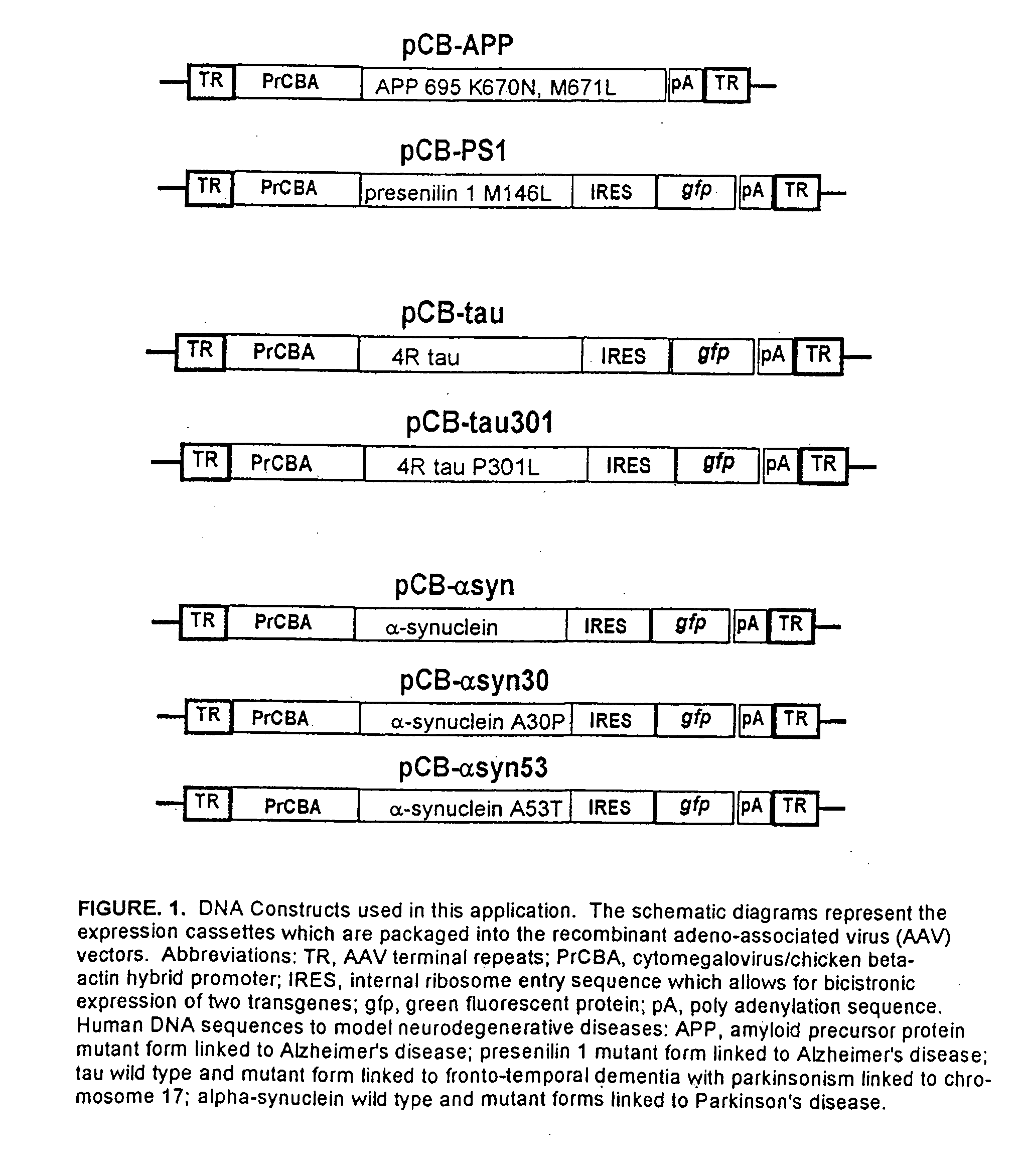
Human disease modeling using somatic gene transfer
a technology of human disease and gene transfer, applied in the field of gene transfer methods, can solve the problems of terminal illness, limited life-span animals, and inducing terminal illnesses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Induction of Tauopathy in Animal Models
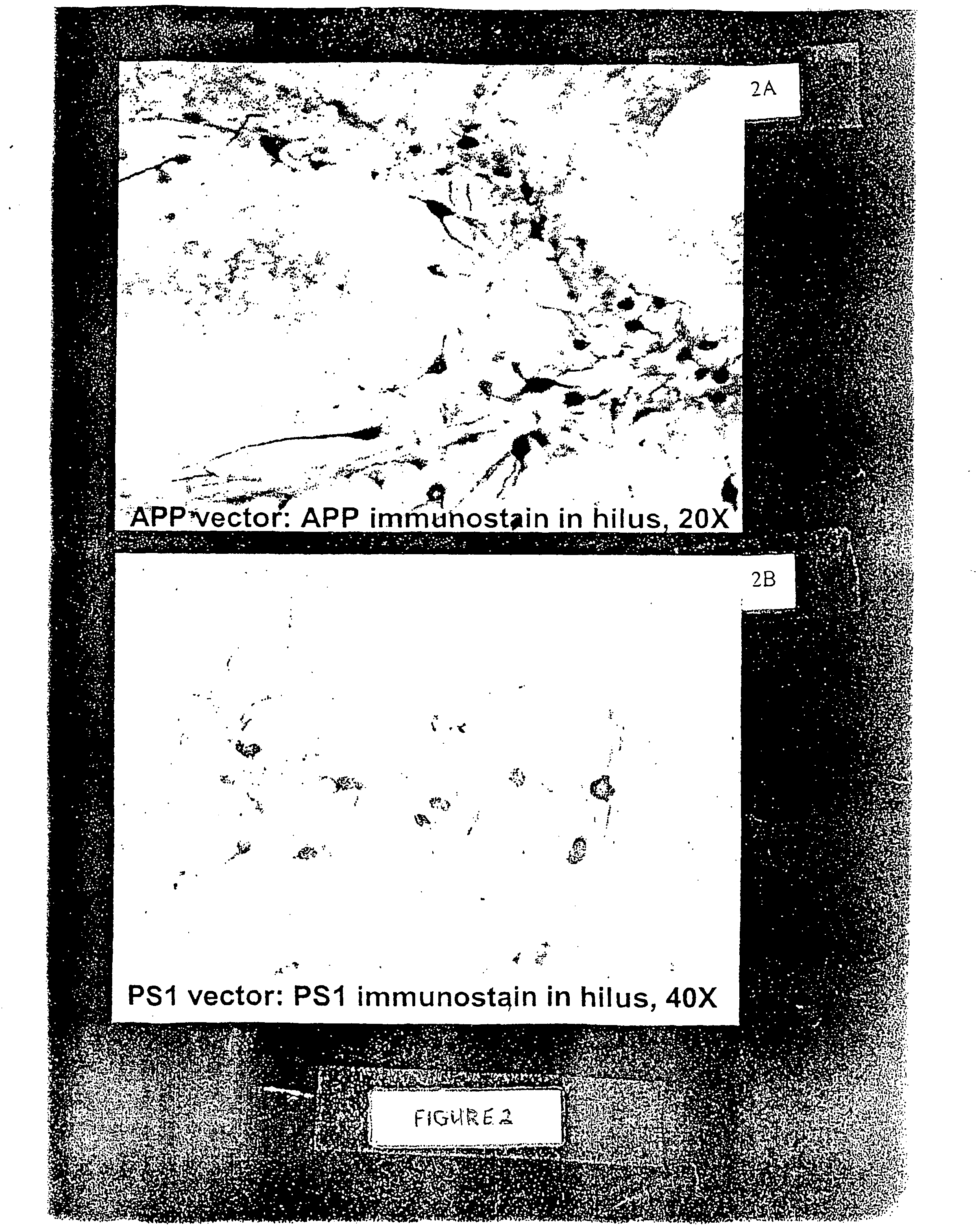
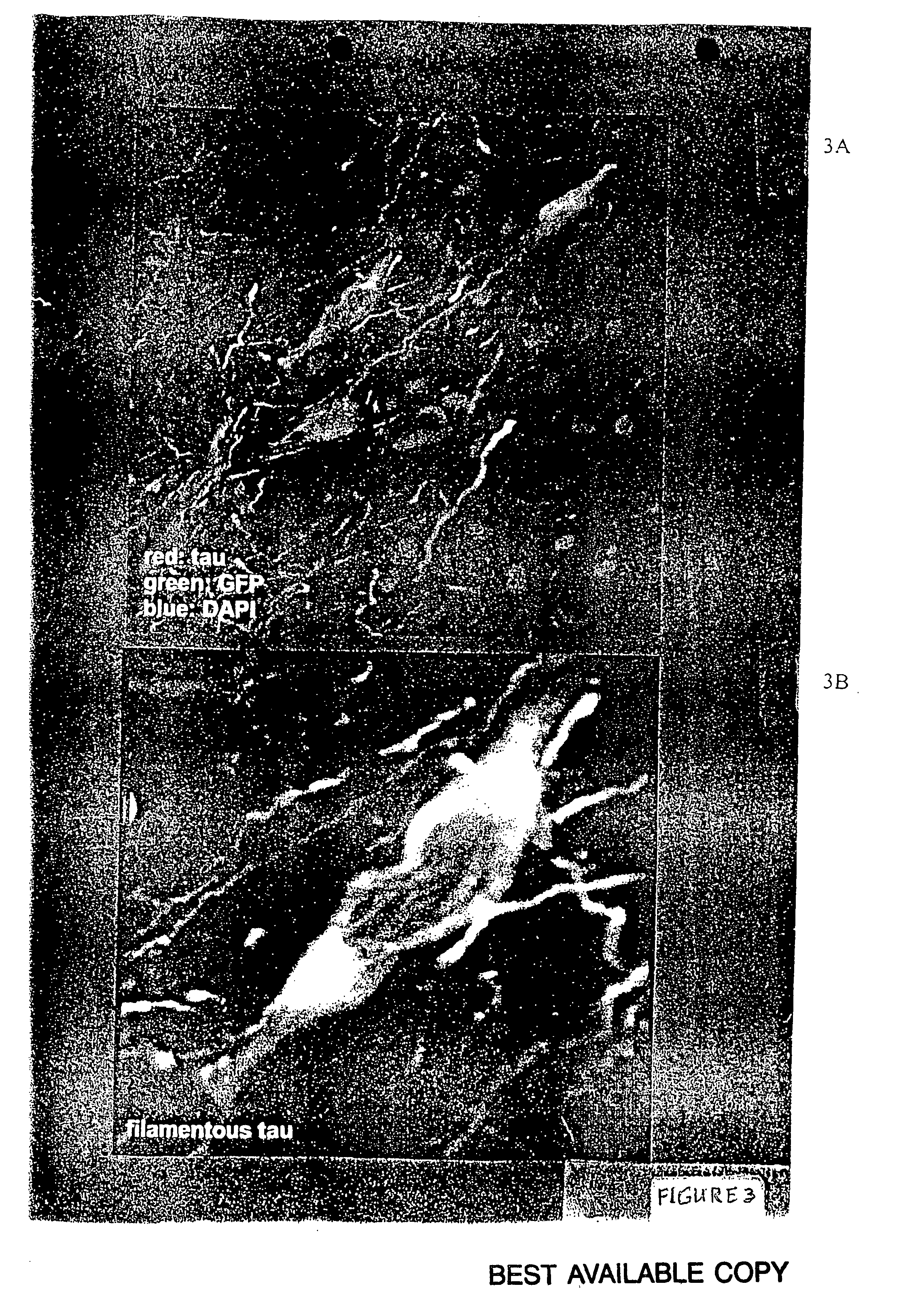
[0055] The present inventors have expressed a mutant form of human tau (P301L) using an AAV vector system in the septal nucleus of the basal forebrain and the hippocampus in the adult rat. The vector-derived tau accumulated in cell bodies and dendrites and formed aggregates as observed by co-localization with the reporter gene, green fluorescent protein (GFP), which was bicistronically expressed by the vector (i.e., GFP filled neurons and tau distribution within cell bodies was clustered). The neurofibrillary pathology observed in this model shows abnormal accumulation of tau in neuron cell bodies and dendrites, filaments immunoreactive for hyperphosphyorylated tau, neuritic immunoreactivity for several antibodies that recognized neurofibrillary tangles in Alzheimer's and FTDP-17, and a dramatic induction of reactive astrogliosis. See FIGS. 2 and 3 provided herewith and the description thereof provided hereinabove.
example 2
SGT as a Method for Supplementing Germline Animal Models
[0056] Another utility of the present vector system is to apply genes in trans to existing germline mouse and other animal models, for example, by expressing tau in current models of amyloidosis to introduce tangles.
example 3
Induction of Parkinson's Disease Associated CNS Lesions in Animal Models
[0057] A gene linked to autosomal dominant Parkinson's disease, alpha-synuclein, harboring the A30P mutation, was expressed in the rat substantia nigra. Transduced neurons in this area had aggregates rich in alpha-synuclein and axons with large varicosities (5-10 micrometers in diameter) that were not found in control vector samples. Overexpression of alpha-synuclein in the nigrostriatial pathway also elevated rates of amphetamine-stimulated locomotor behavior, which is apparently consistent with reduced locomotor response in alpha-synuclein knockout mice (Abeliovich et al., 2000). Accordingly, it is concluded that the somatic transgenic models disclosed herein are useful for studying mechanisms of neurodegenerative disease pathogenesis as well as gene structure-function relationships of tau and alpha-synuclein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com