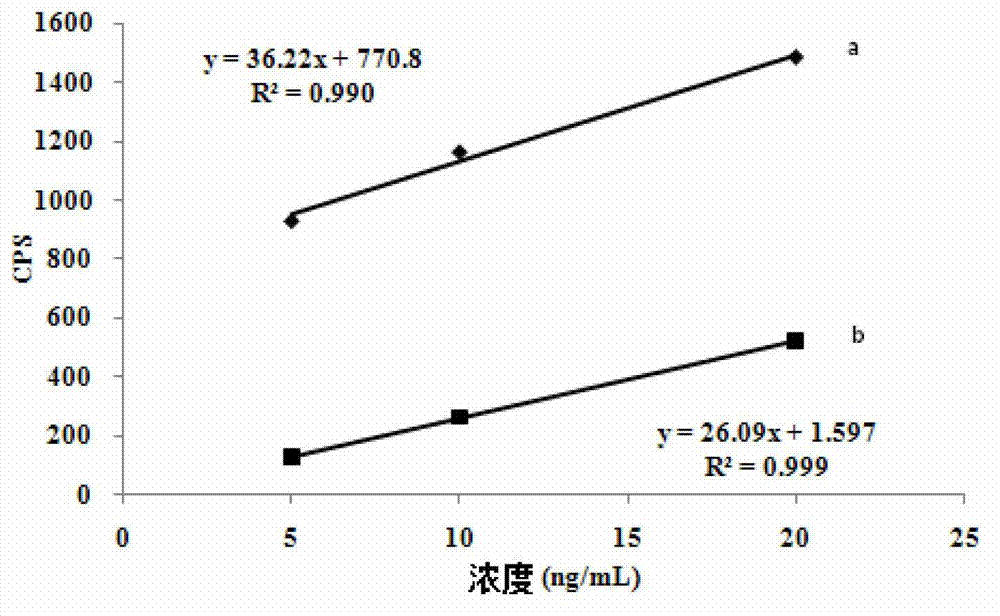
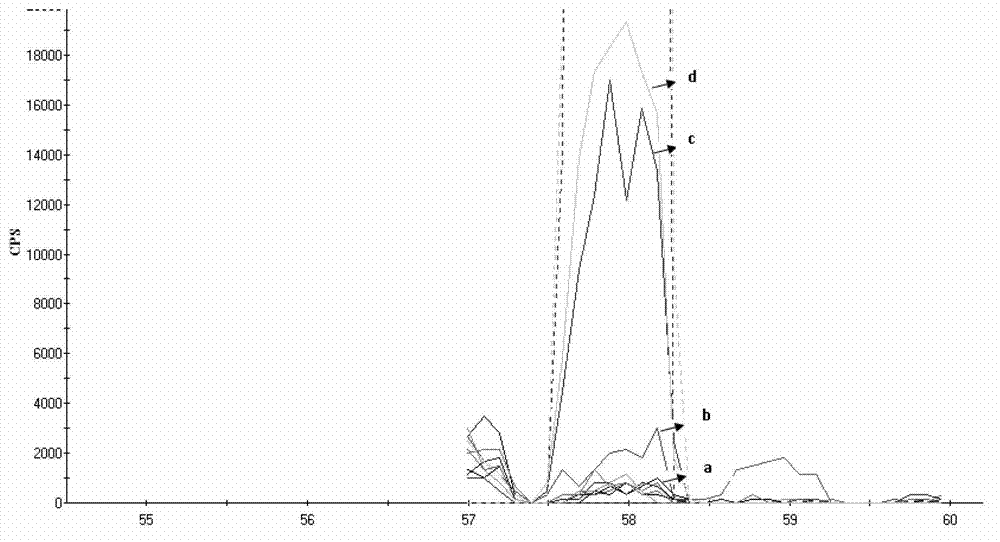
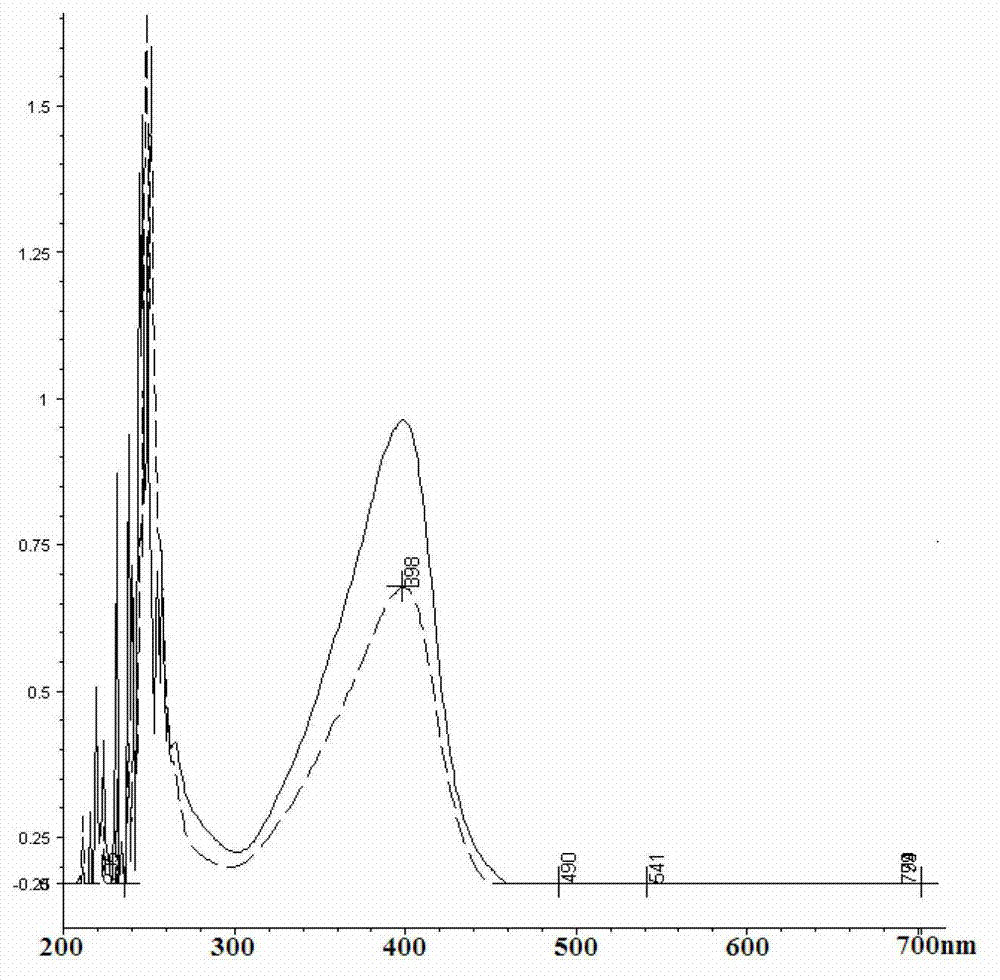
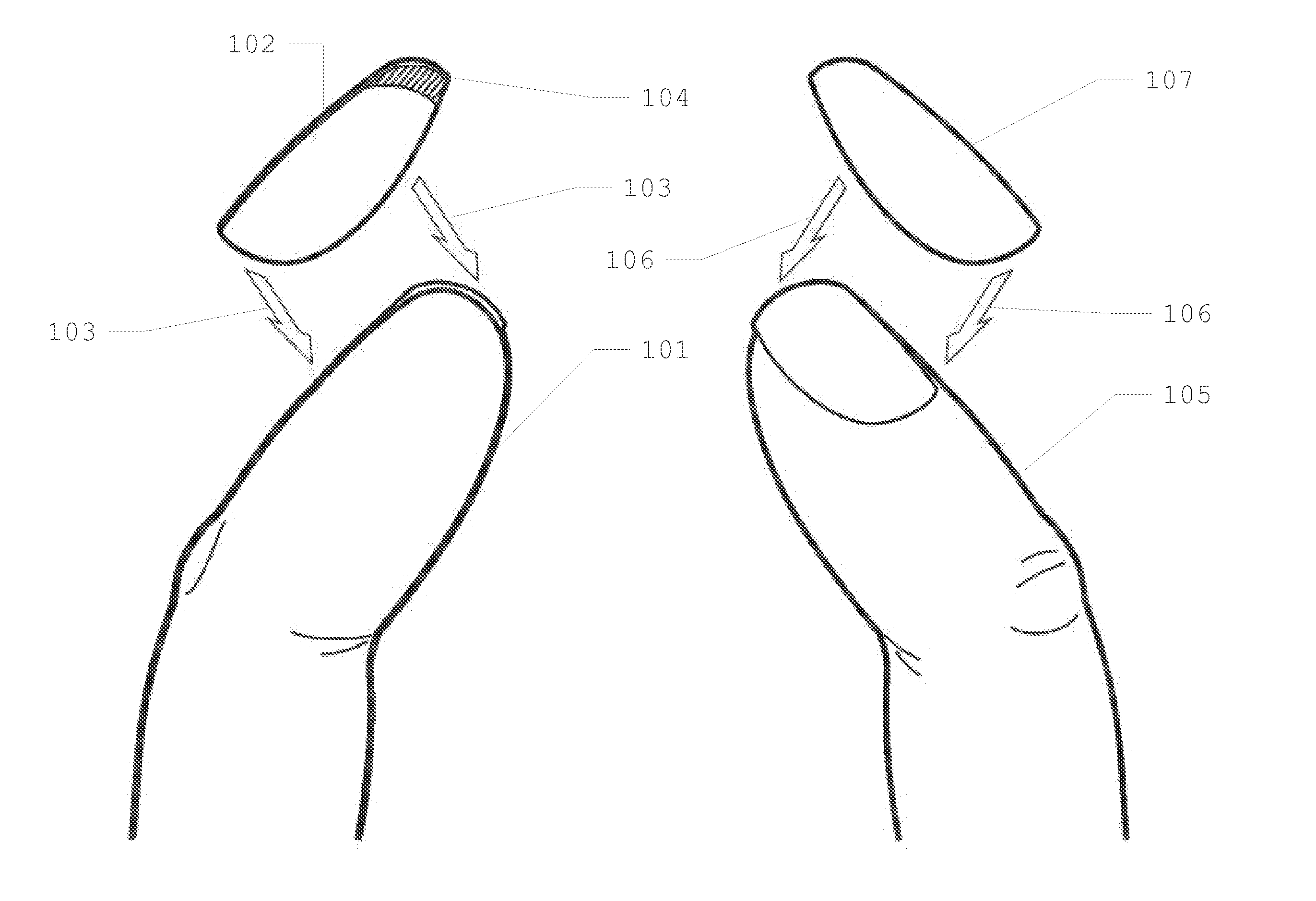
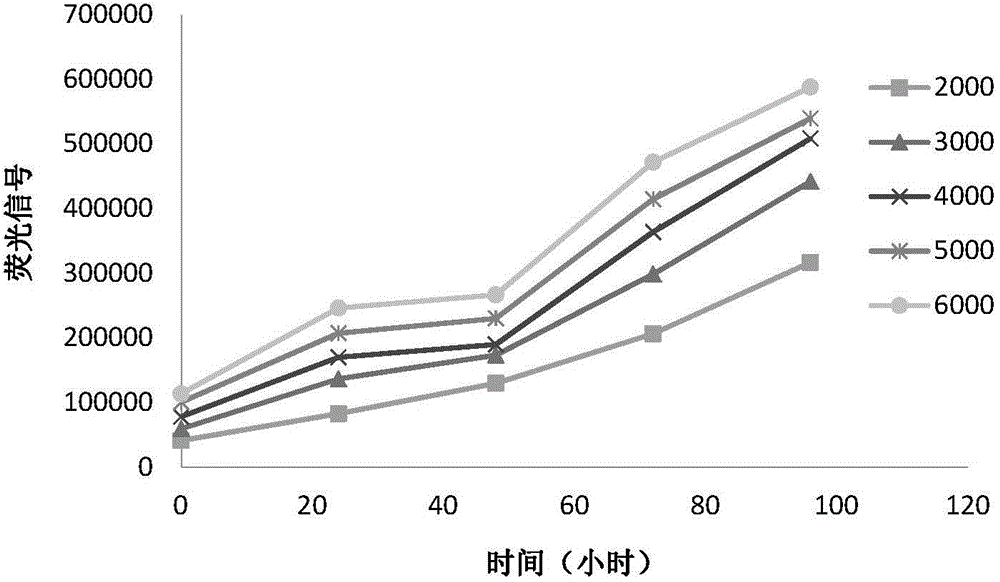
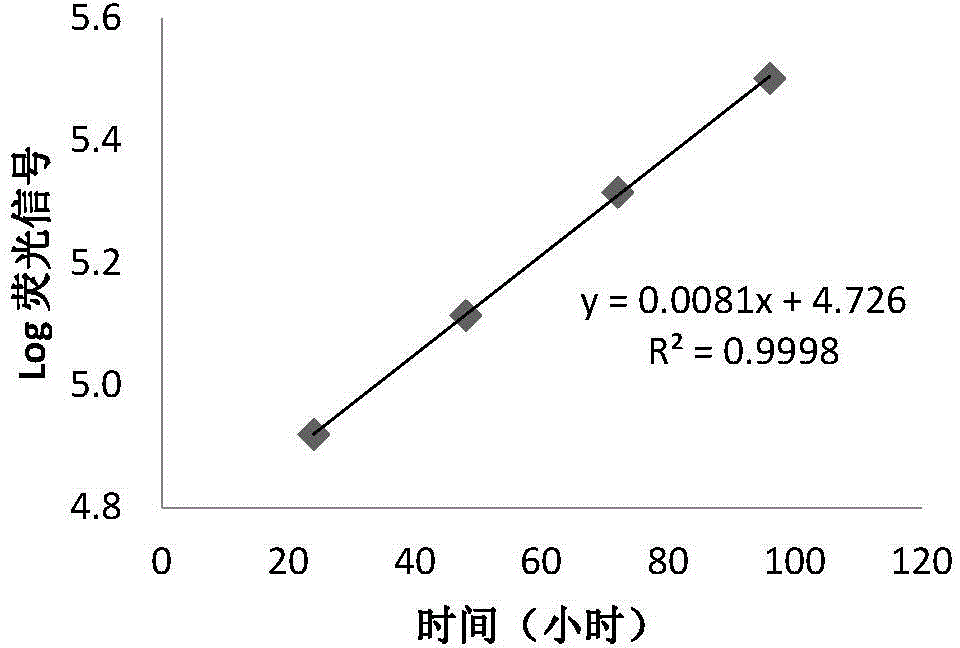
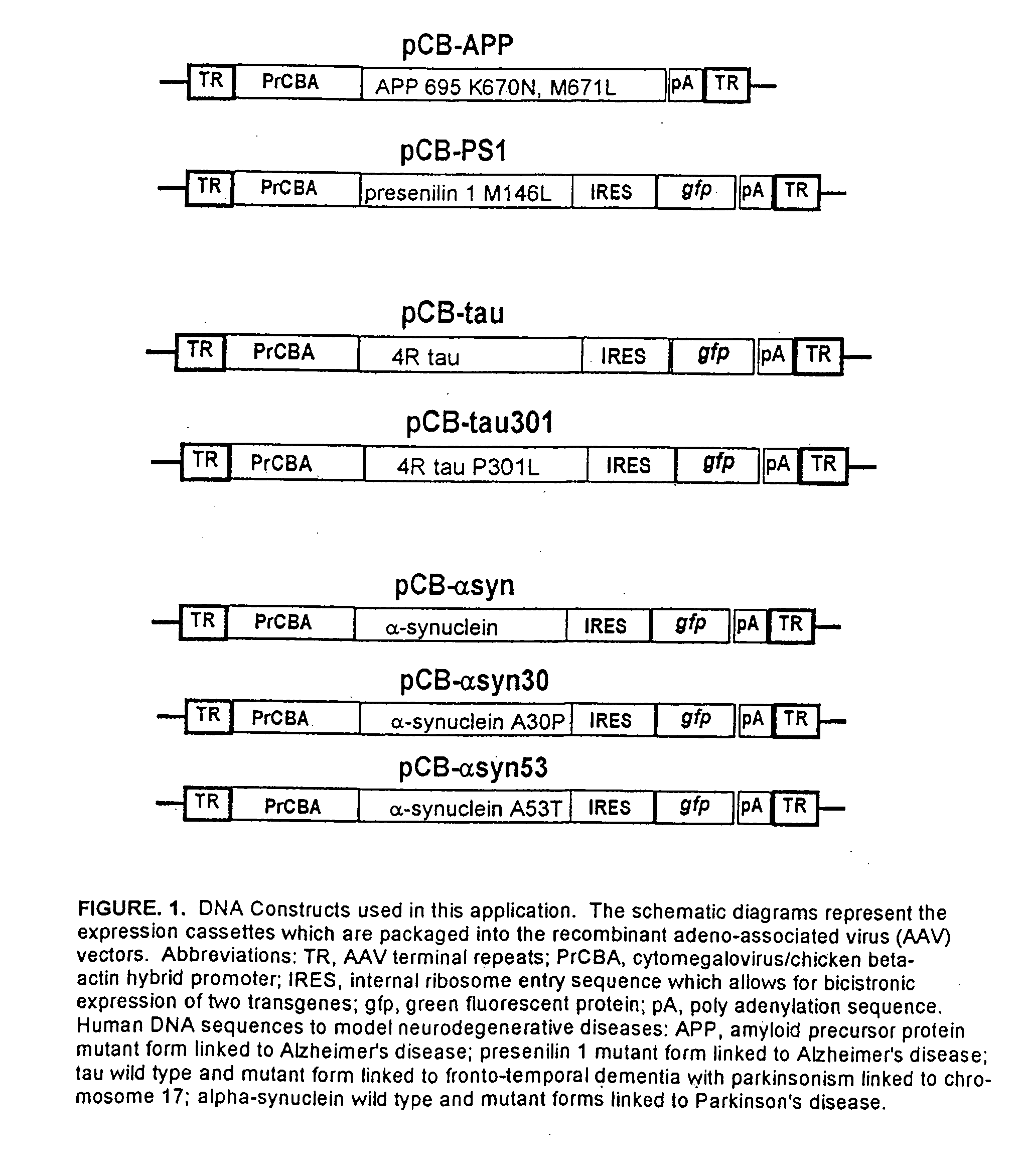
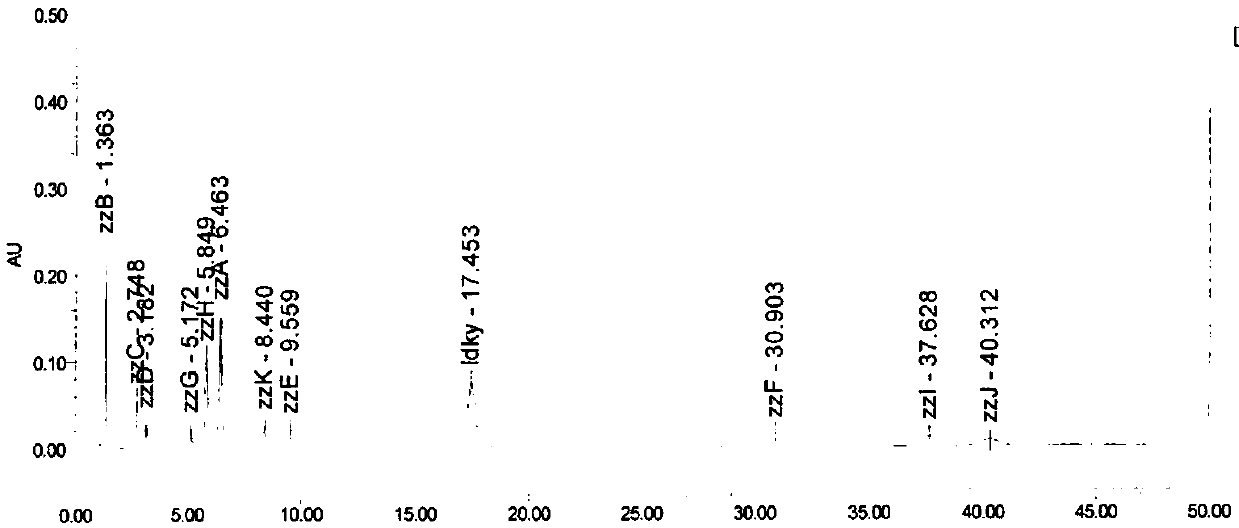
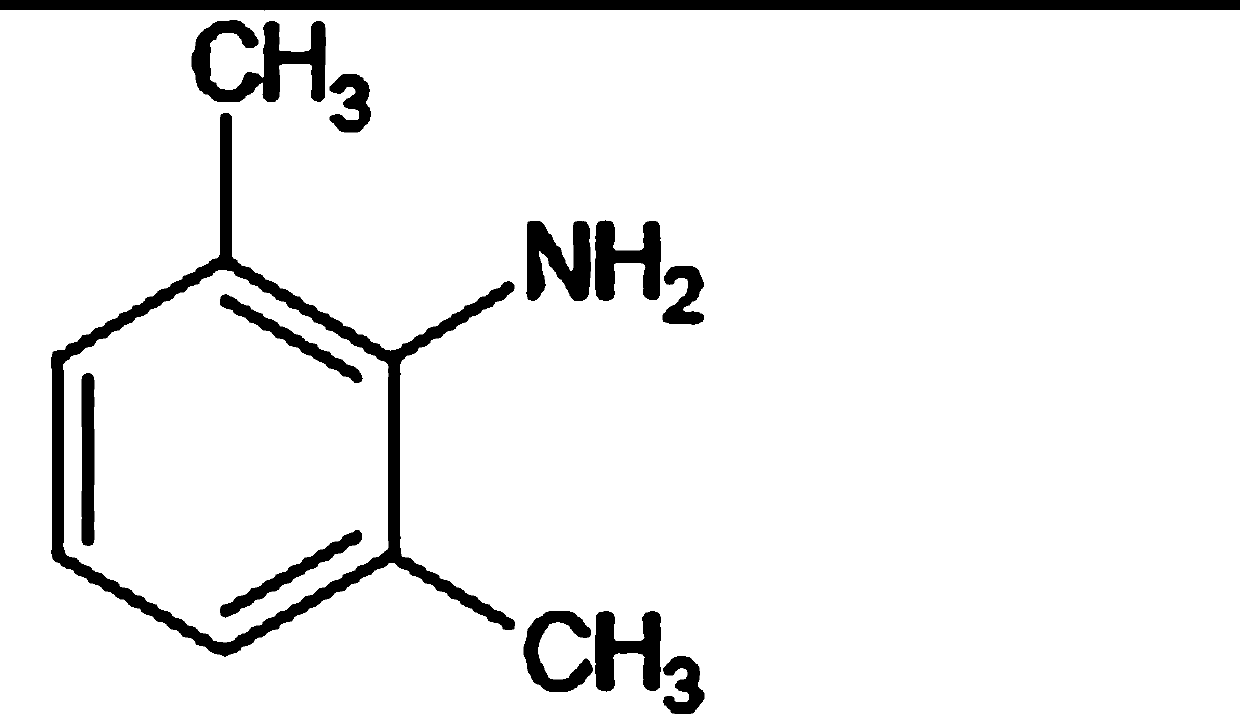
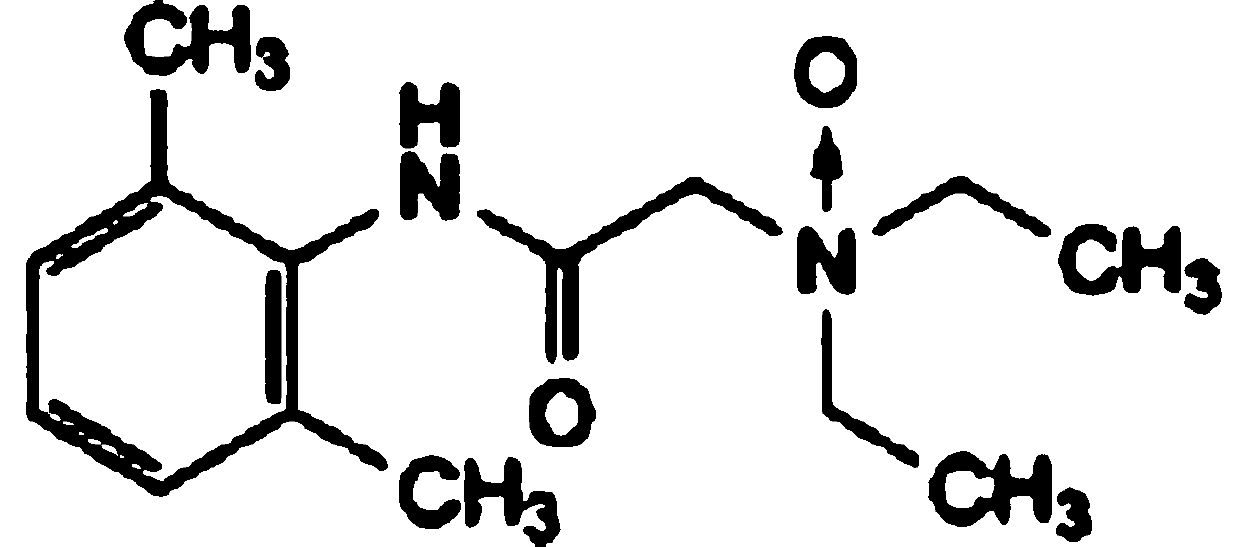
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
142 results about "Drug assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug assays or drug screening assays are tests that are performed to check for doping in sports or the presence of narcotic substances in the blood or in urine.
Application of inductively coupled plasma mass spectrometry in drug testing of hemin
The invention relates to an application of inductively coupled plasma mass spectrometry in drug testing of hemin. A method for measuring concentration of stable isotope iron in animal plasma is carried out by an ICP-MS (Inductively Coupled Plasma Mass Spectrometry) method and comprises the following steps of: providing an inductively coupled plasma source mass spectrometer; determining operating conditions of the ICP-MS; providing stable isotope iron powder, marked hemin, internal standard elements and the like; preparing standard solution and internal standard solution; preparing ICP-MS diluent; establishing a standard curve; measuring the stable isotope iron concentration in the plasma; and adding the ICP-MS diluent into a sample tube containing animal plasma sample containing iron, mixing uniformly and putting the sample tube into a refrigerator to be refrigerated, and determining the