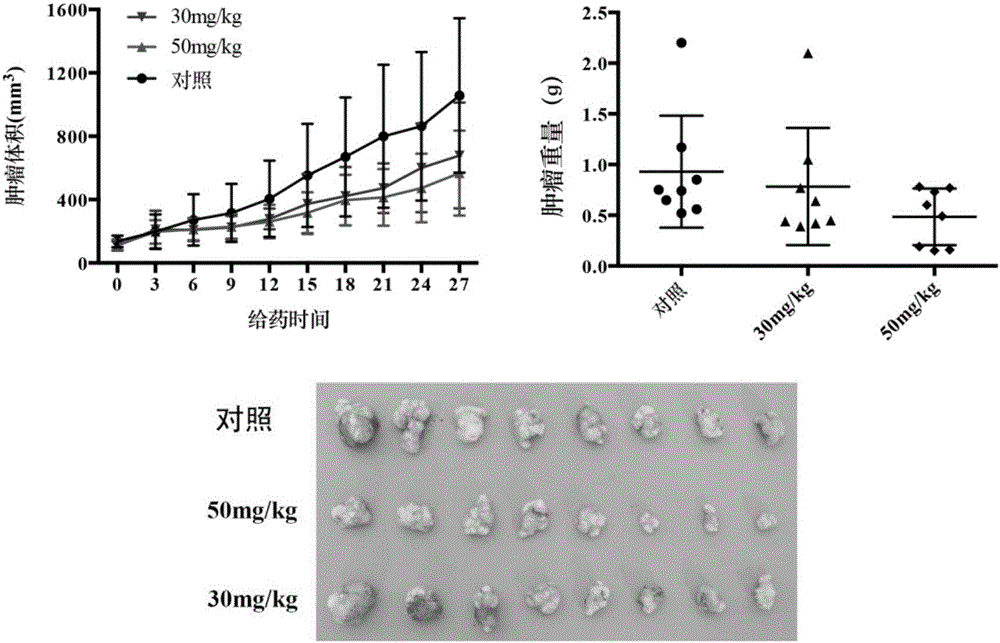
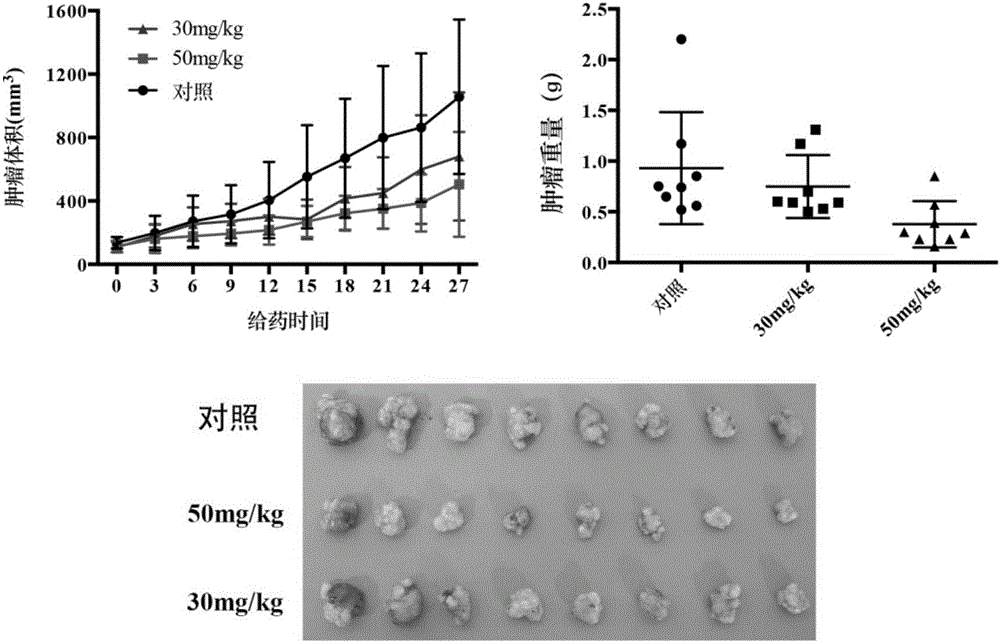
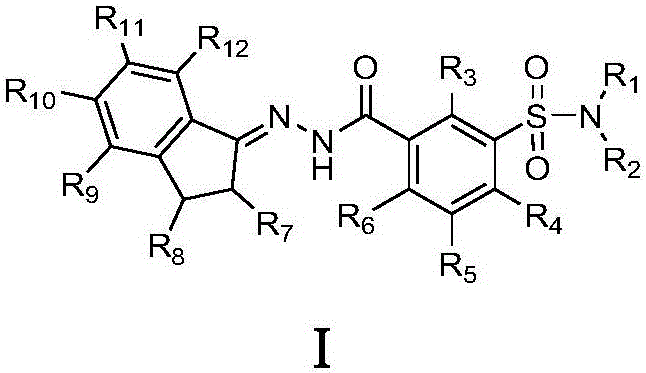
Indene-1-subunit sulfonyl benzoyl hydrazine derivative as well as preparation method and application thereof
A technology of sulfenyl benzoyl hydrazide and derivatives is applied in the field of indene-1-sulfenyl benzoyl hydrazide derivatives and their preparation, and can solve the problems of low selectivity, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The preparation method of the indene-1-ylidene sulfonyl benzoyl hydrazide derivative shown in above-mentioned formula I, its synthetic steps are:
[0059] 1) at In THF (tetrahydrofuran), m-chlorosulfonylbenzoic acid was added dropwise at 0°C, and then reacted at room temperature for 8-12h to obtain intermediate 2;
[0060] 2) dissolving the carboxylic acid in methanol, adding a catalytic amount of concentrated sulfuric acid dropwise, and reacting with intermediate 2 to obtain intermediate 3;
[0061] 3) Dissolving intermediate 3 in methanol, adding 2 to 5 eq of hydrazine hydrate, and reacting at 65 to 70°C for 12 hours to obtain intermediate 4;
[0062] 4) Intermediate 4 is then combined with reaction to obtain the compound of formula I.
[0063] Wherein, in the preparation method of the above-mentioned indene-1-ylidenesulfonylbenzohydrazide derivatives, the m-chlorosulfonylbenzoic acid described in step 1) and The molar ratio is 1:3.
