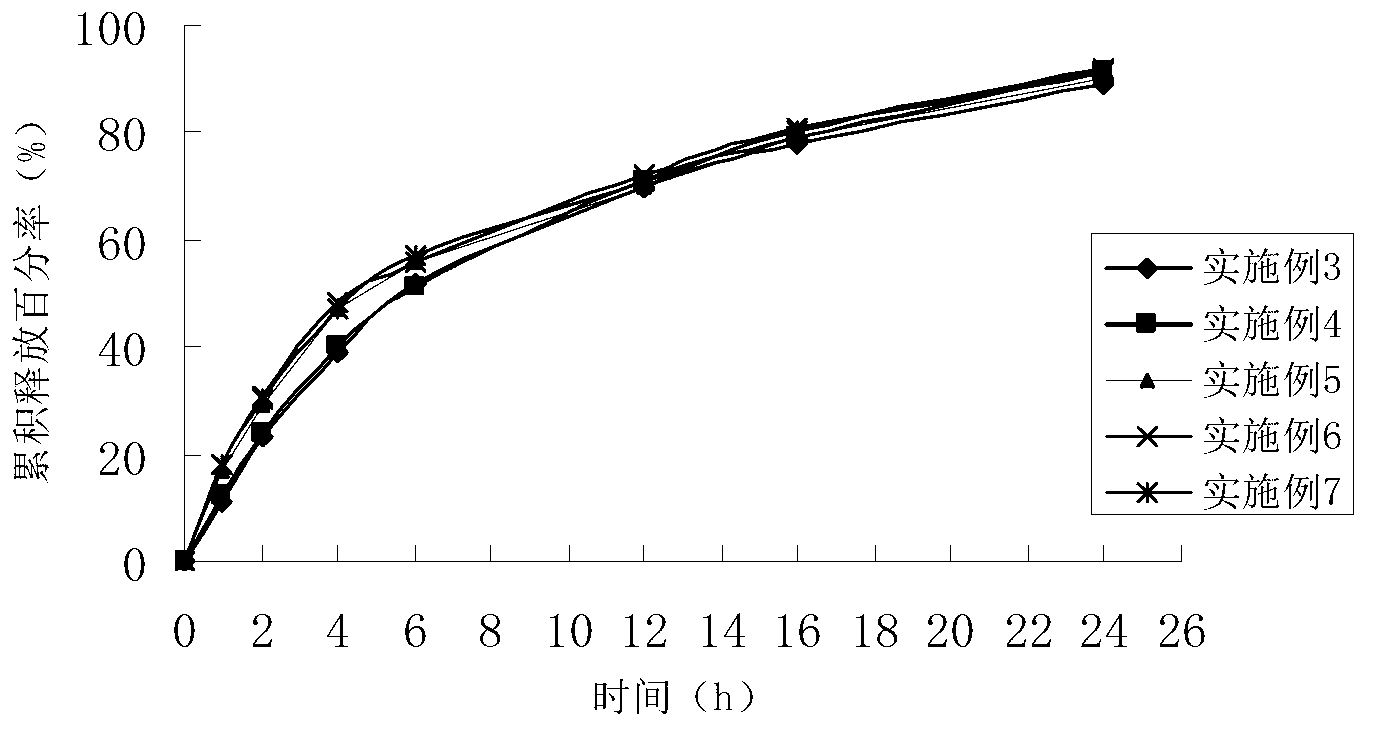
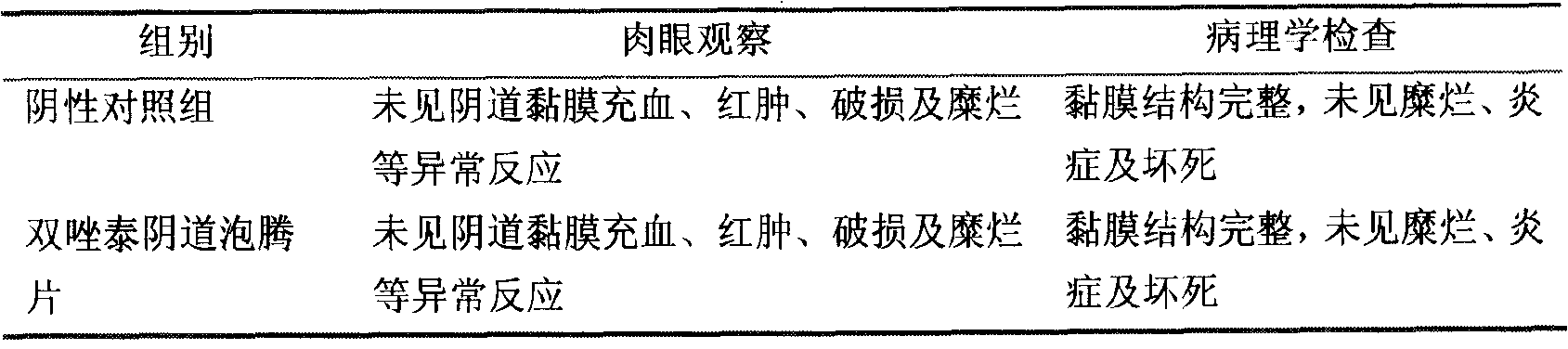