concentration of the iron in the plasma by the obtained standard curve. The application of the inductively coupled plasma mass spectrometry in the drug testing of the hemin has well performances, e.g., the method has well quantification lower limit, accuracy, absolute recovery test, sample stability, medium effect and quality control requirement, and can be taken as a content measurement method for analyzing the content of the stable isotope iron in a biological sample such as the plasma.
Owner:新疆科丽生物技术有限公司
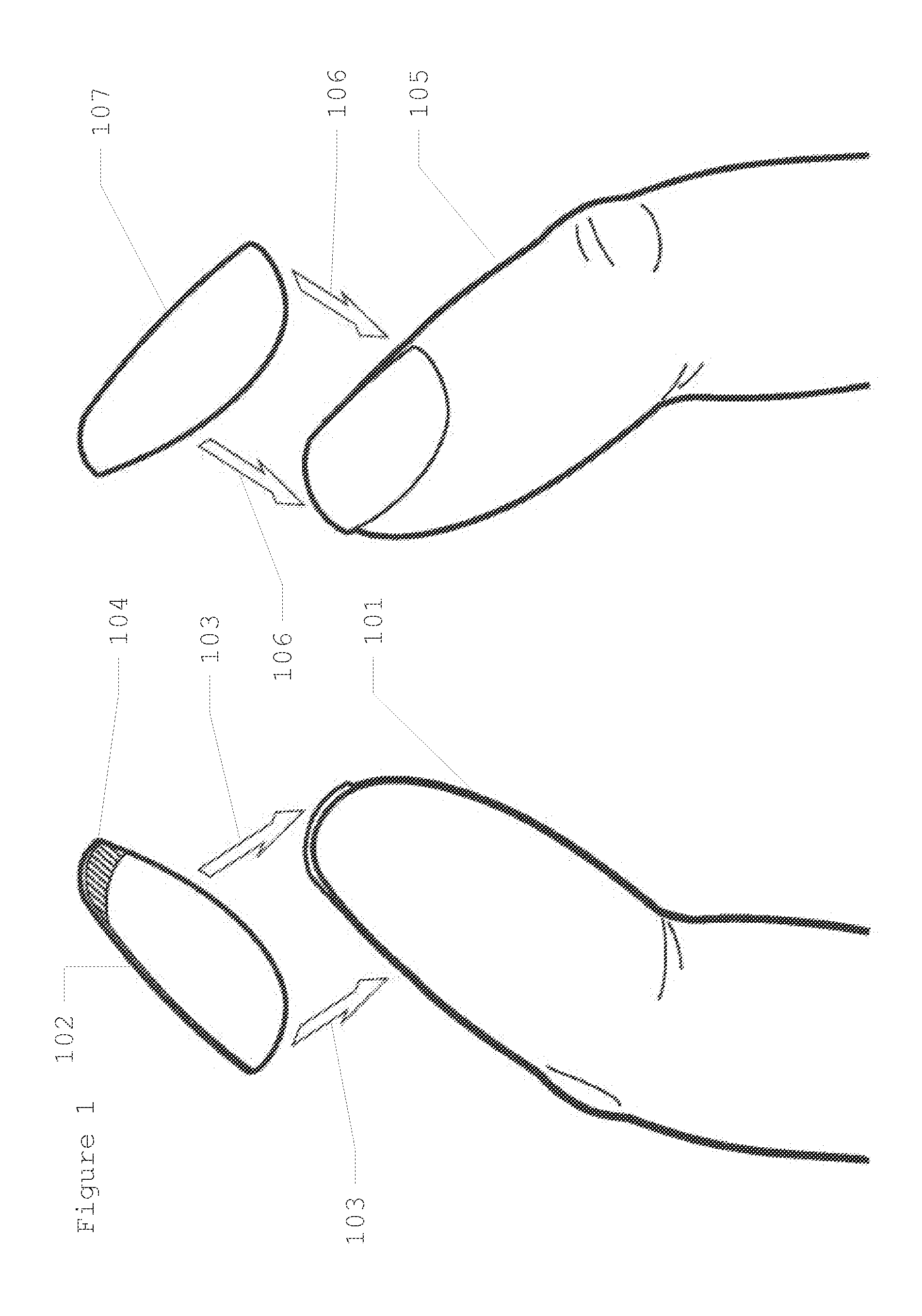
Discrete, Hidden Fingernail Mounted Date Rape Drug Detection Device
InactiveUS20130209325A1Easily alertingQuickly inserted into drinkAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorHydroxybutyric acidPharmaceutical Substances
This invention provides for a test strip to detect common “date rape” drugs, which is hidden and discretely mounted on the underside of a fingernail (hereinafter known as “A Discrete, Hidden Fingernail Mounted Date Rape Drug Detection Device”). The test strip will react positively and detect even the smallest amounts of the most common of these so called family of date rape drugs. These drugs currently include but are not limited to gamma-hydroxybutyric acid (“GEM”), drugs in the benzodiazepine family (such as flunitrazepam, or Rohypnol, or “roofies”), and ketazines. The test strip can be in one embodiment mounted on the underside of a fake fingernail, and in another embodiment mounted on the underside of a true long fingernail. When the test strip detects any one of the drugs mentioned above, a change in color will instantaneously alert the drinker that their drink has been tampered with.
