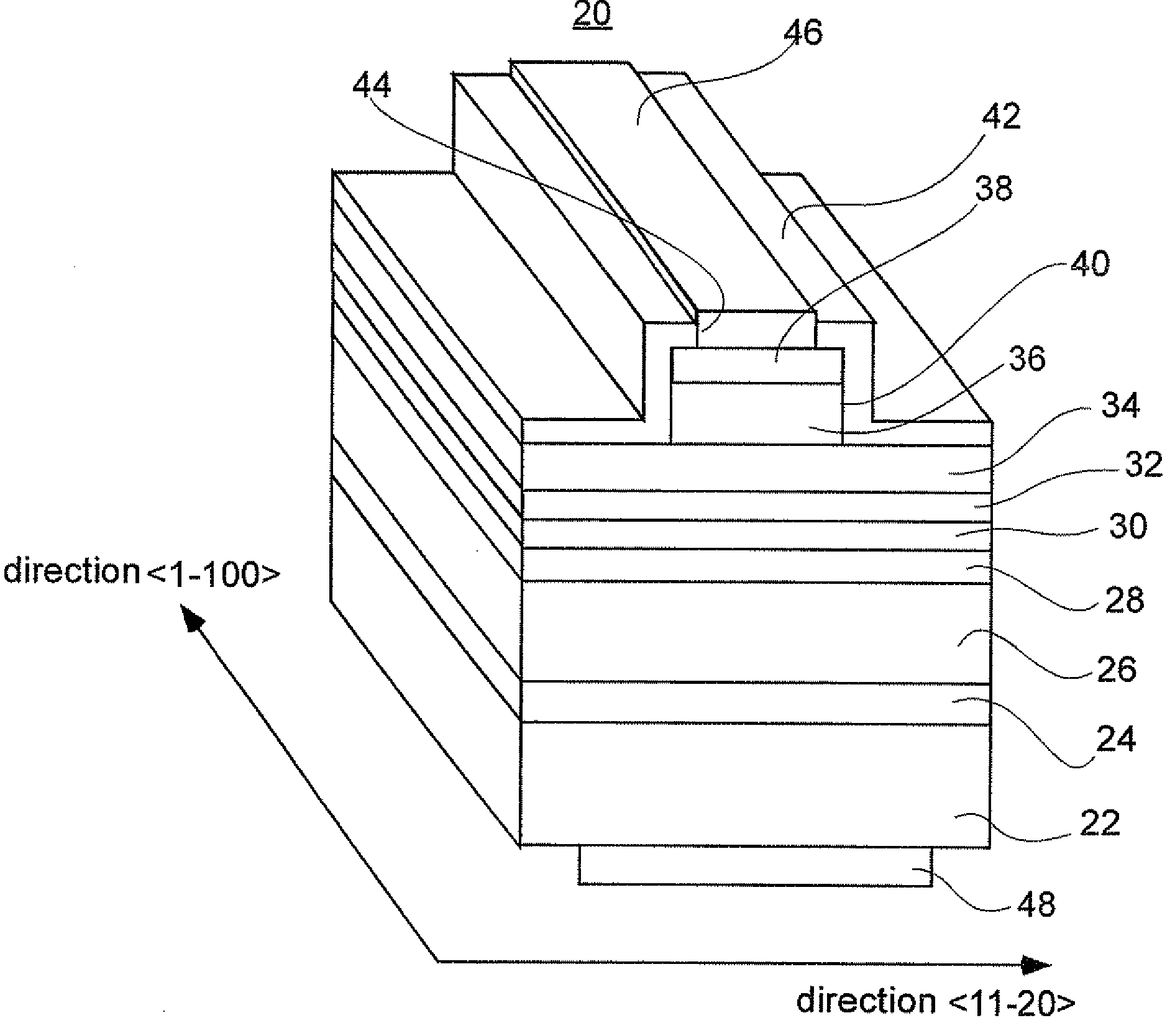
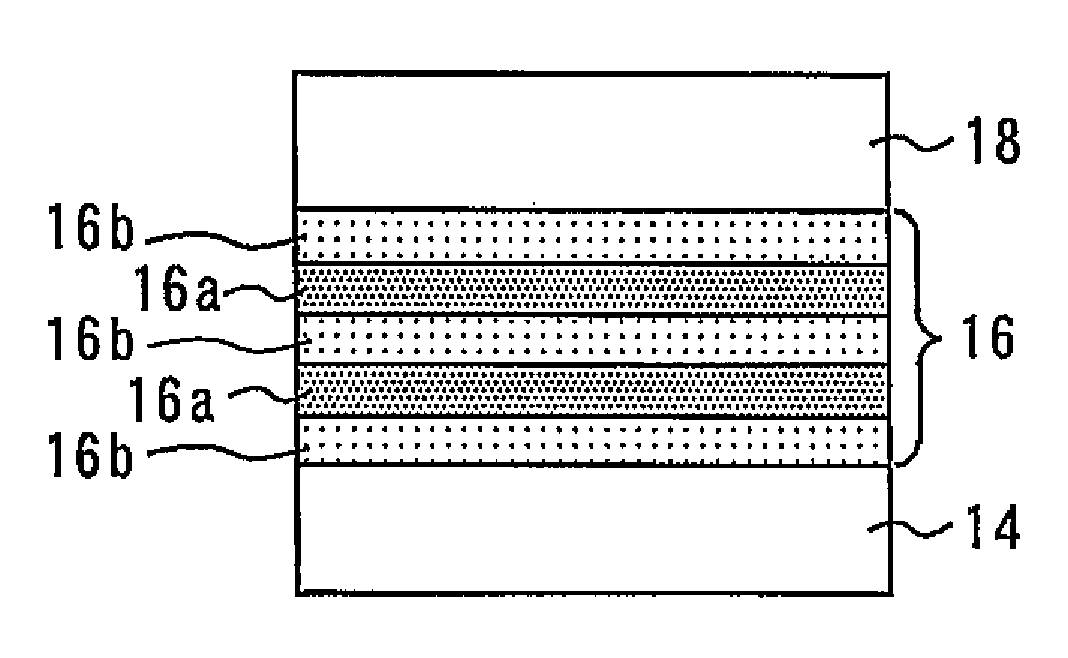
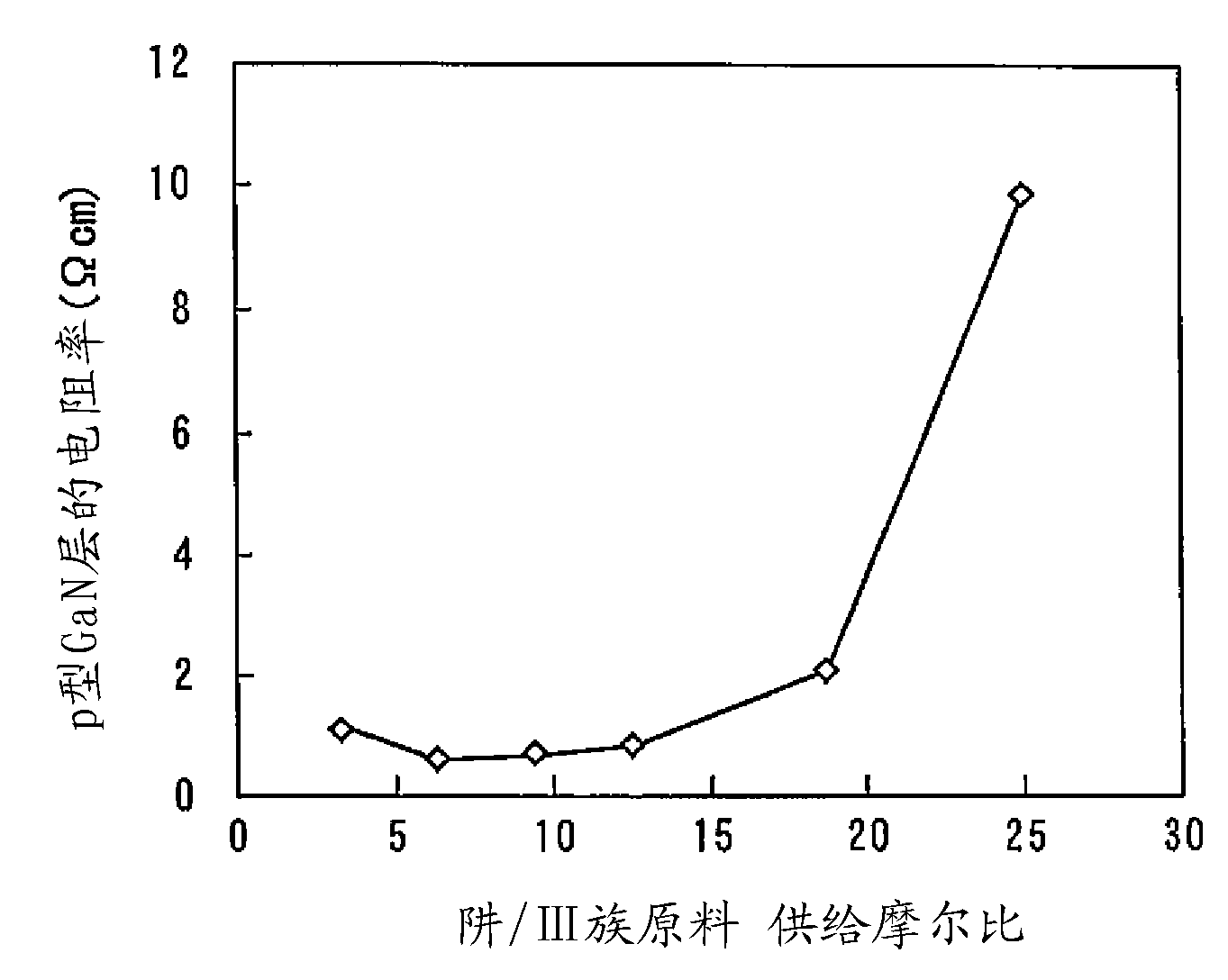
Nitride semiconductor stacked structure and semiconductor optical device, and methods for manufacturing the same
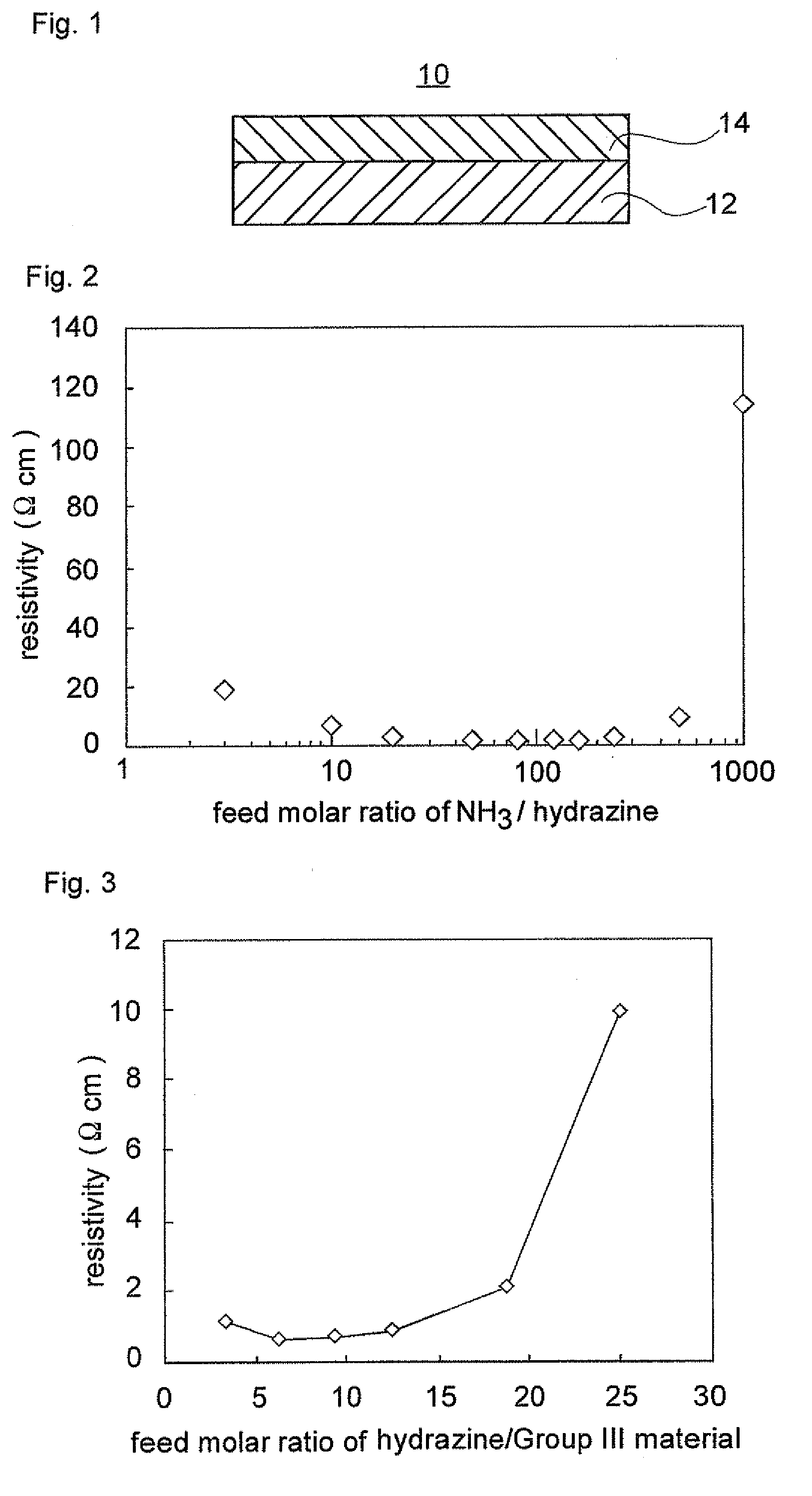
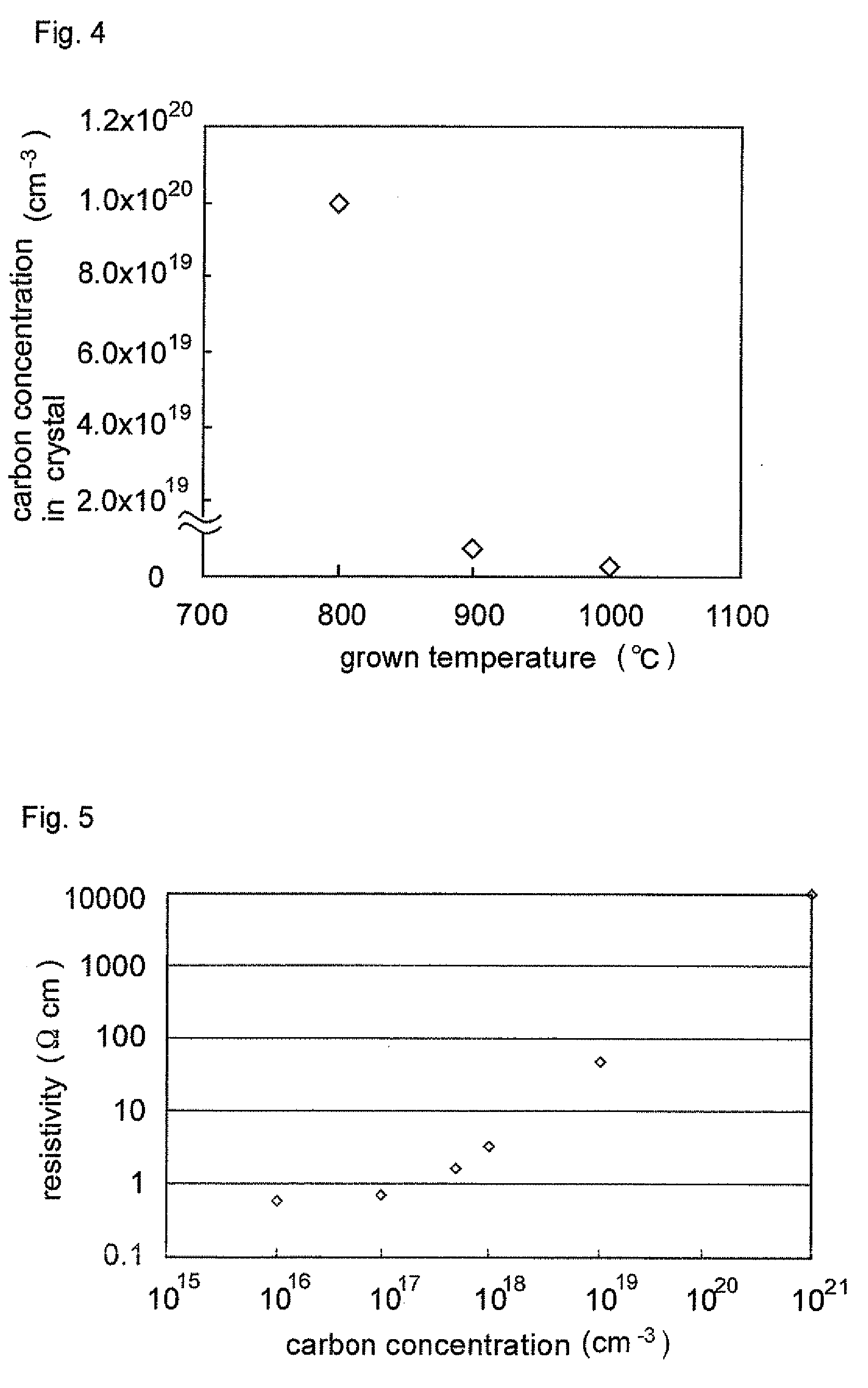
ActiveUS20090008659A1Lower resistanceReduce resistanceOptical wave guidanceLaser detailsAmmoniaImpurity