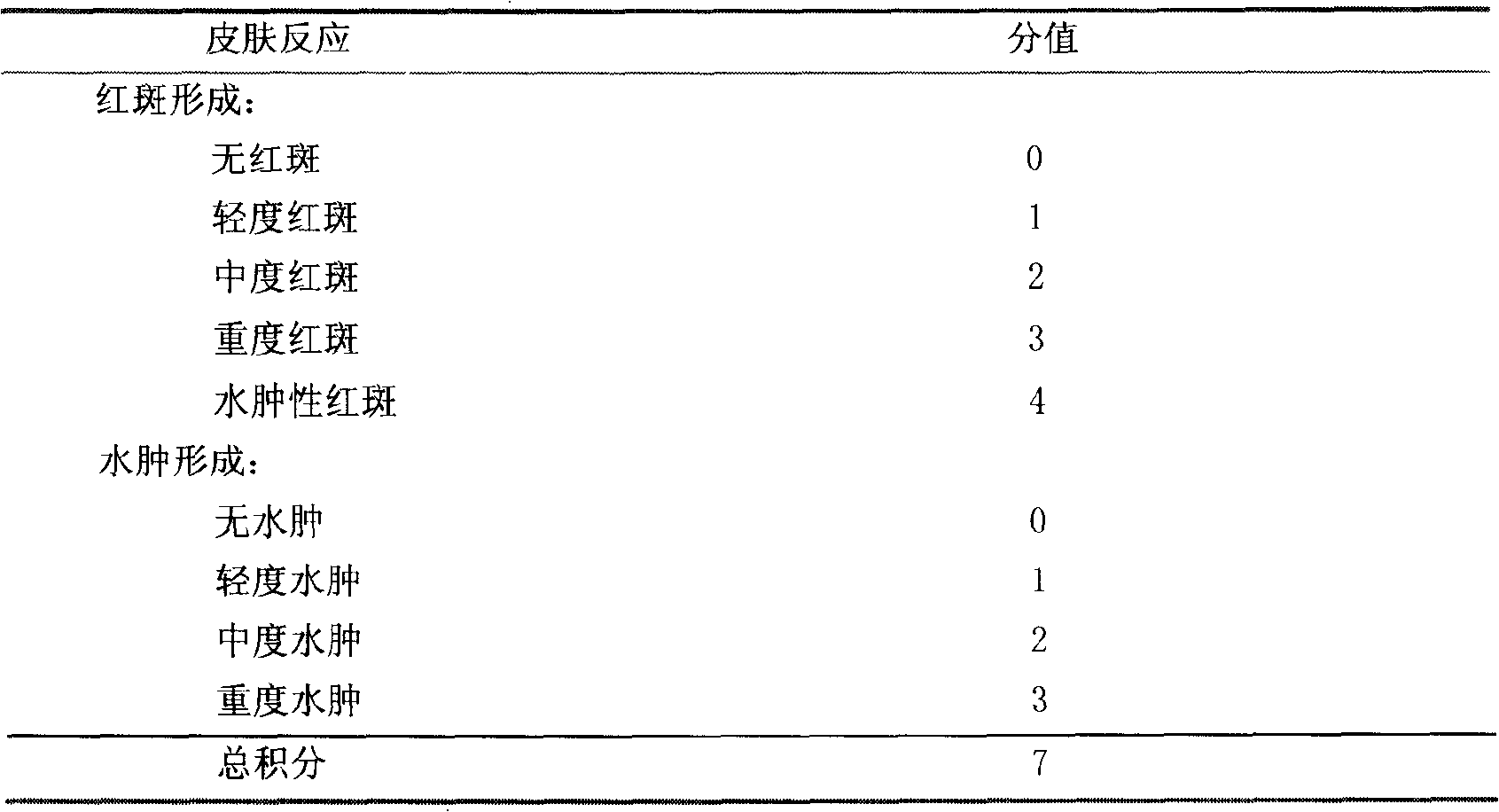
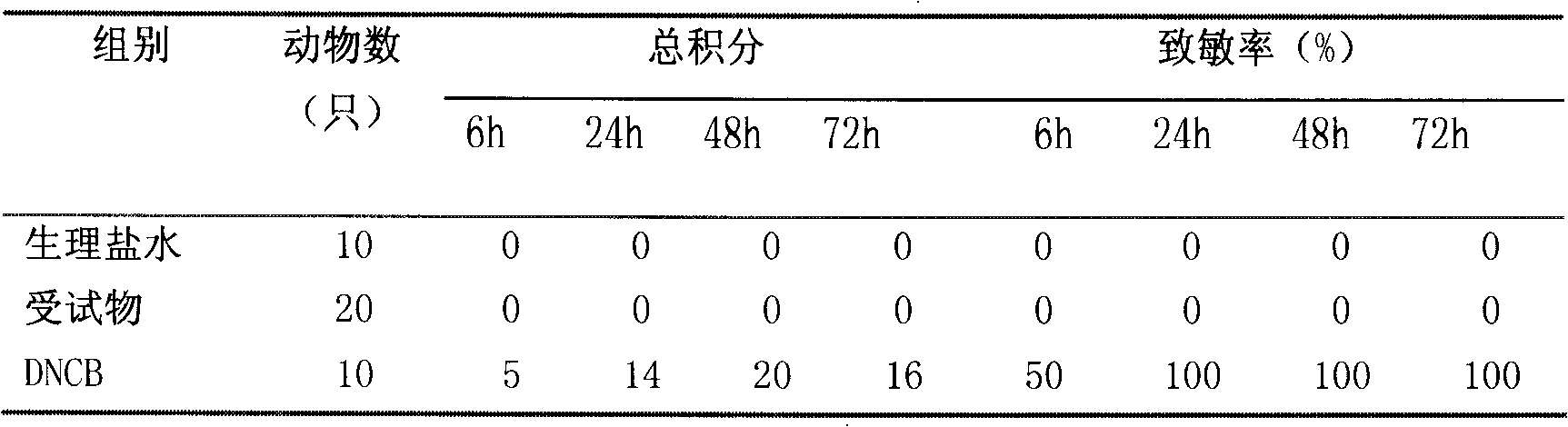