Owner:HAROONI HOOSHMAND
Method for determination of illegally added substances in traditional Chinese medicines and health-care products
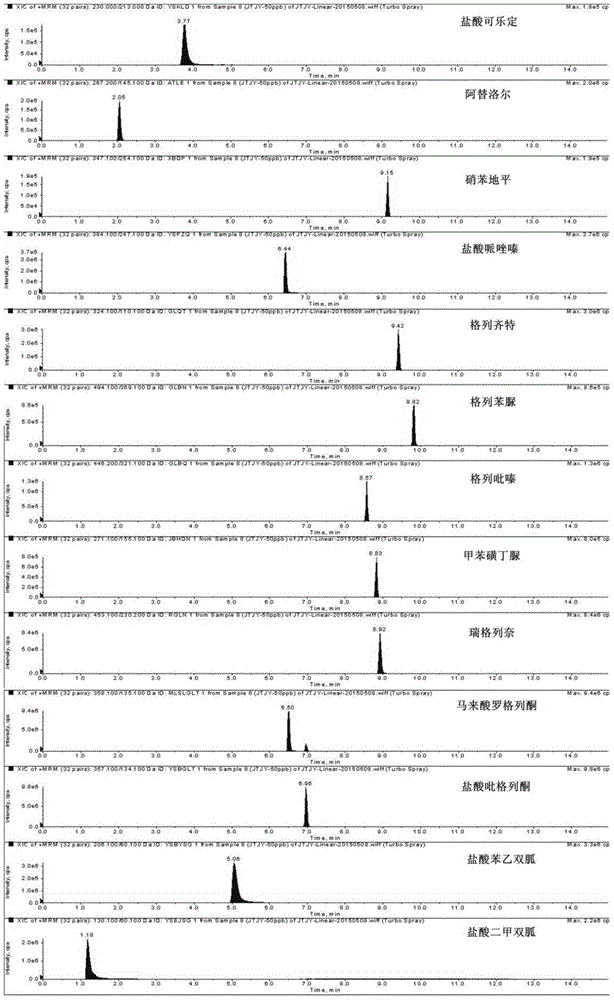
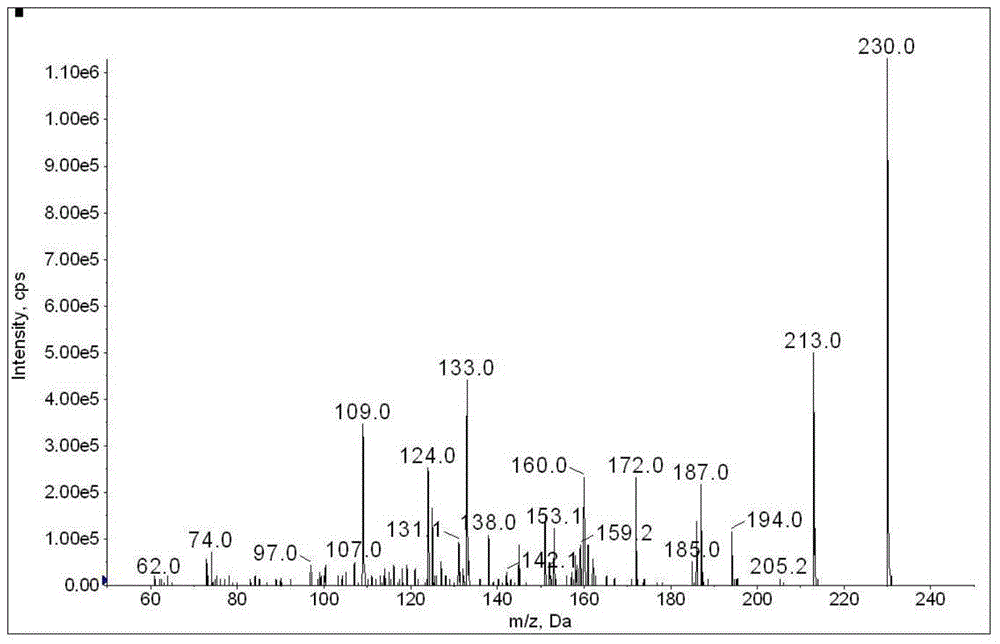
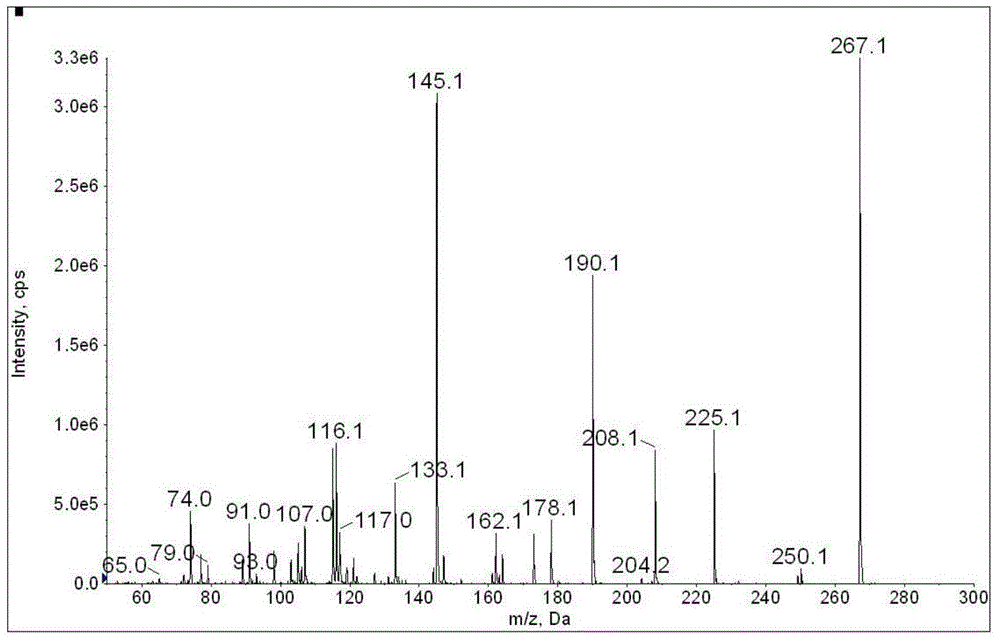
The invention discloses a method for determination of illegally added substances in traditional Chinese medicines and health-care products; with use of high performance liquid chromatography-tandem quadrupole linear ion trap mass spectrometry, the method which is capable of simultaneously qualitative and quantitative detection (multiple reaction monitoring sMRM-information dependent acquisition IDA-enhanced product ion scanning EPI detection) of 13 kinds of illegally added hypoglycemic hypotensive chemical drugs such as clonidine hydrochloride and gliclazide in the traditional Chinese medicines and the health-care products is developed. And through one-time sampling detection, not only can a quantitative result obtained, but also occurrence of a false positive result is effectively avoided through qualitative database screening. The establishment of the method provides a technical support for enacting detection standards of the illegally added hypoglycemic hypotensive chemical drugs in the traditional Chinese medicine and the health-care products.
Owner:SHANDONG ANALYSIS & TEST CENT
Method for determining various types of abuse drugs in human whole blood
InactiveCN105987965AHigh sensitivityImprove accuracyComponent separationHigh concentrationAbuse drugs
The invention belongs to the technical field of drug detection and judicial identification, and in particular relates to the detection of seven abused drugs amphetamine, methamphetamine, methylenedioxyamphetamine, methylenedioxy The method for extracting and measuring methamphetamine, ketamine, pethidine and methadone uses methanol as a dispersant and a protein precipitation solvent, uses dichloromethane as an extraction solvent, and forms a microemulsion system of a three-solvent system in an aqueous solution of pH 13, With the assistance of ultrasound, it is further emulsified for liquid-liquid microextraction, and the extraction process does not need to evaporate the extraction solvent; this method has high enrichment multiple and recovery rate, simple and fast operation, high sensitivity, strong specificity, wide linear range and It has the advantages of low cost and environmental friendliness, and can meet the characteristics of urgent judicial appraisal tasks and high requirements for detection time. The detection system of the invention provides a new technical platform for drug detection.
Owner:FUDAN UNIV
Human bile duct cancer cell line and applications thereof
ActiveCN105255832AStable traitsMicrobiological testing/measurementMicroorganism based processesMammalCancer cell lines
The present invention discloses a human bile duct cancer cell line and applications thereof, wherein the human bile duct cancer cell line is preserved in the China Center for Type Culture Collection, and has the preservation number of CCTCC NO:C201452. The human bile duct cancer cell line applications are the human bile duct cancer cell line is used for preparing the reagent for producing bile duct cancer in immunodeficient mammal. According to the present invention, the human bile duct cancer line has stable character, can be stably and repeatedly passaged, has tumorigenicity in animals, can successfully prepare bile duct cancer animal models, can be used for analyzing in vitro drug sensitivity and drug resistance and drug sensitivity and drug resistance of in vivo animal experiments so as to establish the in vitro and in vivo associated anti-tumor drug activity testing platform, is the ideal human primary bile duct cancer cell line used for basic research and preclinical application, and provides the ideal material for the further tumor occurrence mechanism research and drug testing.
Owner:SHANGHAI LIDE BIOTECH CO LTD +1
Drug detection device
InactiveUS20120070901A1Material analysis by observing effect on chemical indicatorTesting beveragesChange colorColor reaction
The present invention relates to a drug detection device for detecting the presence of a date rape drug in a beverage comprising: a drink consumption device such as a straw; and a chemical reagent, where said chemical reagent may be integrated with the straw and causes the straw to change color upon contact with a date rape drug. In one exemplary embodiment, the drug detection device may be used to test for Gamma Hydoxybutyrate (GHB) or ROHYPNOL® (Flunitrazepam). A stirring straw may be used as a component of the drug detection device as opposing to a conventional straw. The present invention further relates to a method of manufacturing a date rape drug detection device comprising the steps of: integrating a drink consumption device with a chemical reagent; and enabling a visual color reaction within the consumption device upon contact with a date rape drug.
Owner:BRADLEY MICHAEL KEITH +1
Method and kit for detecting 19 drugs and metabolites thereof in blood by liquid chromatography-tandem mass spectrometry
The invention belongs to the technical field of drug detection, and particularly relates to a method and a kit for detecting 19 drugs and metabolites thereof in blood through liquid chromatography-tandem mass spectrometry. The substances to be detected comprise sulpiride, pentafluridol, mianserin, buspirone, tandospirone, hydroxyazine, diazepam, venlafaxine, moclobemide, imipramine, paroxetine, reboxetine, amitriptyline, sertraline, digoxin, clonazepam, clopidogrel, toluenesulfobutyl urea, glimepiride, 1-pyrimidinepiperazine, desmethylvenlafaxine, 6-hydroxy buspirone and normipramine, and the substances to be detected are selected from the group consisting of sulpiride, pentafluridol, mianserin, venlafaxine, metandospirone, metandospirone, hydroxazine, diazepam, venlafaxine, moclobemide, the pharmaceutical composition is prepared from noramitriptyline, nordiazepam and clopidogrel metabolite; the detection method comprises the following steps: calibrating a standard solution, treating a to-be-detected sample, and detecting the to-be-detected sample by adopting high performance liquid chromatography-mass spectrometry. The embodiment of the invention can quickly and accurately measure the content, and the sample treatment method is simple and easy to implement, high in sensitivity and accurate in quantification.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Device and method for detecting beta-agonists drug
InactiveCN102645532AAchieving Simultaneous DetectionReduce testing costsMaterial analysisMedicineAgonist
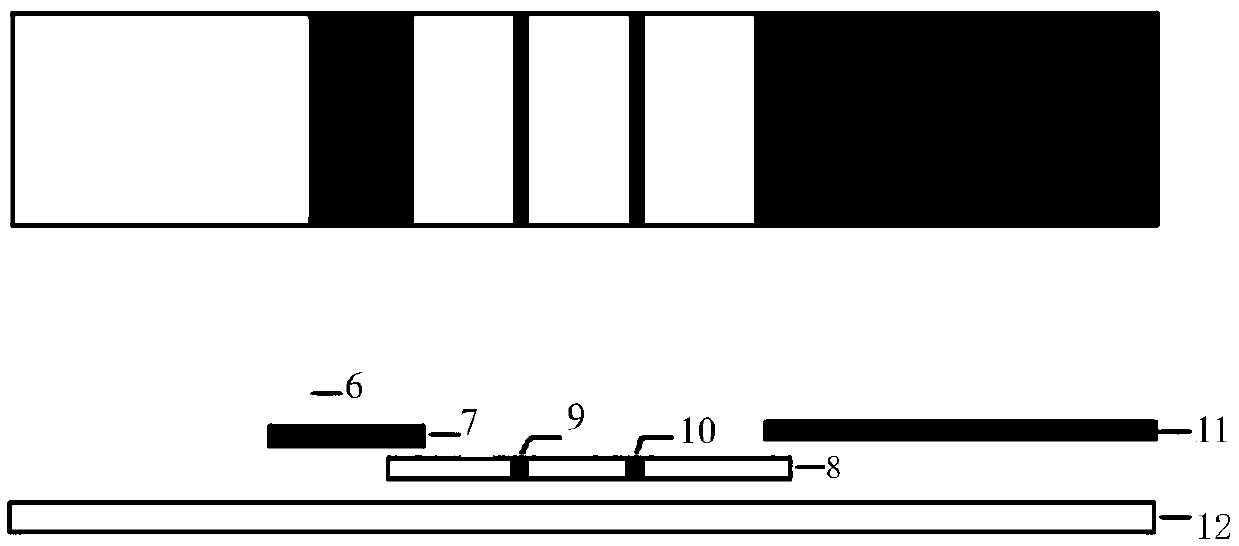
The invention relates to the field of biological detection and discloses a detection device and a detection method of a large class of beta-agonists drugs by applying an immune chromatography technology. According to the invention, an antibody / receptor which is reacted with a large class of the beta-agonists drugs is used for preparing an immune chromatography test strip to realize the simultaneous detection of a large class of the beta-agonists drugs. Compared with the prior art, the detection on a large class of the beta-agonists drugs for one time can be realized, the detection cost is low and the method is convenient and fast.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD +1
Method for determining blood drug level of mizoribine
InactiveCN1804615ADetermination does not interfereStrong specificityComponent separationOther chemical processesPretreatment methodBlood plasma
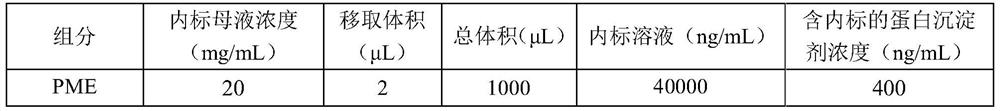
The invention relates to an internal drug assay determination method in the field of medical examination, which relates to a method for measuring human plasma imidazole density. It dose equal indicative elution on acid traveling phase condition after doing first treatment to the tested sample by protein depositing and uses ultraviolet detector to test it after chromatographic column separating.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Preparation of enzyme linked immunosorbent assay kit used for detecting dihydropyridine drug residues
InactiveCN105319353ASimple and efficient operationSensitive detectionMaterial analysisSolid phasesAntigen
The invention discloses preparation of an enzyme linked immunosorbent assay (ELISA) kit used for detecting dihydropyridine drug residues, and a detection method for detecting dihydropyridine drug residues. The enzyme linked immunosorbent assay kit is high in detection sensitivity, accuracy, speed, and specificity; operation is simple; and the detection method is suitable for large-scale sample detection. The enzyme linked immunosorbent assay kit comprises an enzyme labeled plate coated with dihydropyridine drug antigens, a dihydropyridine drug standard substance, a dihydropyridine drug antibody working solution, a dihydropyridine drug enzyme labeled second antibody working solution, a substrate solution A, a substrate solution B, a stopping solution, a concentrated sample diluent, and a concentrated washing liquid. Solid phase indirect competitive enzyme-linked immune response is taken as the principle of the enzyme linked immunosorbent assay kit used for detecting dihydropyridine drugs. According to the detection method, an extracted sample, the dihydropyridine drug enzyme labeled second antibody working solution, and the dihydropyridine drug antibody working solution are added into corresponding enzyme labeled holes for a certain time of incubation; after plate washing, the substrate solution A, and the substrate solution B are added, so that the color of an obtained mixture is changed to be blue under enzyme action; and the stopping solution is added, and the color is changed from blue to yellow. The shade of color developing and the content of dihydropyridine drugs in the standard substance or the sample are inversely related. The direction method can be used for directly detecting dihydropyridine drugs in health care products.