A nitride semiconductor stacked structure having good working efficiency includes a p-type nitride semiconductor layer of low resistance, which is formed from an organometallic compound, compounds including Group V elements, including ammonia and a hydrazine derivative, and a p-type impurity material on a substrate. The p-type nitride layer has a carbon concentration not higher than 1×1018 cm−3.
Owner:MITSUBISHI ELECTRIC CORP
Process for producing thin hafnium or zirconium nitride coatings
InactiveUS20070042224A1Semiconductor/solid-state device manufacturingNatural mineral layered productsGas phaseCompound (substance)
A process for producing hafnium(III) nitride (HfN) or zirconium nitride coatings by means of the CVD method (chemical vapour deposition) from a reactive gas on a substrate surface, the HfN coating or ZrN coating and their use are described. In the process, a hafnium or zirconium tetrakis(dialkylamide) having the general formula Hf(NR1R2)4 or Zr(NR1R2)4 wherein R1 and R2 denote identical or different, straight-chain or branched C1 to C4 alkyl radicals, is used as the Hf precursor or Zr precursor and a hydrazine derivative having the general formula H2N—NR3R4 wherein R3 denotes a straight-chain or branched C1 to C4 alkyl radical and R4 independently denotes a C1 to C4 alkyl radical or H, is used as the reactive gas.
