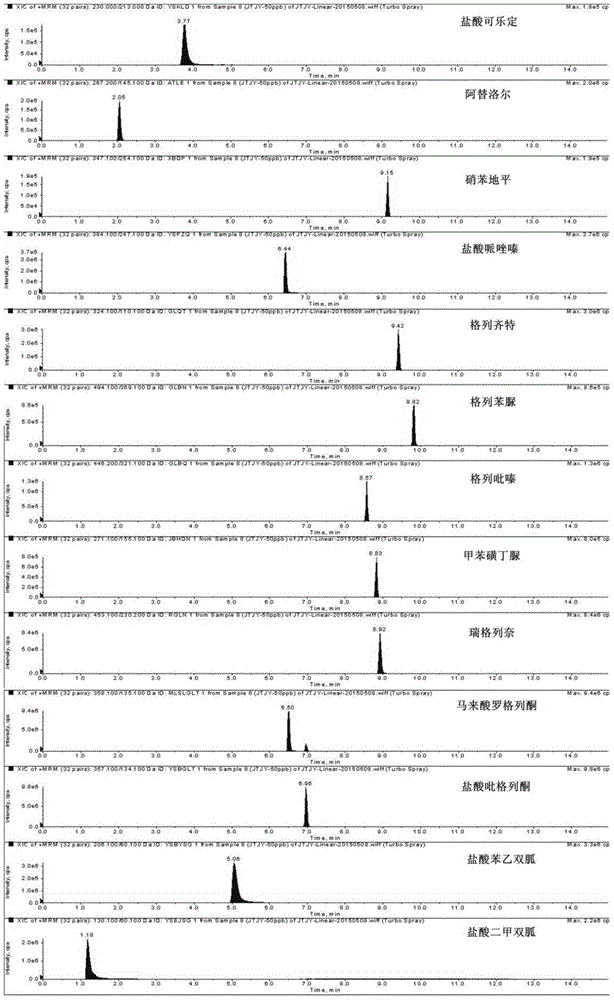
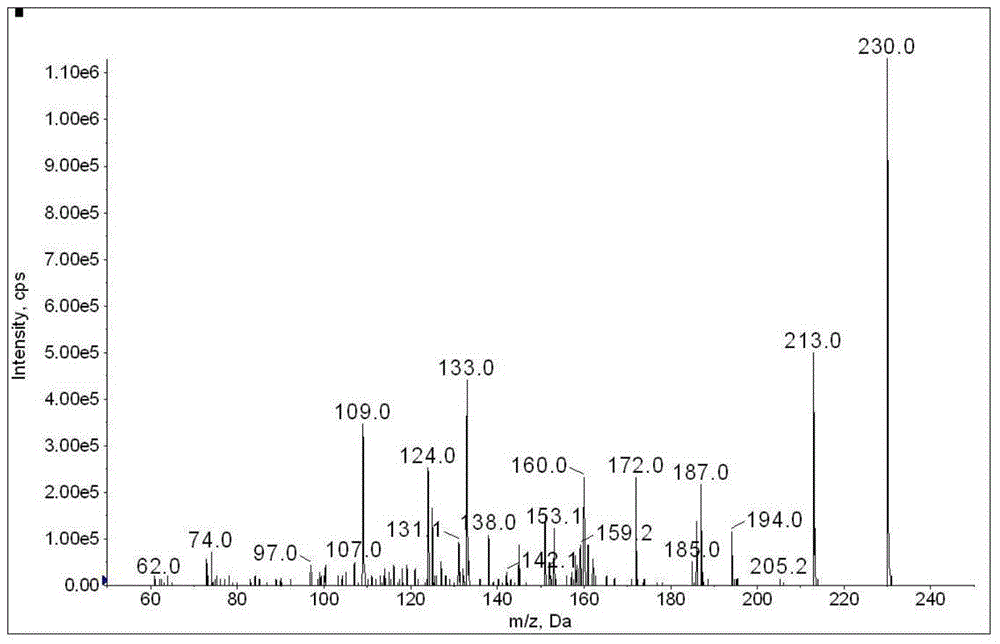
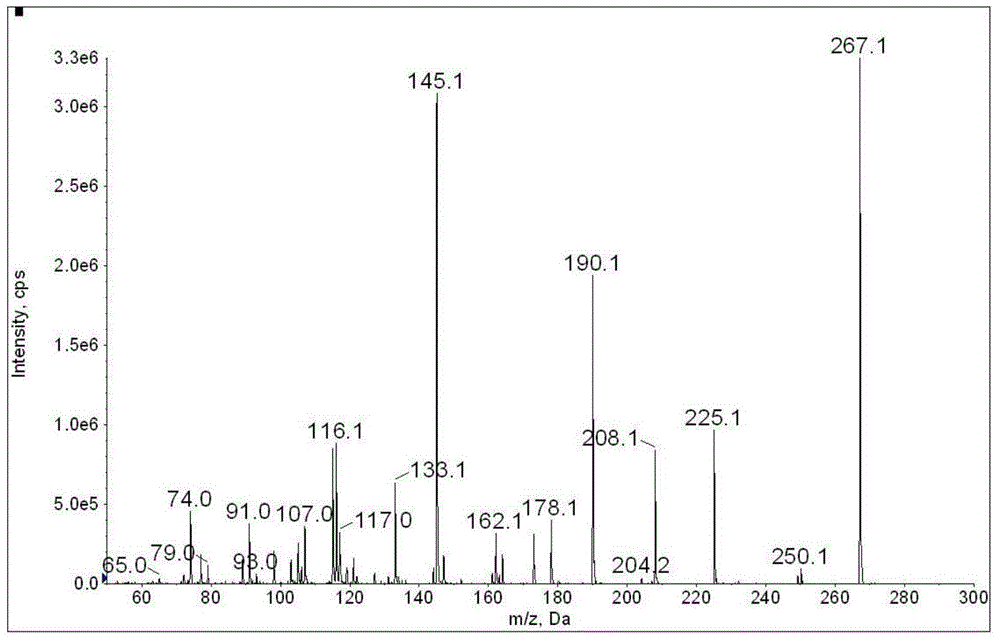
Method for determination of illegally added substances in traditional Chinese medicines and health-care products
A technology of illegally added and health care products, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of endangering the health of consumers, affecting the reputation of traditional Chinese medicine and health food, etc., to avoid false positive results, save time, and operate The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Optimization of mass detection conditions
[0073] The declustering voltage (DP) and collision energy (CE) of 13 chemical drugs were optimized to maximize the transmission efficiency of the sample precursor ions and the higher response intensity of the product ions in the mass spectrum, thereby improving the sensitivity of the detection method.
[0074] The experimental method is as follows: First, in the selected ion detection mode, input the mass-to-charge ratio of the sample precursor ions, optimize the declustering voltage (0V~300V), and ensure the maximum transmission efficiency of the precursor ions, that is, reach the collision cell as much as possible; In the product ion scan mode, investigate the influence of different collision energy on the peak intensity of the parent ion and the product ion, and select the collision energy when the parent ion almost disappears and the product ion intensity is the largest. After optimization, the optimal declustering voltage and ...
Embodiment 2