Owner:JIANGSU WISE SCI & TECH DEV
Drug-sensitive kit and preparation method thereof and bacterial drug sensitivity testing method
ActiveCN110846377AThe test result is accurateShort timeMicrobiological testing/measurementBiological material analysisBacterial diseaseVeterinary Drugs
The invention belongs to the technical field of drug testing and discloses a drug-sensitive kit and a preparation method thereof and a bacterial drug sensitivity testing method. The kit comprises a drug-sensitive plate, liquid culture media and solid culture medium panels. Conventional and latest veterinary drugs are selected for making the drug-sensitive plate, and the different culture medium panels are prepared according to different infection bacteria. The prepared drug-sensitive kit has advantages of accurate testing results, low time consumption, simplicity and convenience in operation and low cost and is suitable for drug sensitivity testing for bacterial diseases in large-scale farms and basic veterinary institutions, and accordingly accuracy in animal medication can be realized.
Owner:NORTHWEST A & F UNIV
Method for detecting residue of sodium pentachlorophenate and application
InactiveCN106841416AIncrease costAchieve the purpose of purificationComponent separationChemistryToxicity
The invention discloses a method for detecting residue of sodium pentachlorophenate, which belongs to the technical field of drug detection. The method for detecting the residue of the sodium pentachlorophenate comprises the following steps: extracting residue of sodium pentachlorophenate in a sample by utilizing sulfuric acid and normal hexane, and then detecting by utilizing a high performance liquid chromatography-mass spectrometry, wherein the concentration of the sulfuric acid solution is 6 percent. The method has the beneficial effects that the method is suitable for detecting the residue of various matrix sodium pentachlorophenate, the used reagent is low in cost and small in toxicity, impurities can be effectively removed, and the operation steps can be simplified; and by adopting the high performance liquid chromatography-mass spectrometry, compared with the single high performance liquid chromatography, the method has the advantages of high sensitivity, good repetition and low detection limit.
Owner:杭州海润泰合检测技术有限公司
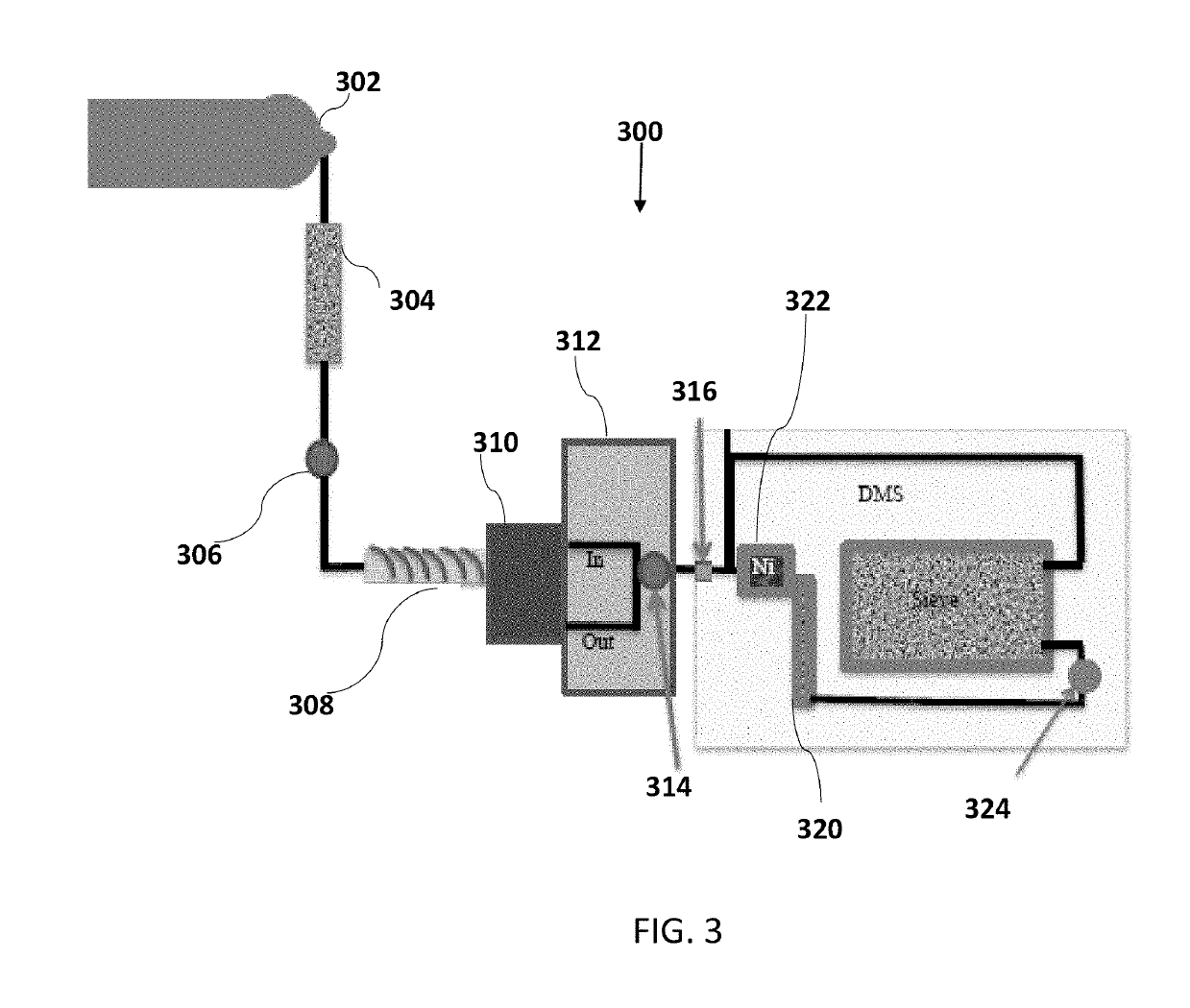
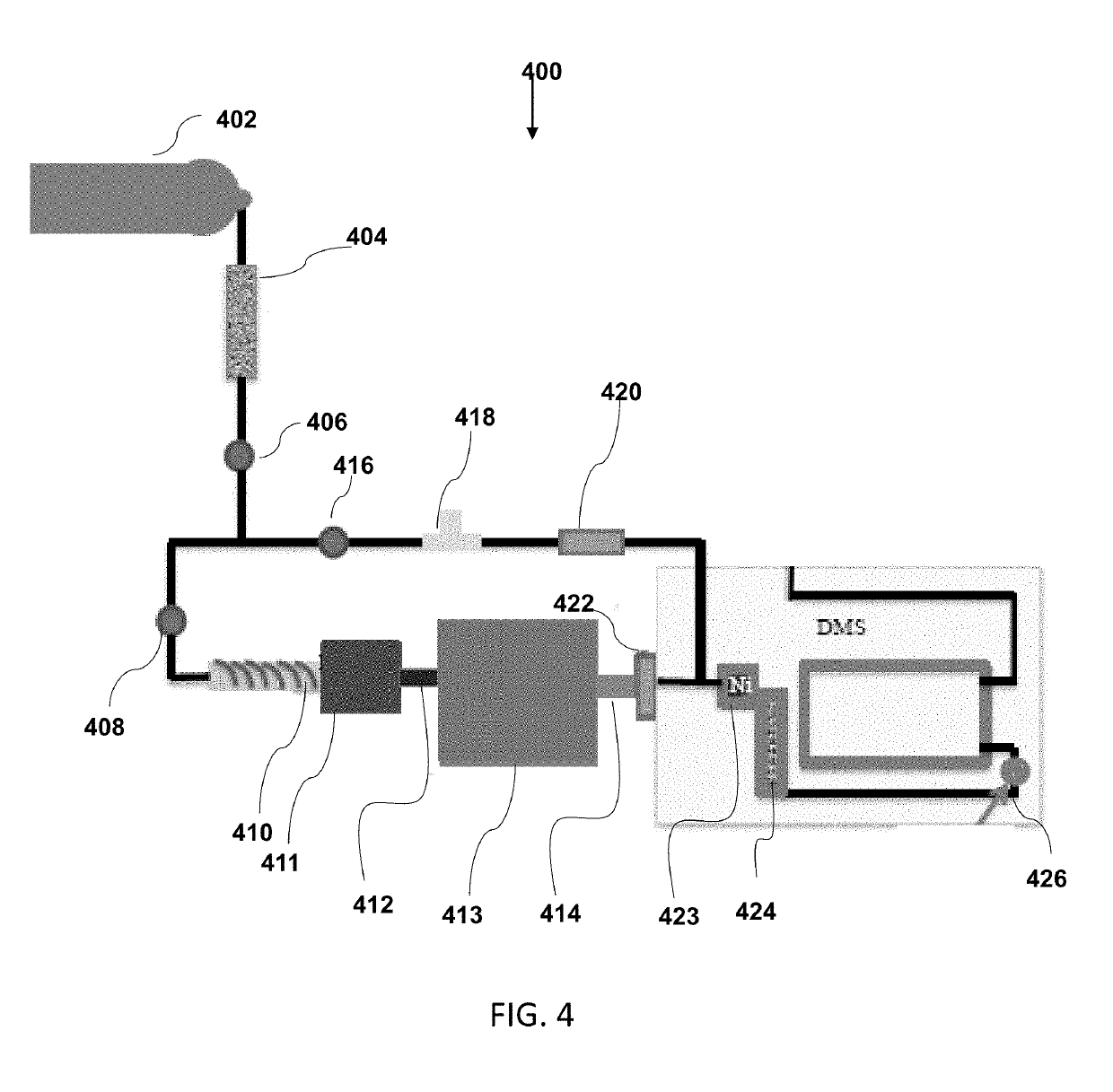
In-vivo drug testing system based on nanoparticle modified hollow-core optical fiber
The invention relates to an in-vivo drug testing system based on a nanoparticle modified hollow-core optical fiber. The system comprises a spectrograph, the hollow-core optical fiber, a laser light source, a lens combination optical path and a computer, wherein the hollow-core optical fiber is the nanoparticle modified hollow-core optical fiber; the laser light source is connected with the hollow-core optical fiber by the output end of the lens combination optical path; one end, modified by nanoparticles, of the hollow-core optical fiber is inserted into a body; the other output end of the lens combination optical path is connected with the spectrograph and the spectrograph is connected with the computer; laser is used for stimulating in-vivo drug molecules to generate optical signal information; then the optical signal information is transmitted to the lens combination optical path through the hollow-core optical fiber and then is transmitted to the Raman spectrograph by the lens combination optical path; and content change information of drugs is judged by analyzing a spectrum through the computer. The system can be used for in-vivo drug testing of animals, human bodies and the like, and provides technical supports for individualized medicines, scientific researches and drug development.
Owner:SHANGHAI UNIV
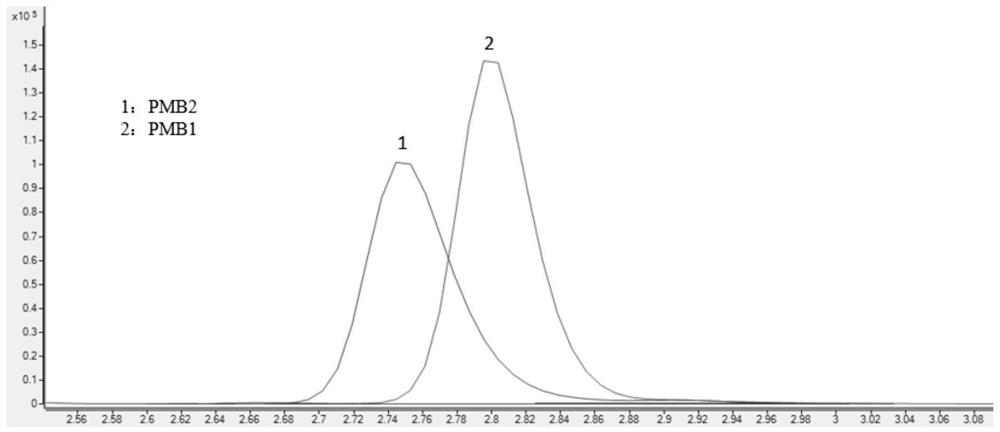
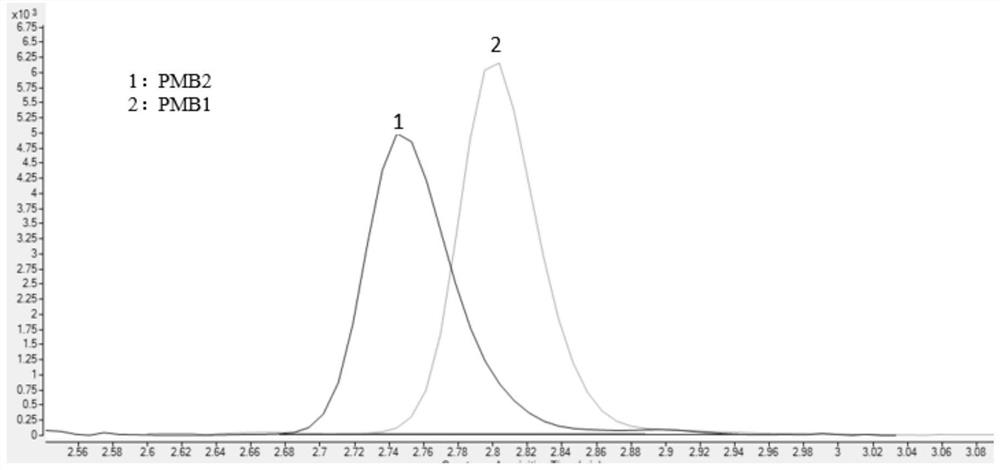
Method for detecting concentrations of polymyxin B1 and polymyxin B2 in serum
InactiveCN111830153AHigh sensitivityStrong specificityComponent separationPolymyxin B1Drug detection
The invention discloses a method for detecting concentrations of polymyxin B1 and polymyxin B2 in serum, and belongs to the technical field of drug detection. The method comprises the following stepsof: taking pretreated serum, separating an object to be detected from a serum matrix by using ultra-high performance liquid chromatography, and calculating the contents of the polymyxin B1 and the polymyxin B2 by using a mass spectrum internal standard quantitative method according to an established calibration curve. The method disclosed by the invention is high in sensitivity, strong in specificity, accurate and simple in pretreatment process; the separation and the detection of the polymyxin B1 and the polymyxin B2 in the serum are completed within 5.0 minutes; the accuracy and precision basically meet the requirements; the method can be used for quantitatively analyzing polymyxin B1 and polymyxin B2 drugs in serum clinically; and a simple and rapid detection method is provided for monitoring the concentrations of the polymyxin B1 and polymyxin B2 drugs clinically.