Owner:H C STARCK GMBH
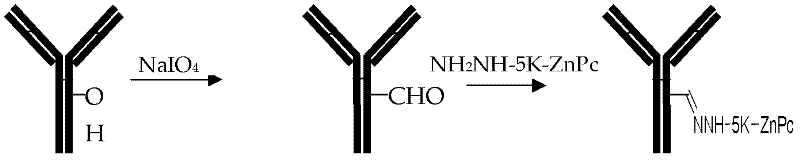
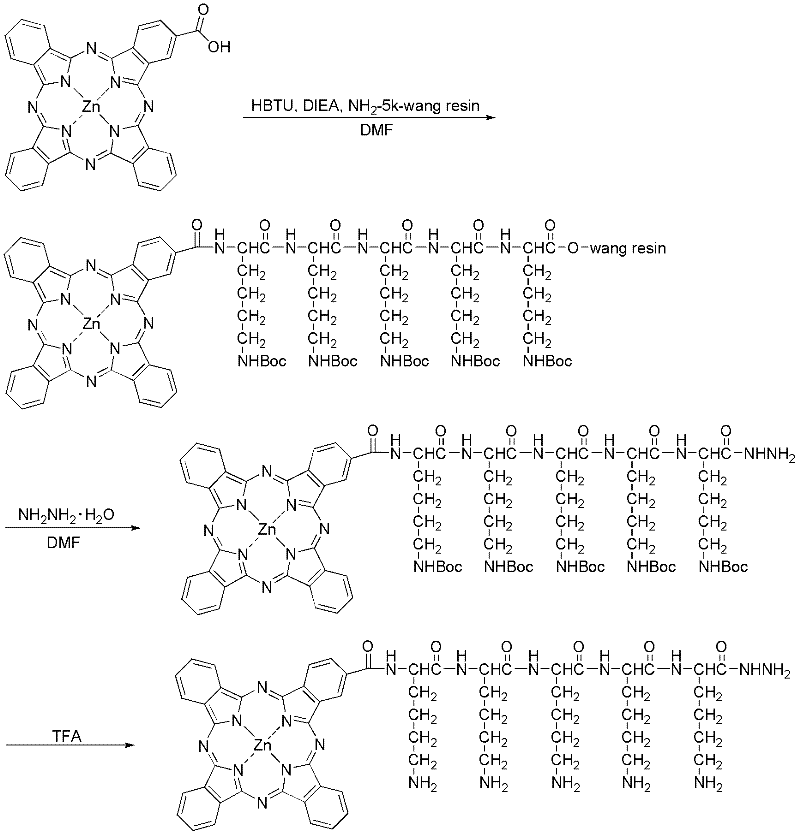
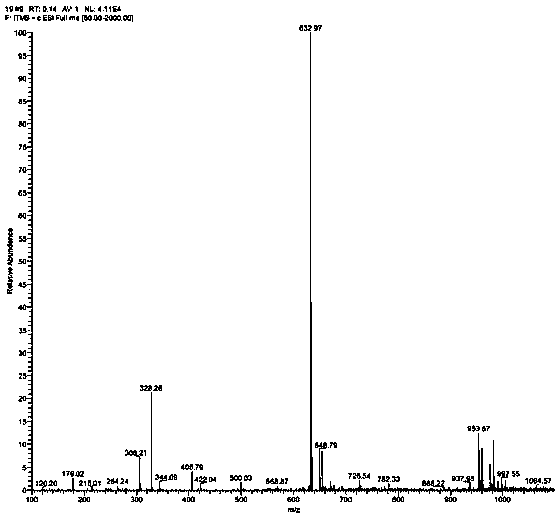
Preparation method and application of tumor-targeted photosensitive immunoconjugate
ActiveCN102585003AImprove anti-tumor activityLow costEnergy modified materialsImmunoglobulins against cell receptors/antigens/surface-determinantsTumor targetPentamer
The invention provides a preparation method of high-activity zinc phthalocyanine-monoclonal antibody tumor-targeted photosensitive immunoconjugate and a technical method for anti-tumor application of the immunoconjugate. Monocarboxyl zinc phthalocyanine with a single structure is reacted with lysine hydrazine pentamer to form a water-soluble monocarboxyl zinc phthalocyanine hydrazine derivative with high reaction activity; meanwhile, the monoclonal antibody with anti-tumor activity is selected; under a neutral condition, glycosyl non-related to activity of the antibody is mildly oxidized into an aldehyde group with sodium periodate; and at 4 DEG C, excess monocarboxyl zinc phthalocyanine hydrazine derivative is extremely easy for reacting with the aldehyde group and the reaction product is further reduced into stable photosensitive immunoconjugate to obtain the high-purity photosensitive immunoconjugate. The preparation method has the characteristics of low temperature, neutral conditions and no use of any organic solvent. In-vitro anti-tumor experiments prove that the conjugate has extremely high selectivity and anti-tumor activity as well as application value.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Method for preparing 7-dehydrocholesterol
The invention relates to a method for preparing 7-dehydrocholesterol, which comprises the following steps: 1) protecting hydroxyl on the 3-site of cholesterol; 2) oxidizing carbon on the 7-site of the product obtained in step 1) into carbonyl; 3) carrying out a hydrazone reaction on the product obtained in step 2) and a hydrazine derivative; 4) reacting the product obtained in step 3) with a strongly alkaline reagent; and 5) reacting the product obtained in step 4) with water or an acid. The method has the advantages of simple operation process, less secondary reaction, high yield and less corrosion to equipment, thereby having high industrial production values.