[0079] A method that can simultaneously qualitatively and quantitatively detect 13 kinds of antihypertensive or antihypertensive chemical drugs illegally added to Chinese medicines and health products, including the following:
[0080] 1. HPLC-MS / MS Coupled Instrument
[0081] 1.1 HPLC conditions
[0082] Chromatographic column: ZORBAX Eclipse XDB-C18 (4.6mm×50mm, 1.8μm, Agilent Technologies Co., Ltd.). Mobile phase: Phase A is an aqueous solution containing 0.1% formic acid (volume ratio), phase B is acetonitrile. Gradient elution procedure: 0~2min, phase B keeps 10%; 2~8min, phase B rises from 10% to 80%; 8~10min, phase B keeps 80%; 10.1min-15min, phase B drops to 10% ; Stop after 15min. Flow rate: 0.4 mL / min. Column temperature: 35°C, injection volume: 5.00μL.
[0083] 1.2 MRM quantitative detection conditions
[0084] Ion source: Electrospray ion source (+ESI), using positive ion mode for detection. Dry gas: N 2 , Collision gas: high-purity nitrogen. Curtain air pressure: 35....
Embodiment 3
[0106] Example 3 A health product testing example
[0107] Using the method of 1 and 2 in Example 2, weigh 0.20g of a health care product, and process it according to the pretreatment method of Example 2. The test solution is diluted 1000 times and then injected with 5μL for detection. The mass spectrometry results are as follows Figure 15 with Figure 16 (EPI spectrum) as shown. After testing, glibenclamide was illegally added to the sample, and the qualitatively tested EPI spectrum was consistent with the standard library. After calculation, the content of glibenclamide in the sample was 8.67 mg / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com