Owner:NANJING QLIFE MEDICAL TESTING LAB CO LTD
All-in-one sampling detector for DNA sample collection and saliva alcohol and drug detection
PendingCN108414513ASuitable for useSimple structureMaterial analysis by observing effect on chemical indicatorSurgical needlesAlcohol contentSaliva sample
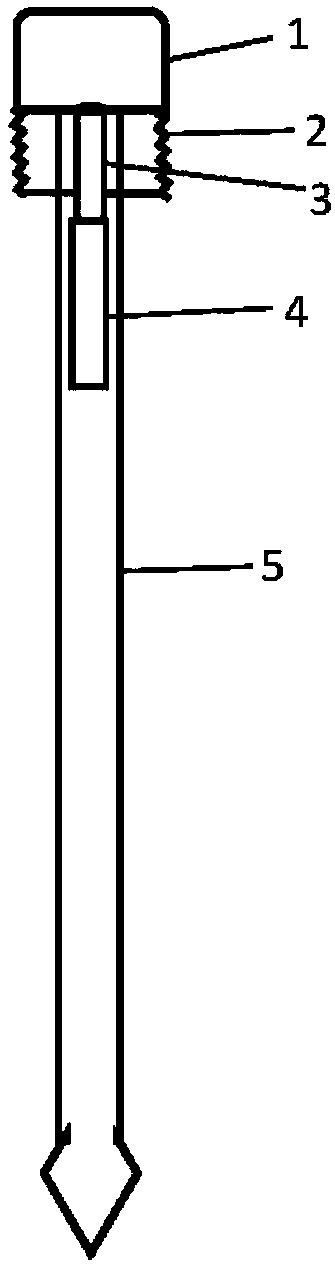
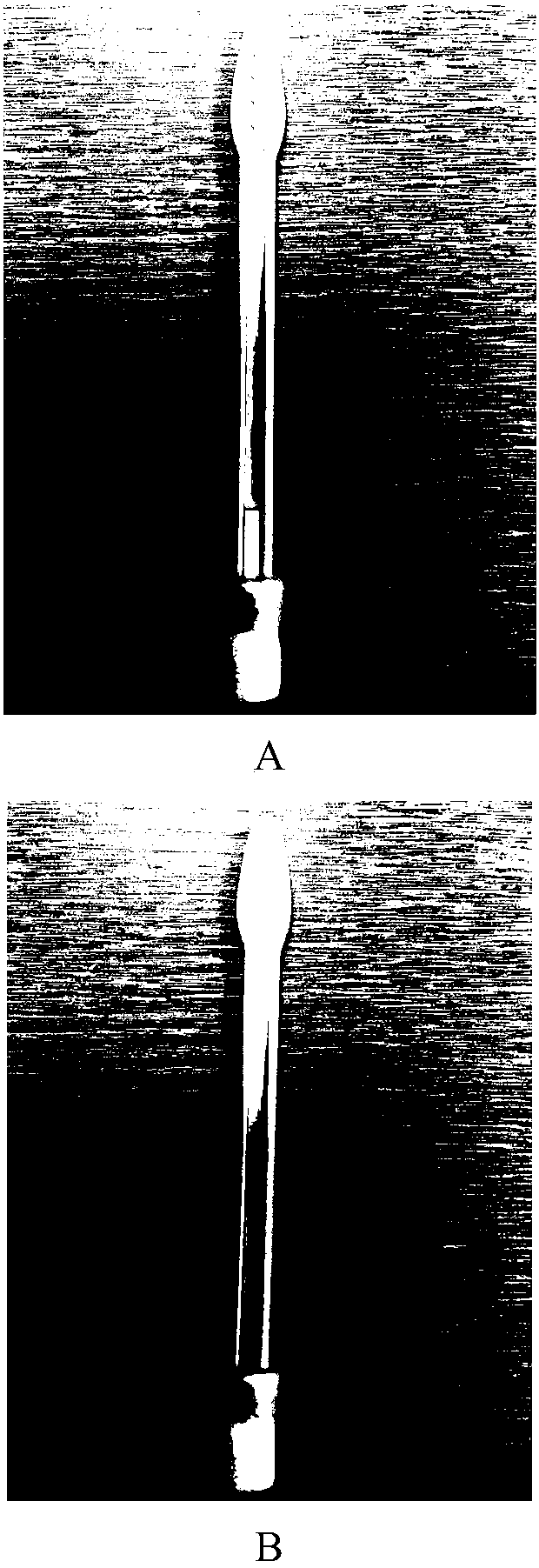
The invention provides a novel all-in-one sampling detector for DNA sample collection and saliva alcohol and drug detection. The all-in-one sampling detector consists of a sampling detection rod and adrug detection card, wherein the sampling detection rod is used for saliva and DNA sample collection and alcohol detection. According to the invention, a detection card which can be used for detection of a drug in a saliva sample is prepared based on colloidal gold competitive inhibition method immunochromatography. The all-in-one sampling detector provided by the invention is convenient to use and simple to operate, and can complete DNA sample collection and detection of three indicators of saliva alcohol content while completing drug saliva detection. This product is simple in structure andsmall in size, is suitable for on-site use during law enforcement, and is also suitable for individuals and families to use at any time; painless collection is realized without any trauma or invasiveness, so that a user does not feel any discomfort; the detector is disposable, and has no risk of cross-infection. The collected DNA sample is suitable for genetic analysis and detection and other medical examination.
Owner:北京中生朗捷生物技术股份有限公司
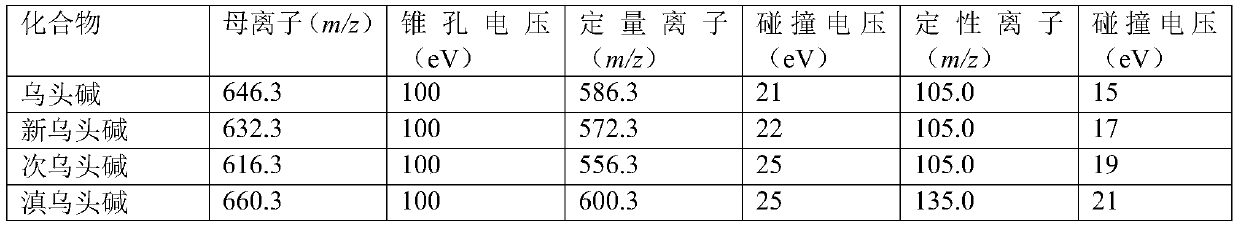
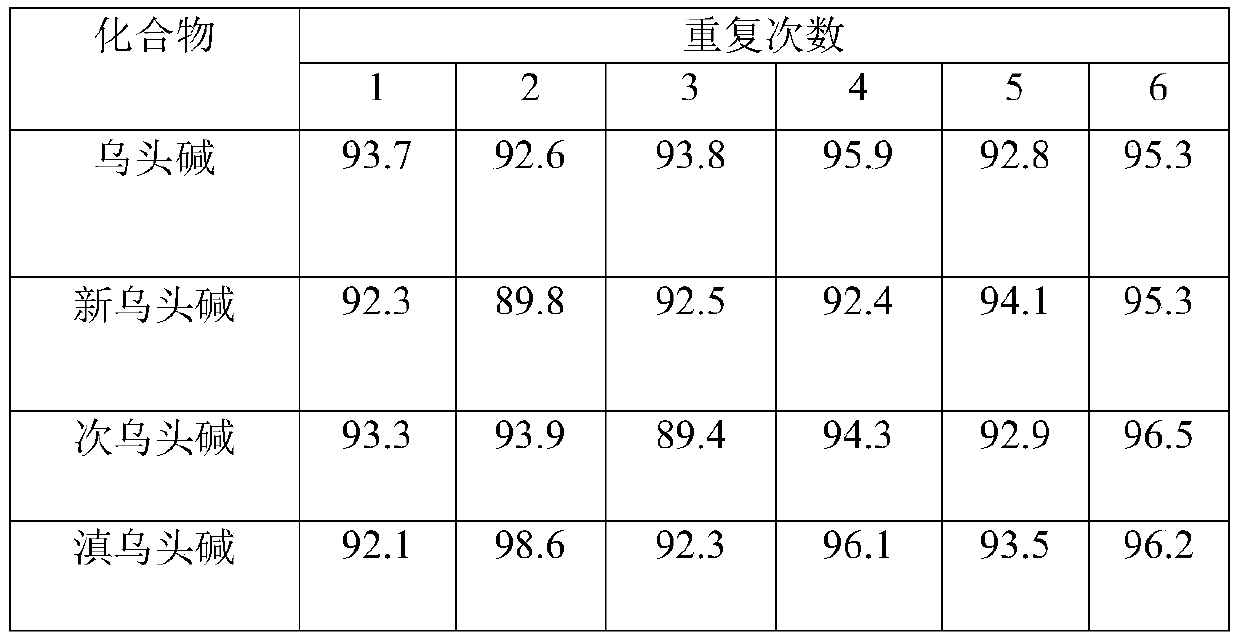
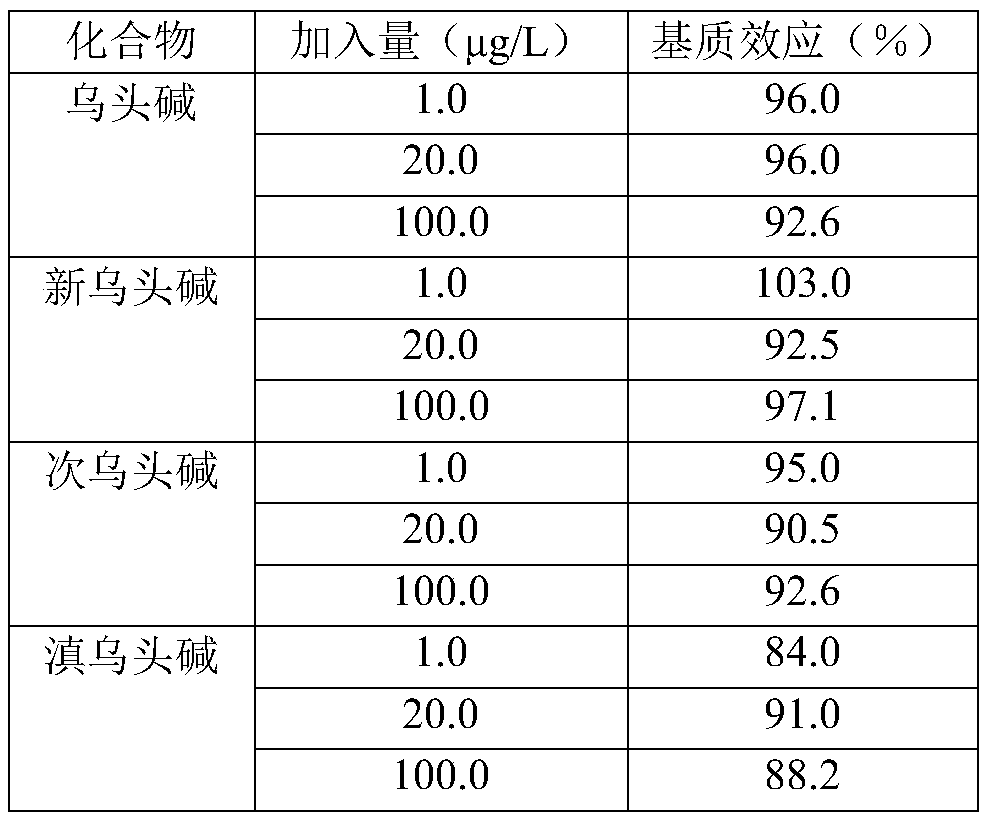
Method for determining four diester aconitum alkaloids in blood
InactiveCN111435128AGuaranteed specificityReduce the impactComponent separationSorbentPharmaceutical drug
The invention relates to the technical field of drug detection, and provides a method for determining four diester aconitum alkaloids in blood in order to solve the problems of many interferents, longdetection time and low accuracy of an aconitum alkaloid detection method. The method comprises the following steps: (1) adding a stabilizer into a plasma sample, and storing the plasma sample at lowtemperature for later use; (2) adding an extraction solvent for extraction to obtain an extracting solution; (3) adding a magnetic graphene-based adsorbent for purification treatment to obtain a purified solution; and (4) filtering the purified liquid, and performing HPLC-MS / MS on-machine detection and analysis. According to the invention, the method for detecting four diester aconitum alkaloids in a blood sample is established through pretreatment of a blood sample and optimization of detection conditions, the method is rapid and accurate, the result is objective and easy to analyze, the content change of the four diester aconitum alkaloids in a poisoned patient can be monitored in real time, and a scientific basis can be provided for clinical diagnosis and treatment of aconitum alkaloidpoisoning.
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
Particle-based drug detection method and device embodiments
ActiveUS20190195899A1Preparing sample for investigationMaterial analysis by electric/magnetic meansPharmaceutical drugDrug detection
Disclosed herein are embodiments of methods for detecting the presence of and amount of drugs in a sample, particularly a particle sample obtained from a subject. In particular disclosed embodiments, the particle samples are skin particle samples, saliva particle samples, and / or mucous samples isolated from a subject and analyzed using thermal desorption methods combined with a selected detection method.
Owner:WASHINGTON STATE UNIVERSITY
Method for directly determining three degradation products of tryptophan in compound amino acid injection
InactiveCN111537637AEasy to analyzeNo distractionComponent separationAmino Acid InjectionGradient elution
The invention belongs to the technical field of drug detection, and particularly relates to a method for directly determining three degradation products of tryptophan in a compound amino acid injection. The three degradation products of tryptophan refer to dioxindole alanine, kynurenine and 2-hydroxytryptophan. The method comprises the following specific steps: (1) preparing the following chromatographic conditions of a liquid chromatograph: a chromatographic column being an octadecylsilane chemically bonded silica chromatographic column; a mobile phase comprising a mobile phase A being a phosphate buffer solution and a mobile phase B being acetonitrile; the flow rate being 0.95-1.05 mL / min; the column temperature being 25-35 DEG C; the sample size being 5-100 [mu]L; gradient elution; andthe detection wavelength being 254 nm; (2) preparing a test solution; (3) preparing a reference substance solution; and (4) determining. The method has the advantages of strong specificity, high sensitivity and good repeatability, solves the technical problems of interference of other amino acid components in the prescription, inaccurate determination result caused by derivative reaction in otherdetection methods, and the like, and ensures the product quality.