Owner:ANHUI BBCA FERMENTATION TECH ENG RES
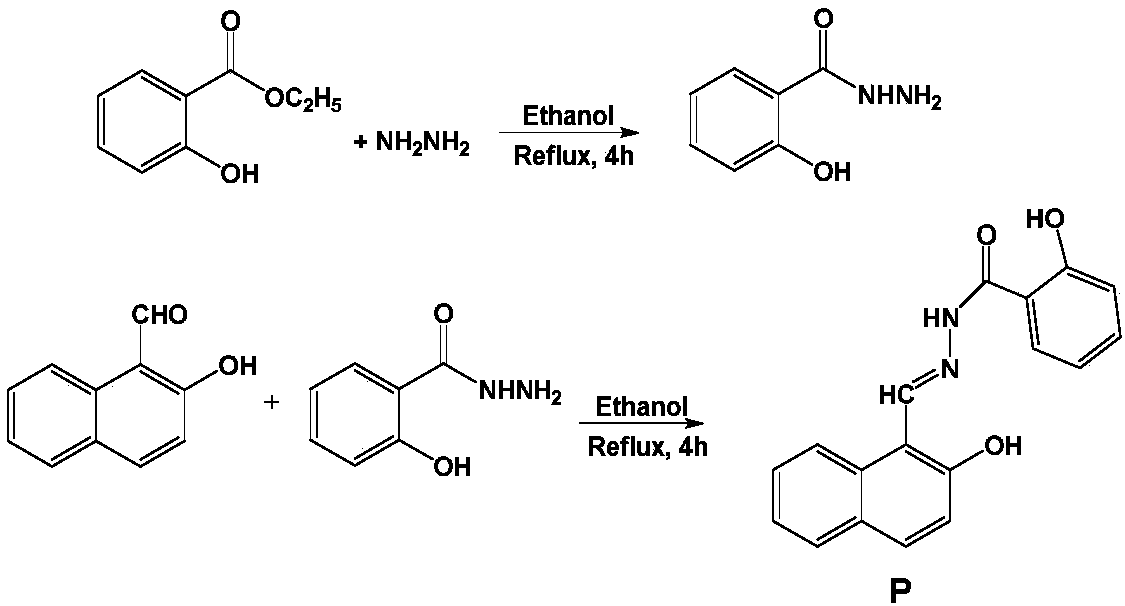
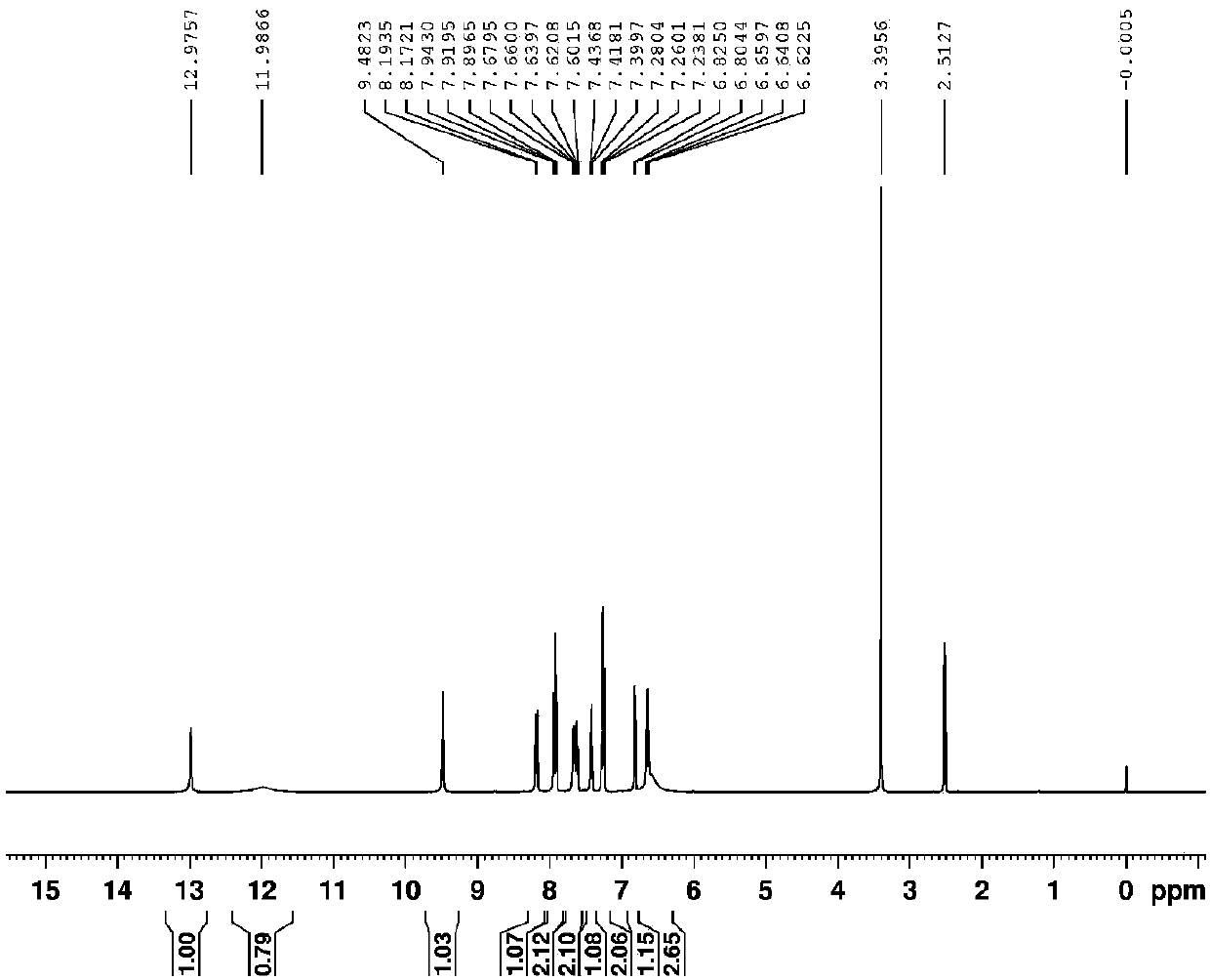
Benzoyl hydrazine derivative P and synthesis and application thereof
InactiveCN107759489AGood choiceThe synthesis steps are simpleHydrazone preparationFluorescence/phosphorescenceEthyl salicylateFiltration
The invention relates to detection of a fluorescence method on metal ions, and particularly relates to a benzoyl hydrazine derivative P which identifies and detects two different metal ions Mg<2+> andAl<3+> separately in different pH conditions. The structural formula of the benzoyl hydrazine derivative P is as shown in a formula (1). The preparation method of the benzoyl hydrazine derivative P comprises the following steps: firstly, carrying out hydrazinolysis on ethyl salicylate; carrying out a reaction with o-hydroxy naphthaldehyde at a molar ratio of 1 to 1 for 4 hours at 80 DEG C in anhydrous ethanol; cooling the mixture and separating out a solid and carrying out suction filtration; washing the mixture with a lot of water and anhydrous ethanol successively; and drying the solid to obtain a light yellow solid pure product P. The benzoyl hydrazine derivative P can be used for detecting Mg<2+> and Al<3+> separately in different pH conditions as a fluorescent probe. The benzoyl hydrazine derivative P is obtained by means of an effective synthetic means and shows relatively good selectivity on the metal ions Mg<2+> and Al<3+> separately. On a basis of an optimized experimental condition, the metal ions Mg<2+> and Al<3+> can be detected separately. The formula is as show in the description.
Owner:HAINAN MEDICAL COLLEGE
Hydrocracking catalyst, and preparation method and application thereof
ActiveCN103785441AHigh strengthImprove nitrogen toleranceMolecular sieve catalystsHydrocarbon oil crackingMolecular sieveHydrogen
