Method for directly determining three degradation products of tryptophan in compound amino acid injection
A technology for compound amino acids and degradation products, which is applied in the field of direct determination of the three degradation products of tryptophan in compound amino acid injections. It can solve the problems of easy interference with the determination of 2-hydroxytryptophan and complex ingredients of compound amino acid prescriptions, and achieve high sensitivity. High performance, strong specificity, good analysis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Chromatographic conditions
[0042] Chromatographic column: Shimadzu InertSustain AQ C18 column (250×4.6mm, 5μm), detection wavelength: 254nm, flow rate 1.0mL / min, column temperature: 30°C, injection volume 20μL.
[0043] 2. Solution preparation
[0044] 2.1 Mobile phase A: phosphate buffer (0.01mol / L diammonium hydrogen phosphate solution, adjust the pH value to 7.6±0.1 with trifluoroacetic acid).
[0045] Mobile Phase B: Acetonitrile.
[0046] Gradient elution conditions:
[0047]
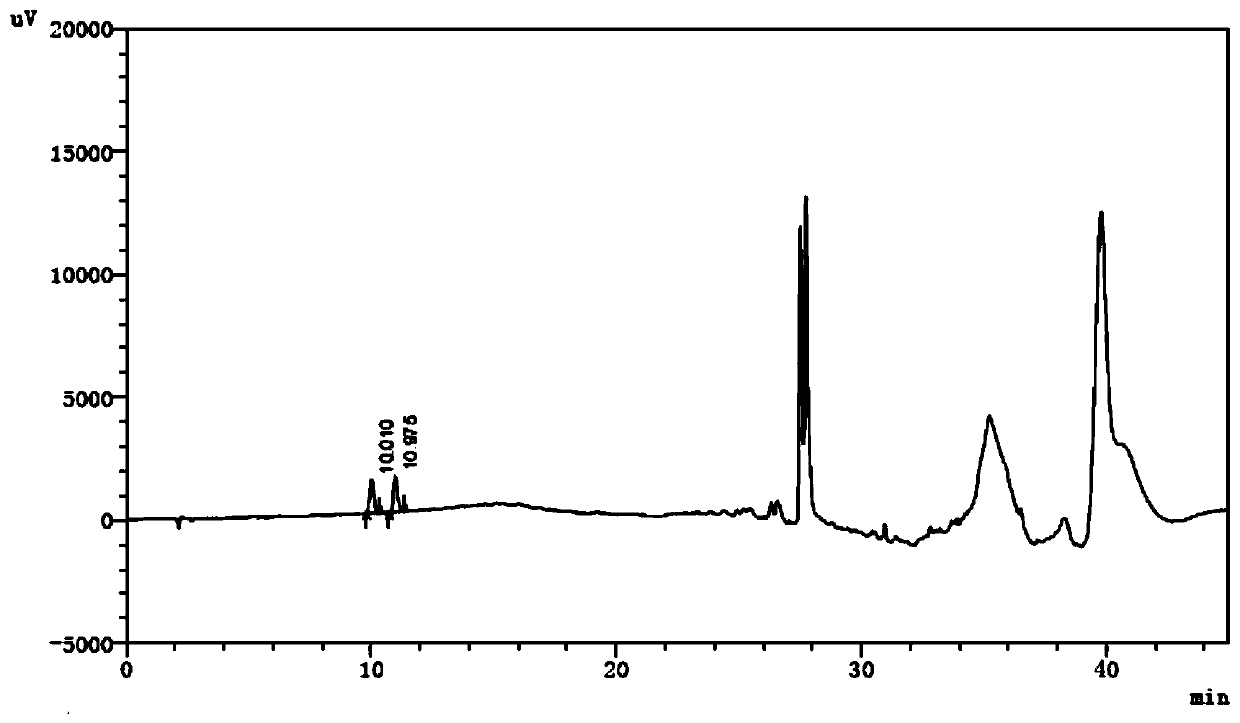
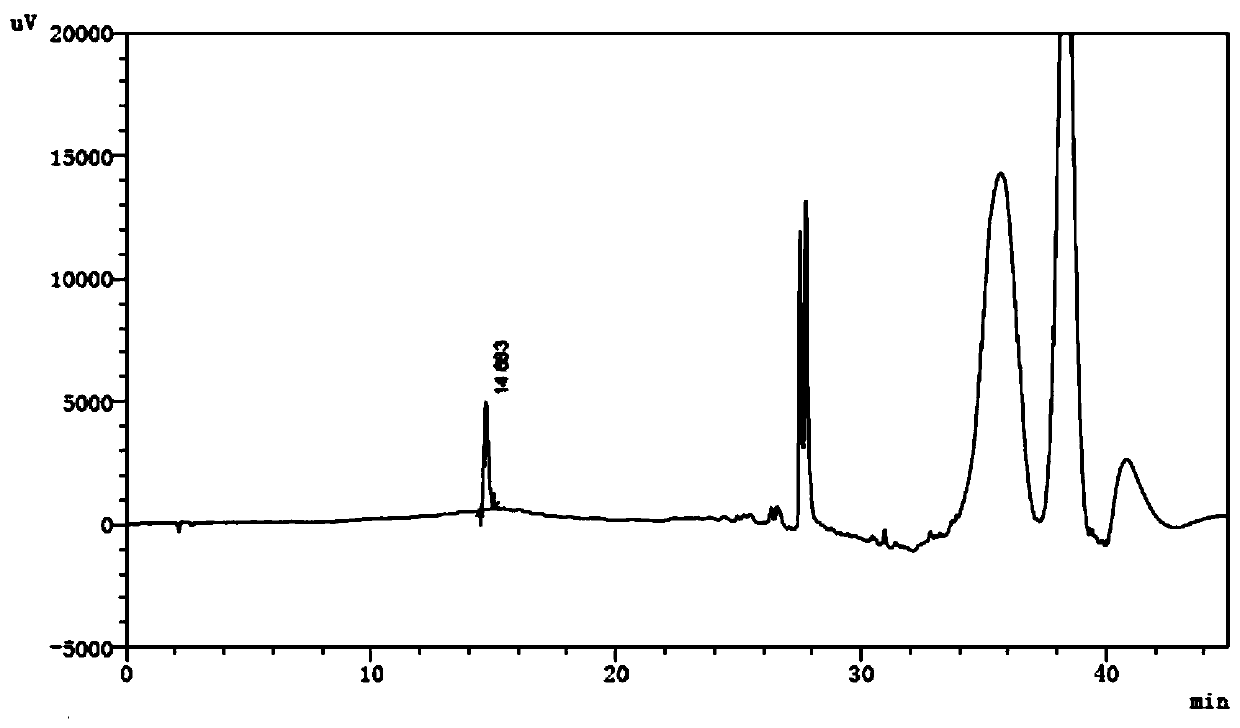
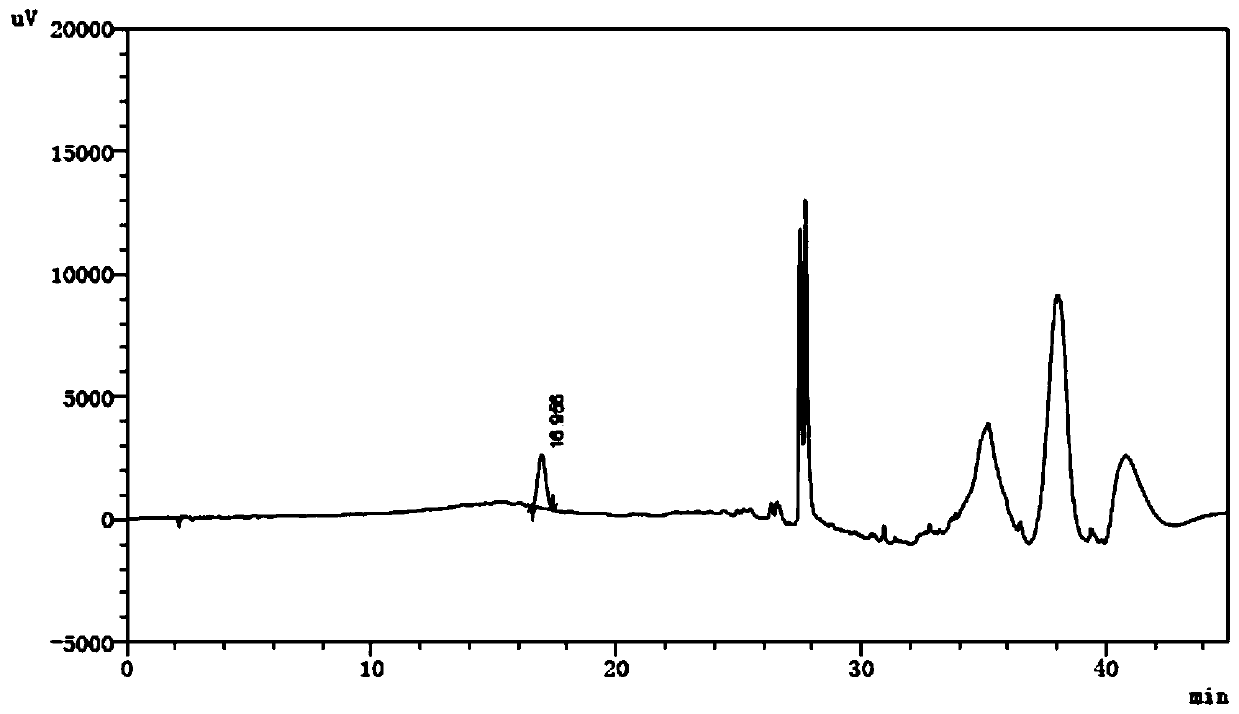
[0048] 2.2 The test solution: Precisely measure the compound amino acid injection, dilute with water to make a solution containing tryptophan 0.95mg / mL, as the test solution.
[0049] 2.3 Reference substance solution: Accurately weigh the appropriate amount of reference substances of dioxindole alanine, kynurenine and 2-hydroxytryptophan respectively, add acetonitrile to dissolve and quantitatively dilute with water to make a mixed solution each containing 1.43 μg / mL , as a refer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com