Owner:SHANDONG QIDU PHARMA
High performance liquid chromatography detection method of recombinant protein drug charge heterogeneous impurities
ActiveCN111175404AEfficient separationHigh detection sensitivityComponent separationProtein targetGradient elution
The invention belongs to the technical field of drug detection and particularly discloses a high performance liquid chromatography detection method of recombinant protein drug charge heterogeneous impurities. The method comprises steps of adding a certain proportion of organic solvent into a mobile phase, adding organic phase gradient elution on the basis of salt gradient elution, and selecting chromatographic conditions, such as chromatographic column, mobile phase A and mobile phase B components, column temperature, detection wavelength and gradient elution conditions, thereby obtaining thehigh performance liquid chromatography detection method. Tests prove that the method can effectively separate the target protein from the charge heterogeneous impurities, and the method has advantagesof good repeatability, simplicity, rapidness, high detection sensitivity, low cost and the like, in addition, the detection method is high in universality, and charge heterogeneity impurities of insulin recombinant protein drug samples can be effectively separated and detected.
Owner:DONGGUAN HEC BIOPHARMACEUTICAL R&D CO LTD
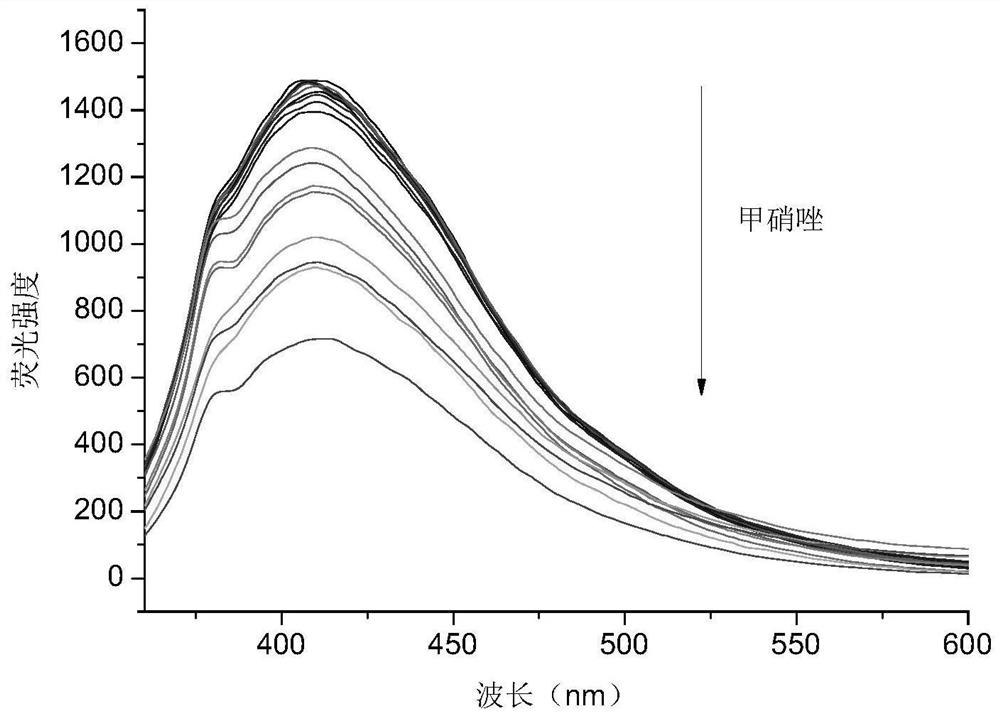
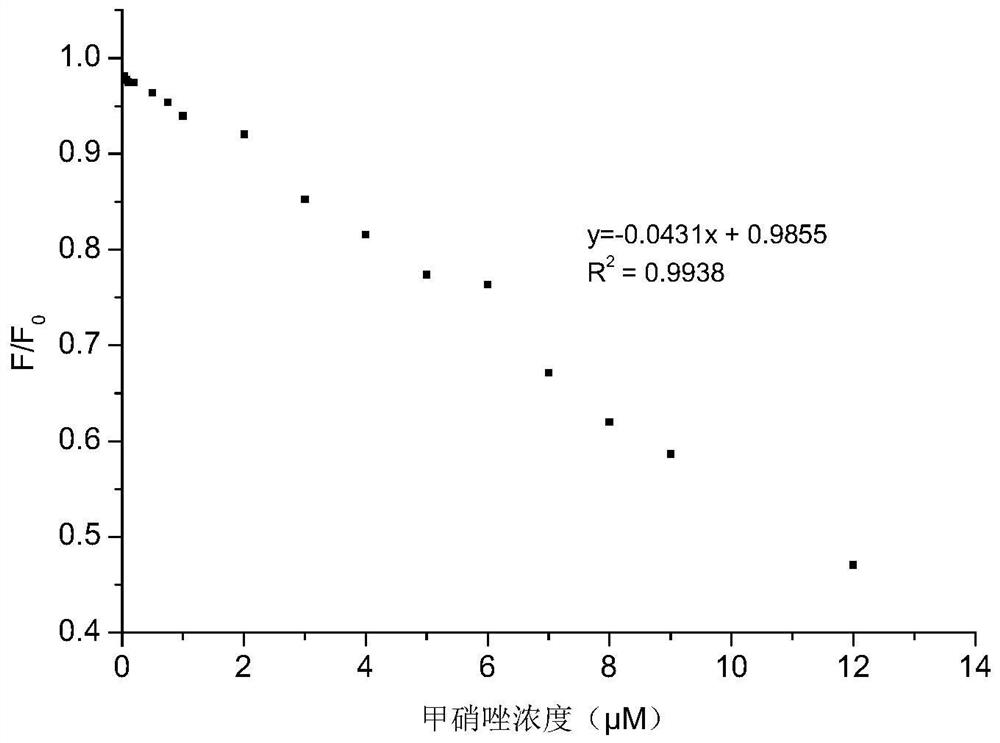
Carbon dot powder with high fluorescence property, preparation method of carbon dot powder and application of carbon dot powder in detection of nitroimidazole drugs
ActiveCN113025322AAbundant resourcesEasy to operateFluorescence/phosphorescenceLuminescent compositionsNitroimidazoleFreeze-drying
The invention belongs to the technical field of preparation of nano composite materials, and particularly relates to carbon dot powder with high fluorescence performance, a preparation method of the carbon dot powder and an application of the carbon dot powder in detection of nitroimidazole drugs. The preparation method comprises the steps: uniformly mixing dried defatted rice bran with deionized water and a nitrogen source, carrying out a hydrothermal reaction, centrifuging the reacted solution, passing the supernatant through a membrane, optionally dialyzing, and freeze-drying to obtain carbon dot powder. The higher the content of the doped nitrogen element is, the quantization yield is greatly increased, and the quantization yield can be increased to 32.44% from 0.73% to the maximum; An adopted carbon source is defatted rice bran, raw materials are common, cheap and easy to obtain, and the preparation method of carbon dots is simple to operate and relatively green and environment-friendly; the quantization yield of the prepared nitrogen-doped carbon dots is increased by ten times or more compared with the quantization yield of non-doped nitrogen source carbon dots. The carbon dots have high detection sensitivity on the nitroimidazole drugs, has a wide linear range, and is suitable for trace detection of the nitroimidazole drugs.
Method for detecting residual solvent in antiangitide by utilizing gas chromatographic method and application
PendingCN113607869AEffective quality controlStrong specificityComponent separationGas liquid chromatographicPhysical chemistry
The invention relates to the technical field of drug detection, and particularly discloses a method for detecting a residual solvent in antiangitide by using a gas chromatographic method and application. According to the method for detecting the residual solvent in antiangitide by utilizing the gas chromatographic method, the residual solvent is dimethyl sulfoxide, a direct sample injection mode is adopted, and a programmed heating mode is as follows: the initial temperature is 50-60 DEG C and is kept for 1-2 minutes; and heating is performed to 210-220 DEG C at the heating rate of 20 DEG C / min, the temperature is kept for 4-6 minutes, and the process is finished after 13-16 minutes. The gas chromatographic detection method is high in sensitivity and good in specificity, stability and accuracy, the quality of antiangitide can be effectively controlled, and the quality of antiangitide is stable, controllable and safe.
Owner:BEIJING SAISHENG PHARMA
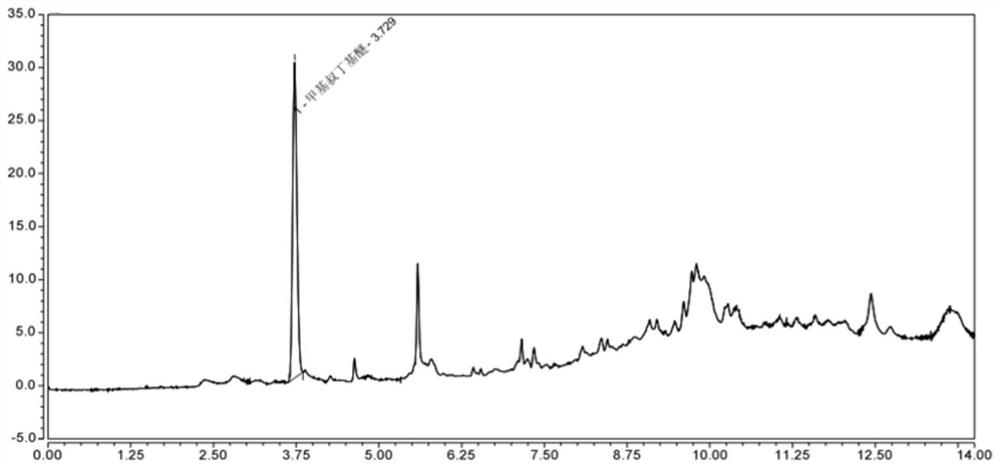
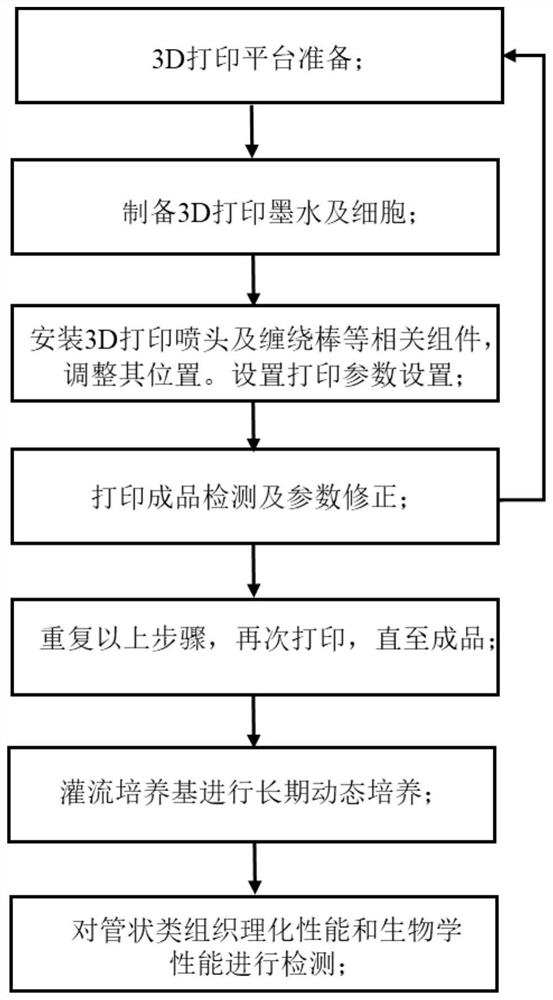
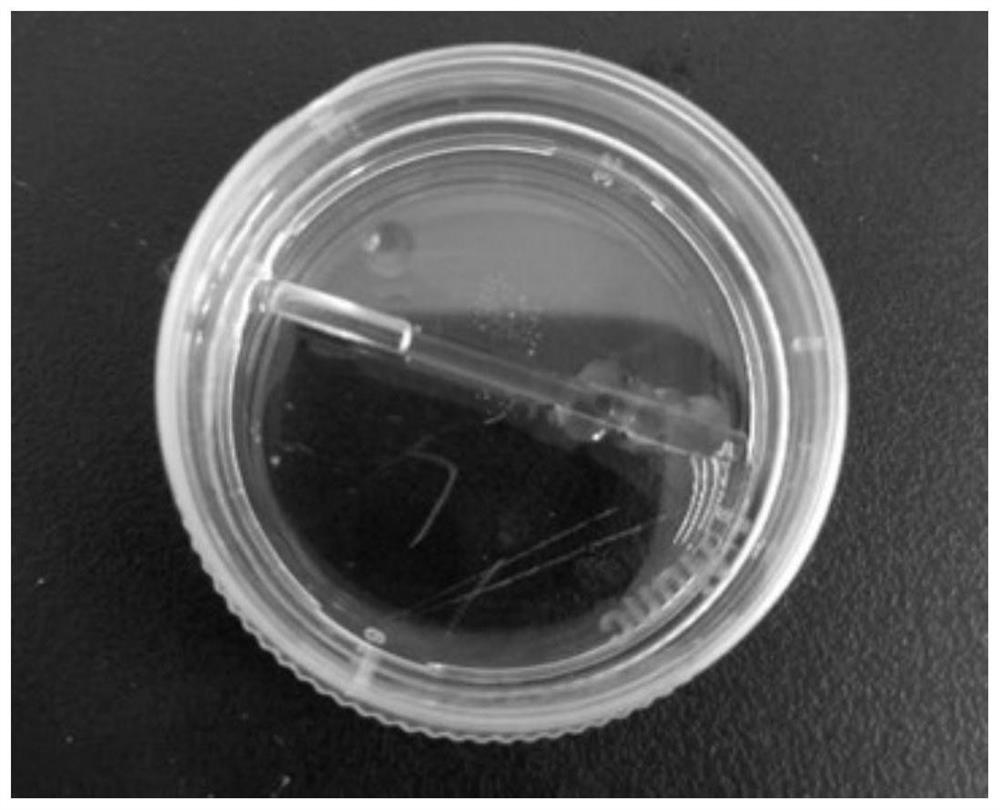
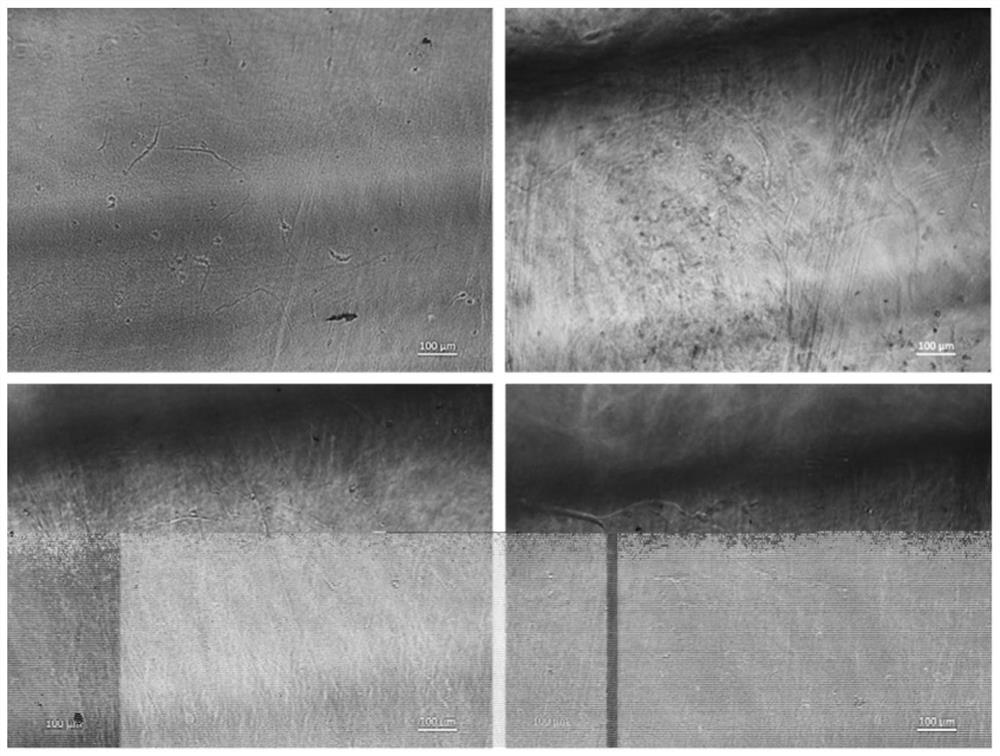
Tubular tissue structure body and constructing method thereof
PendingCN112206074AFlexible designVarious printing methodsTubular organ implantsHuman bodyComputer printing
The invention provides a tubular tissue structure body and a constructing method thereof. The method comprises the following steps: A, preparing printing ink; B, culturing cells for printing in vitroto obtain a cell culture solution; and C, respectively connecting the printing ink and the cell culture solution with different spray heads of a biological printer, jointly printing the printing ink and the cell culture solution on a winding rod to form seamless tubular tissue, and then removing the winding rod to obtain the hollow tubular tissue structure body. According to the method, a tubularstructure with a controllable structure and cell / material components is formed through a horizontal winding type cell 3D printing technology. The tubular structure is close to a human vessel in shapeand size, has mechanical and biological properties meeting the requirements of a human body, has clinical application potential, and can be applied to the aspects of drug detection, tissue engineering, regenerative medicine, in-vitro physiological model / pathological model / pharmacological model construction, tissue / organ / human body chips and the like.