The invention discloses a hydrocracking catalyst, comprising an acidic component and active metal component compounds, wherein the acidic component at least includes a molecular sieve, the active metal component compounds comprise compounds of Mo and / or W and compounds of Ni and / or Co, Mo and W are group VI-B elements, and Ni and Co are group VIII elements. The hydrocracking catalyst is prepared through loading of active components, dipping with a hydrazine derivative, drying and hydrogen activation. A preparation method for the hydrocracking catalyst comprises the following steps: (1) preparing a molecular sieve and a porous inorganic refractory oxide carrier and carrying out molding; (2) loading the active metal component compounds on the carrier; (3) carrying out dipping with the hydrazine derivative and drying; and (4) carrying out hydrogen activation so as to obtain the finished catalyst. The catalyst has good comprehensive performance and good nitrogen resistance, is used for treating heavy hydrocarbon materials and can realize maximum production of intermediate distillate oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
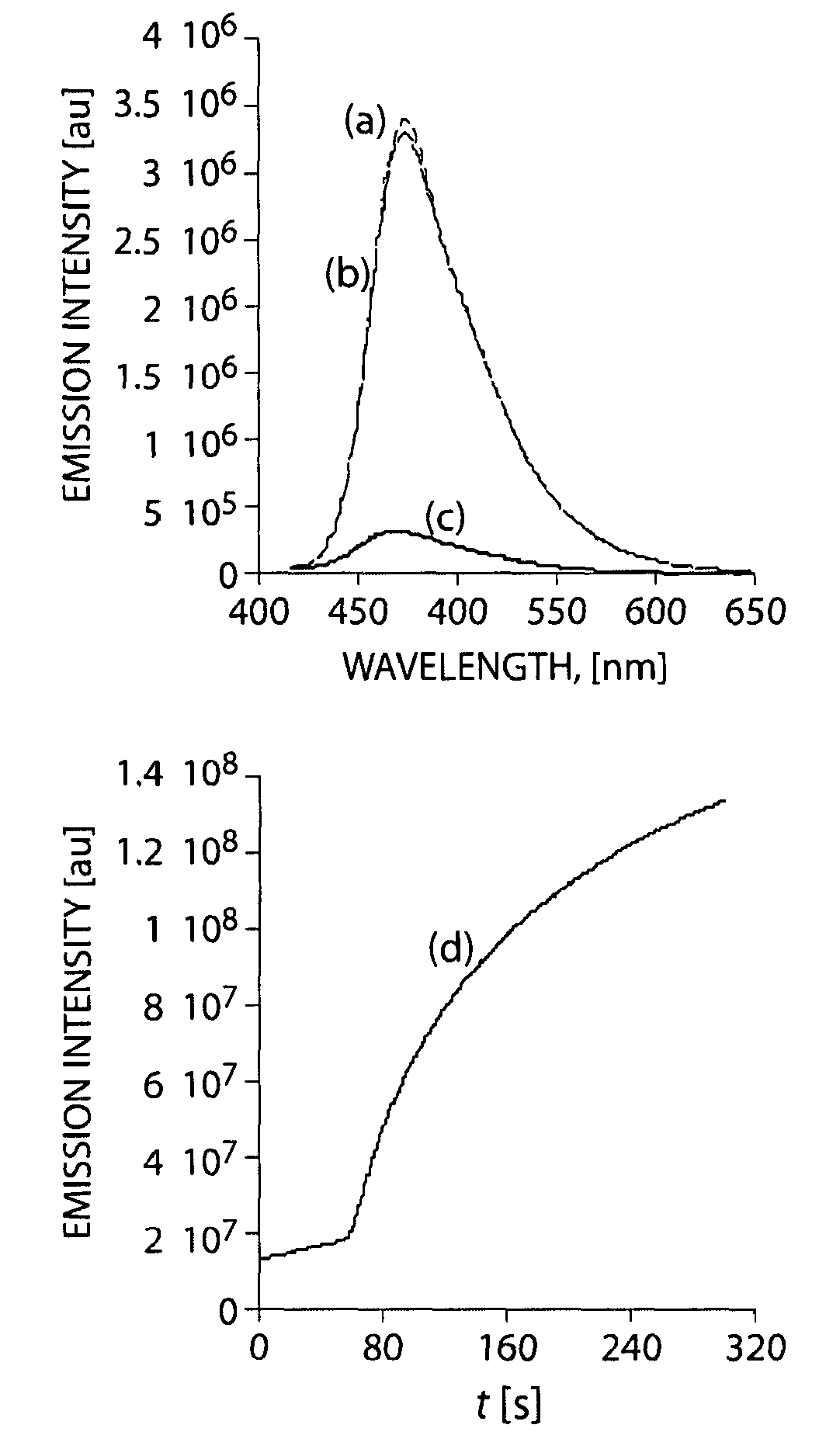
Luminescent detection of hydrazine and hydrazine derivatives
InactiveUS8158437B2High luminous intensityChemiluminescene/bioluminescenceBiological testingHydrazine compoundGas phase
The present invention generally relates to methods for modulating the optical properties of a luminescent polymer via interaction with a species (e.g., an analyte). In some cases, the present invention provides methods for determination of an analyte by monitoring a change in an optical signal of a luminescent polymer upon exposure to an analyte. Methods of the present invention may be useful for the vapor phase detection of analytes such as explosives and toxins. The present invention also provides methods for increasing the luminescence intensity of a polymer, such as a polymer that has been photobleached, by exposing the luminescent polymer to a species such as a reducing agent.
Owner:MASSACHUSETTS INST OF TECH
Treatment solution for reducing oxidation films on surface of copper and copper alloys and treatment method thereof
ActiveCN102191494AAffect healthNo harmMetallic material coating processesReduction treatmentHydroxylamine Hydrochloride