[0064] Wherein, in the ab...
Embodiment 1
[0069] Example 1 Preparation of 3-(morpholinesulfonyl)benzoic acid (intermediate 2-1)
[0070]
[0071] Dissolve morpholine (300mg) in 5mL tetrahydrofuran, add a solution of m-chlorosulfonylbenzoic acid (250mg) in tetrahydrofuran (3mL) dropwise at 0°C, after the addition, react at room temperature for 12h, and purify by column chromatography to obtain 160mg of a white solid , yield 52%.
[0072] 1 H NMR (400MHz, CDCl 3 ): δ11.46(s, 1H), δ8.15(s, 1H), δ7.96(d, J=7.6Hz, 1H), δ7.84(t, J=7.7Hz, 1H), δ7. 35 (d, J=7.6Hz, 1H), δ3.64 (brs, 4H), δ3.11 (brs, 4H) ppm.
Embodiment 2
[0073] Example 2 Preparation of 3-(N-methylpiperazinesulfonyl)benzoic acid (intermediate 2-2)
[0074]
[0075] Dissolve N-methylpiperazine (340mg) in 5mL tetrahydrofuran, add m-chlorosulfonylbenzoic acid (250mg) in tetrahydrofuran (3mL) dropwise at 0°C, after the addition, react at room temperature for 12h, and purify by column chromatography 166mg of white solid was obtained, the yield was 53%.
[0076] 1 H NMR (400MHz, CDCl 3 ): δ11.46(s, 1H), δ8.15(s, 1H), δ7.96(d, J=7.6Hz, 1H), δ7.84(t, J=7.7Hz, 1H), δ7. 35 (d, J=7.6Hz, 1H), δ3.64 (brs, 4H), δ3.11 (brs, 4H), δ2.26 (s, 3H) ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average volume | aaaaa | aaaaa |
| Tumor volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com