Owner:REGENOVO BIOTECH +1
Reagent detection kit for detecting multiple drugs for drug alert
InactiveCN114229204AEasy to findShorten the timeMachines using electric/magnetic effectsShock-sensitive articlesStructural engineeringPharmaceutical Substances
The invention discloses a reagent detection kit for detecting various drugs for drug alert, and relates to the field of drug alert. The reagent detection box for detecting various drugs for drug alert comprises a refrigeration box body, a first fixing seat and a second fixing seat are fixedly connected to the inner wall of the refrigeration box body and the inner wall of a reaction box body respectively, and a plurality of reagent insertion holes are formed in the upper surface of the first fixing seat and the upper surface of the second fixing seat; the outer surfaces of the top ends of the reagent tubes are movably sleeved with limiting lantern rings; and the lifting mechanism is in transmission connection with two of the limiting lantern rings. According to the reagent detection box for drug warning for detection of various drugs, the semiconductor chilling plate and the heating plate are arranged, the semiconductor chilling plate has a refrigeration effect on reagent tubes placed in the reagent insertion holes, the lifting mechanism is arranged, the reagent tubes are moved to a proper height, and at the moment, marks on the outer surfaces of the reagent tubes can be observed.
Owner:郭慧
Method for determining lidocaine hydrochloride related substances by high performance liquid chromatography
The invention discloses a method for determining lidocaine hydrochloride related substances by high performance liquid chromatography, and relates to the field of drug detection, the chromatographic conditions are as follows: octadecylsilane chemically bonded silica bonded with polar groups is selected as a filler as a stationary phase; wherein the particle size of the chromatographic column is 5[mu]m, the detection wavelength ranges from 225 nm to 235 nm, the flow rate is 0.8 to 1.2 ml / min, the column temperature is 30-40 DEG C, and the mobile phase is prepared from the following componentsin parts by mass: 50-80 parts of 0.035 mol / L phosphate buffer solution and 20-50 parts of organic solvent. The adopted detector is a high performance liquid chromatograph, more impurities can be detected by the method, and methodological verification proves that the detection result is accurate and reliable.
Owner:TIANJIN PHARMA GROUP XINZHENG
Method for determining photodegradation impurities in levofloxacin raw material and levofloxacin preparation
InactiveCN111458436AImprove securityEfficient use ofComponent separationMonopotassium phosphateSilica gel
The invention belongs to the field of drug detection, and particularly relates to a method for determining photodegradation impurities in a levofloxacin raw material and a levofloxacin preparation. The method comprises the following steps: (1) preparing a levofloxacin photodegradation impurity standard solution; (2) injecting the standard solution into a high performance liquid chromatograph for determination, and constructing a standard curve by taking the concentration of the standard solution as an abscissa and the peak area as an ordinate; (3) preparing a to-be-detected sample solution; and (4) injecting the to-be-detected sample solution into a high performance liquid chromatograph for determination, recording the peak area of the to-be-detected sample solution, and calculating the content of the photodegradable impurities by using the standard curve. According to the method, high performance liquid chromatography is adopted, amino silane bonded silica gel is used as a stationaryphase, a mixed solution of acetonitrile and 0.05mol / L monopotassium phosphate solution is used as a mobile phase, and an isocratic elution mode is adopted. The method is high in specificity, high in precision, good in accuracy and capable of effectively detecting the content of photodegradation impurities in levofloxacin raw materials and preparations.