The invention discloses a treatment solution for reducing the oxidation films on the surfaces of copper and copper alloys and a treatment method thereof. In the treatment solution, one or more of ascorbic acid, hypophosphite, glucose, hydrazine, hydrazine derivatives, hydroxylamine and hydroxylamine derivatives are used as reducing agents to reduce the oxygen element in the oxidation films formedon the surfaces of copper and copper alloys and keep the copper element. The treatment solution has the following advantages: the acid fog with a lot of H2, NO2 and the like can not generated in the reduction treatment to seriously influence the fitness of the operator; and after treatment, the copper ion content of the treatment solution is less than 0.5mg / l, thus the water treatment system can not be polluted; and the treatment solution is environmentally-friendly. In addition, the oxidation films formed on the surfaces of copper and copper alloys can be reduced to bright copper and copper alloys in only 1-5 minutes, thus the treatment solution is the treatment solution with low cost,, high treatment speed, remarkable effect and environmental friend and has good market application prospect, and the method is convenient to use.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Method for manufacturing nitride semiconductor device
InactiveCN102130425ASimple process manufacturingOptical wave guidanceLaser detailsPower semiconductor deviceHydrogen
Owner:MITSUBISHI ELECTRIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

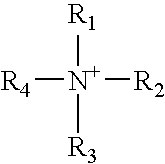






![Preparation method and use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine Preparation method and use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f02ee7b4-8d75-4fef-aaad-07072076b17e/120330220101.PNG)
![Preparation method and use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine Preparation method and use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f02ee7b4-8d75-4fef-aaad-07072076b17e/120330220109.PNG)
![Preparation method and use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine Preparation method and use of N-(1-naphthoyl)-N'-[rhodamine B-9-(2-benzoyl)]hydrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f02ee7b4-8d75-4fef-aaad-07072076b17e/120330220140.PNG)