Owner:SHANDONG QIDU PHARMA
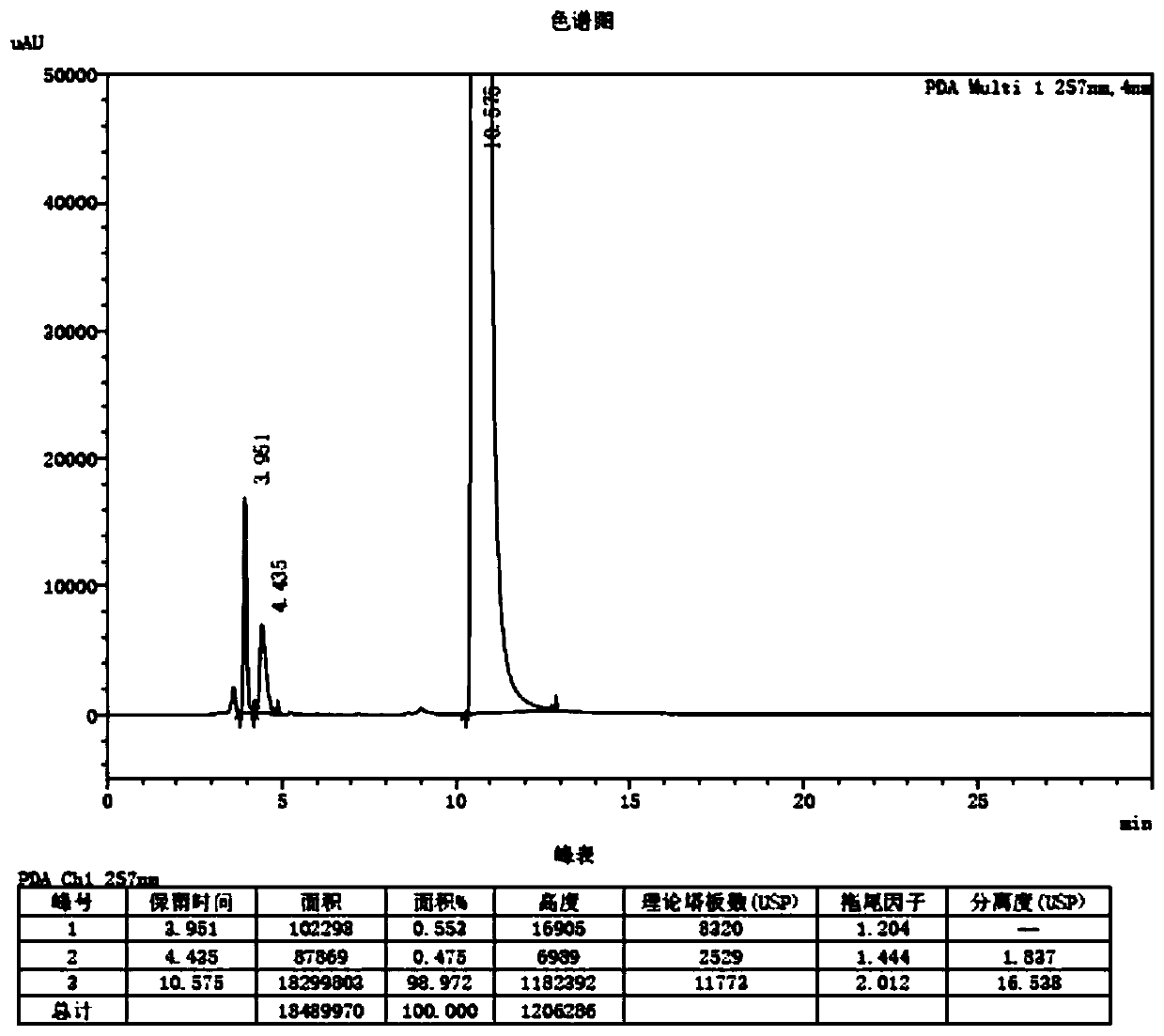
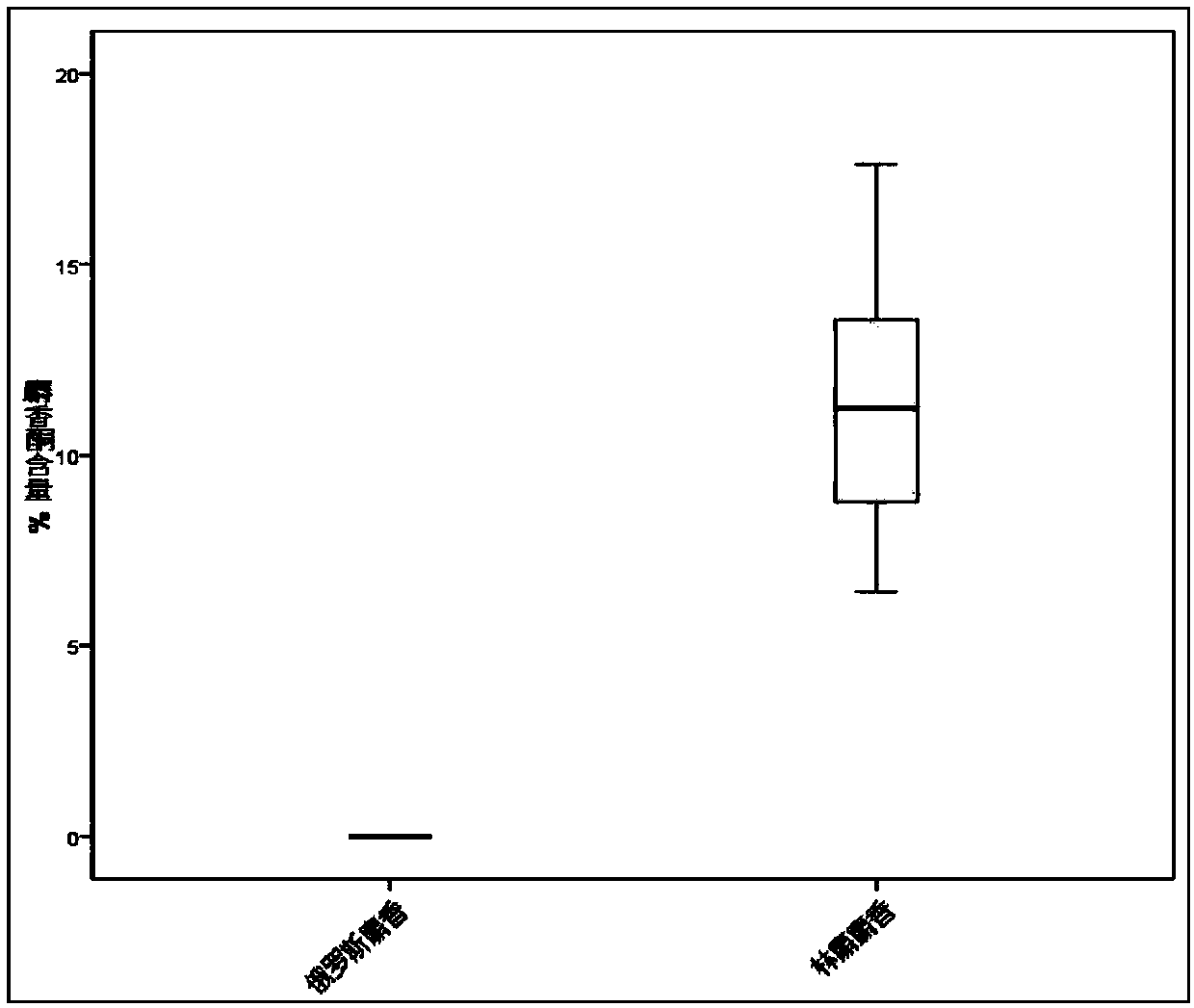
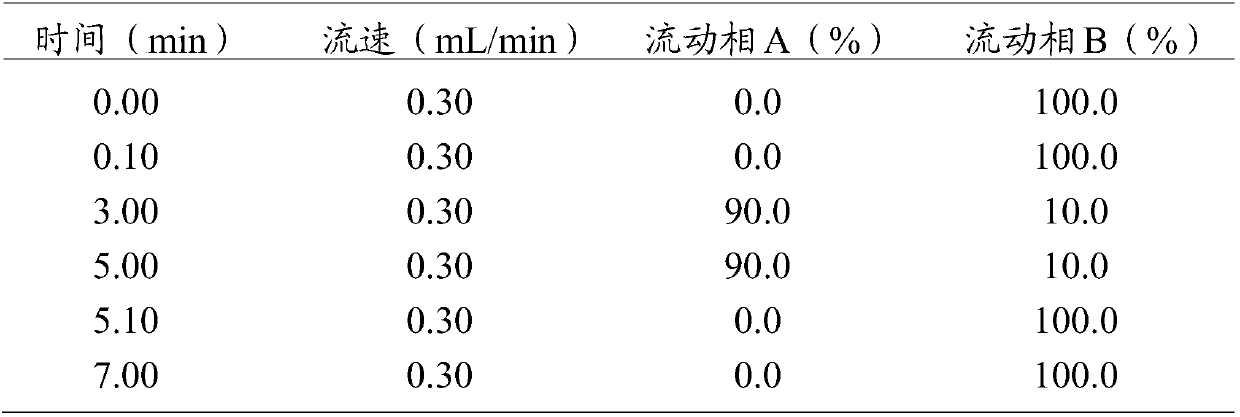
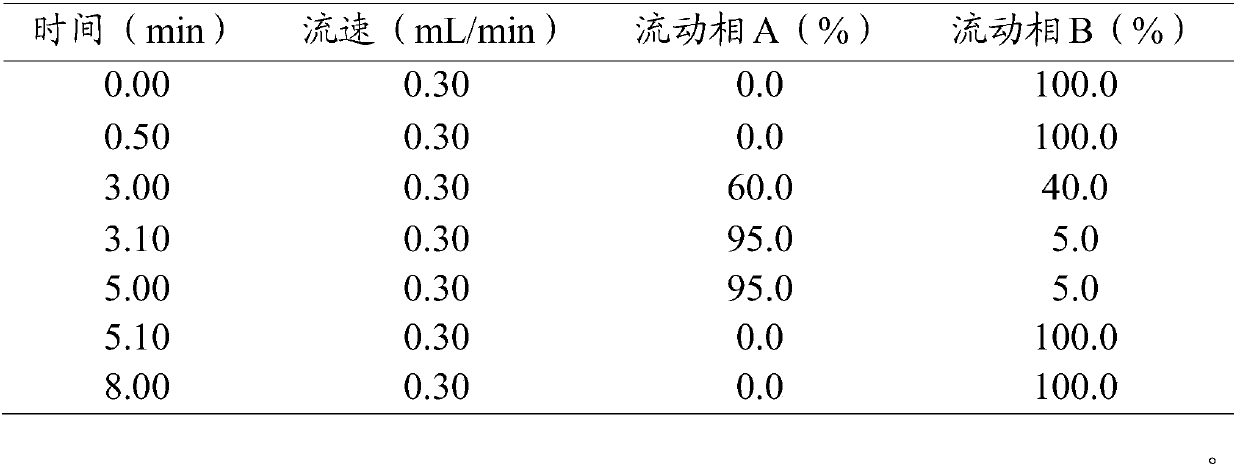
Trace identification method for natural Russian musk and natural forest musk
ActiveCN107561191AImprove extraction efficiencyImprove accuracyComponent separationAnalysis dataMedicine
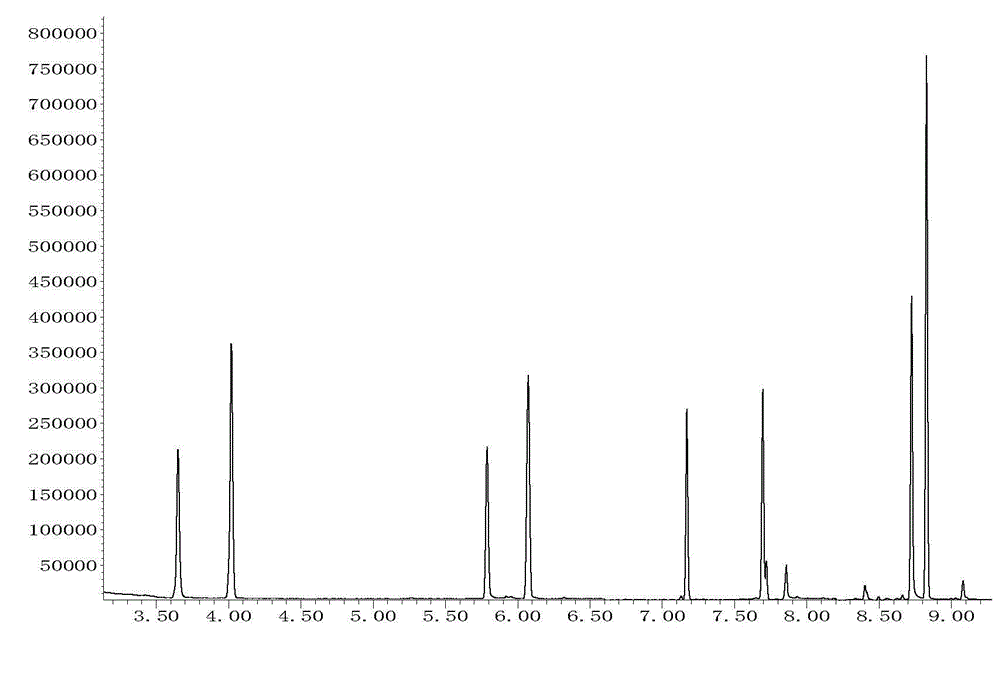
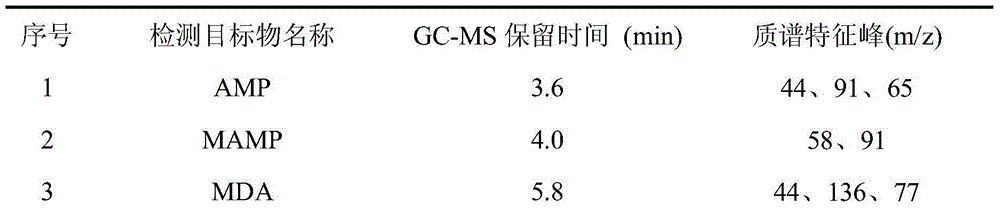
The invention relates to the fields of pharmaceutical analysis and traditional Chinese medicine identification and particularly relates to a trace identification method for natural Russian musk and natural forest musk. The method comprises a sample pretreatment step and a combined GC-MS chromatographic analysis and data treatment step. GC-MS color spectra of the natural Russian musk and the natural forest musk are contrastively analyzed, and the type of the musk can be determined by virtue of the significant difference of characteristic peaks of muscone in two types of musk and the comparisonof analysis data of the contents of muscone in two types of musk, so that the natural Russian musk and the natural forest musk can be conveniently, rapidly and accurately analyzed and judged. The operation specification and conditions of the method are specific, and the influence caused by the subjective factor of people is low, so that the accuracy and reliability of a result are remarkably improved; and furthermore, the method is simple, convenient and feasible, low in requirements on the professional level and practical experience of an operator and is vey suitable for being popularized andused in the field of pharmaceutical detection.
Owner:BEIJING FORESTRY UNIVERSITY +1
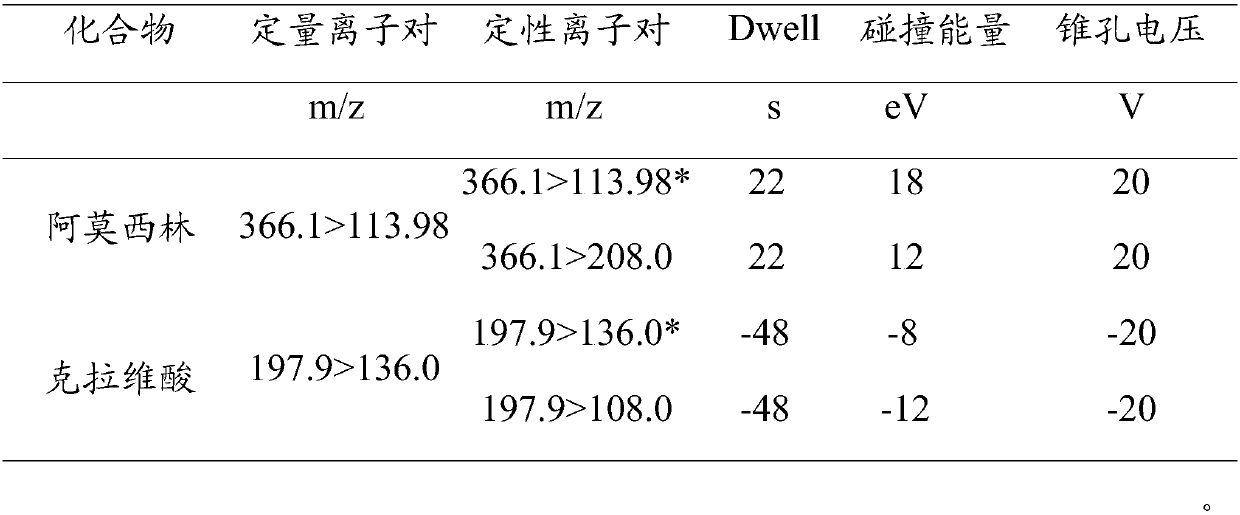
Method for detecting residual quantity of amoxicillin and clavulanic acid in tissues
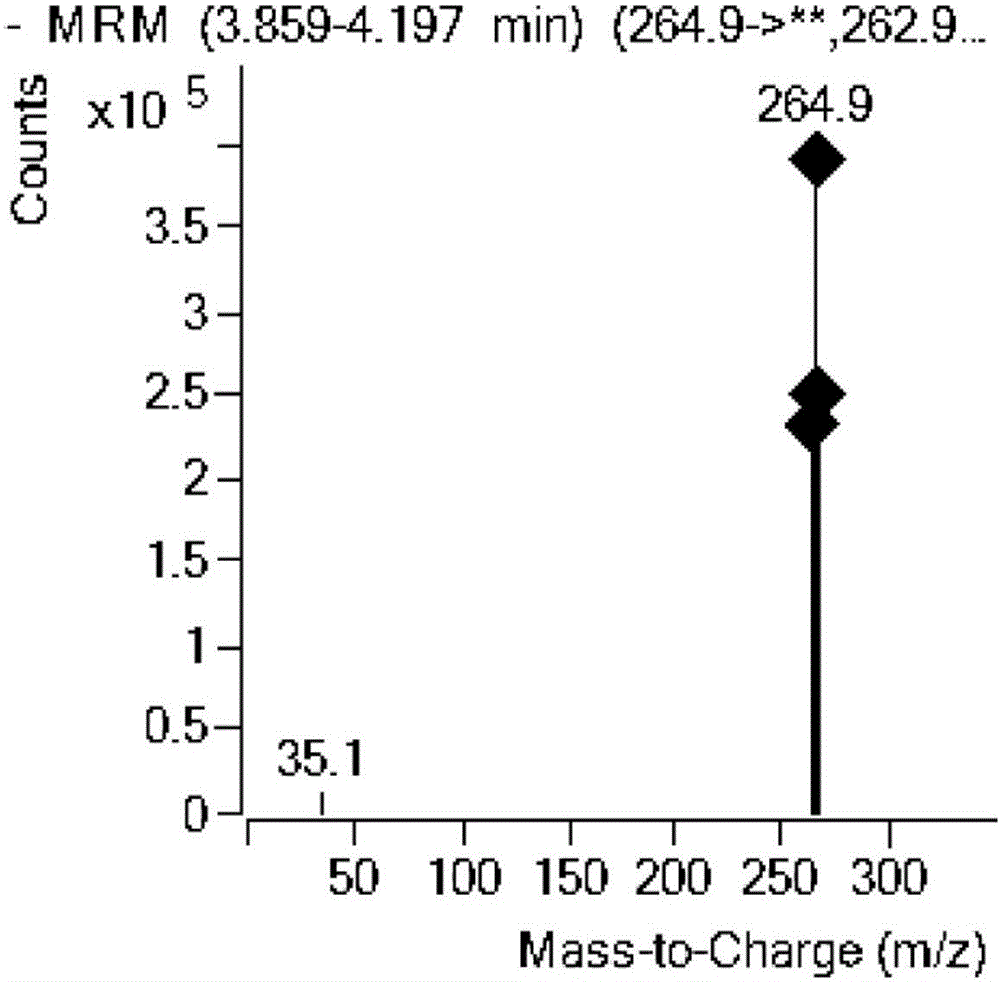
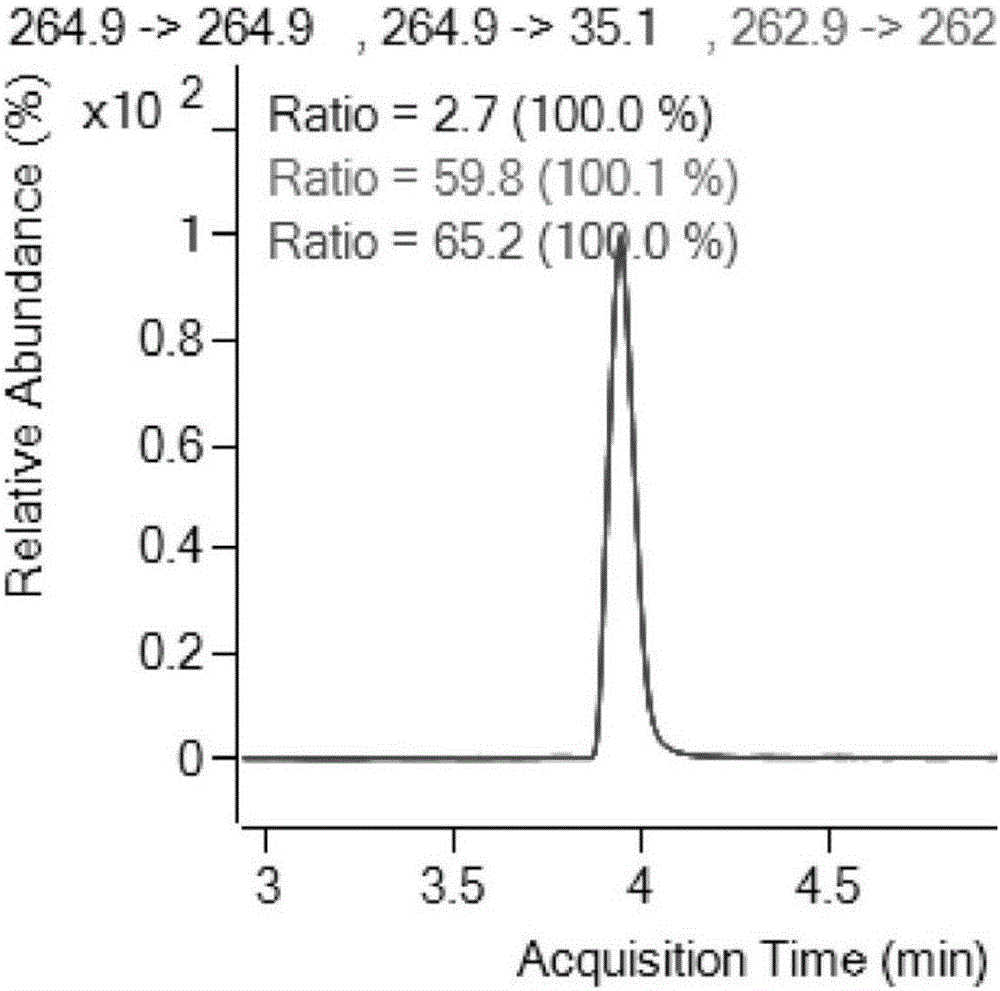
The invention relates to the field of drug detection, in particular to a method for detecting the residual quantity of amoxicillin and clavulanic acid in tissues, which comprises the following steps:1) preparing an amoxicillin and clavulanic acid reference substance solution and a to-be-detected tissue extract sample solution; 2) carrying out high performance liquid chromatography-tandem mass spectrometry detection, and quantifying by an external standard method or qualifying by a contrast method; wherein the chromatographic conditions are as follows: a chromatographic column is ACQUITYHSS T3, a mobile phase A is acetonitrile, and a mobile phase B is 0.1% formic acid water; wherein the mass spectrum conditions of amoxicillin detection comprise multi-reaction monitoring, positive ion scanning and capillary voltage of 3kV; mass spectrum conditions of clavulanic acid detection include multi-reaction monitoring, negative ion scanning and capillary voltage of-3kV. The method has good sensitivity, accuracy and precision, and qualitative and quantitative detection of amoxicillin and clavulanic acid residues is effectively realized.
Owner:内蒙古联邦动保药品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com