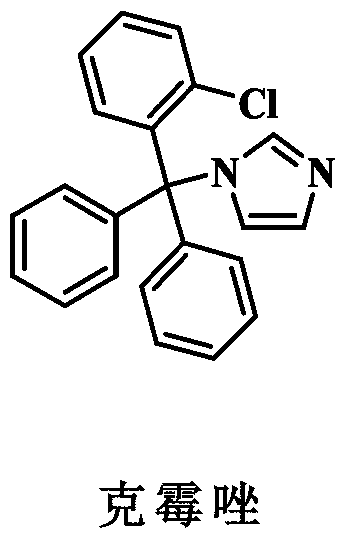
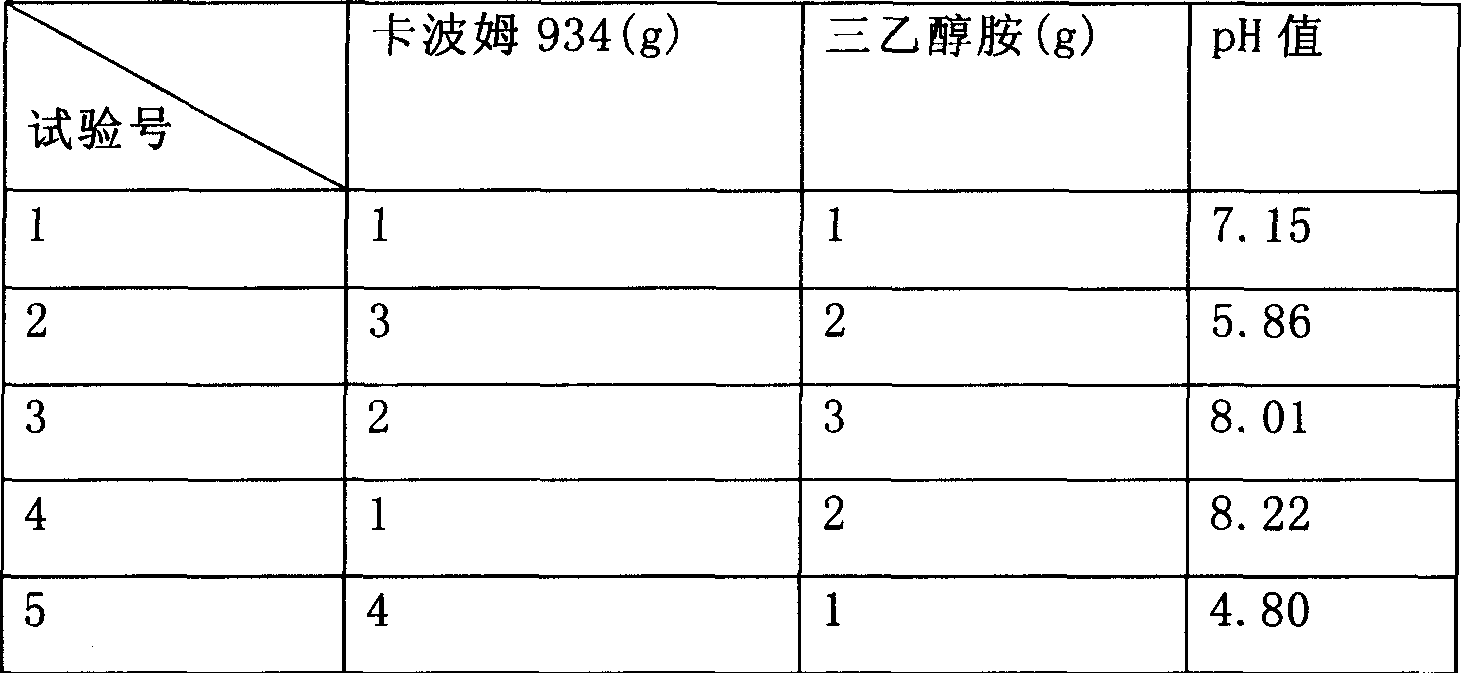
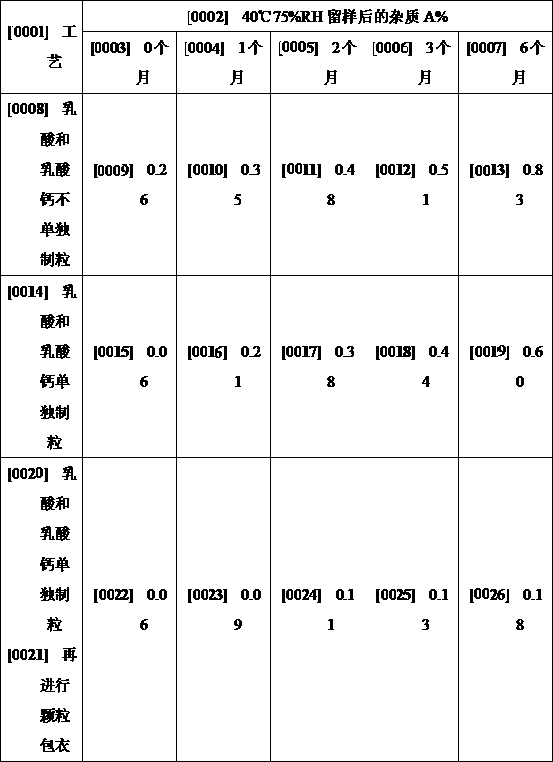
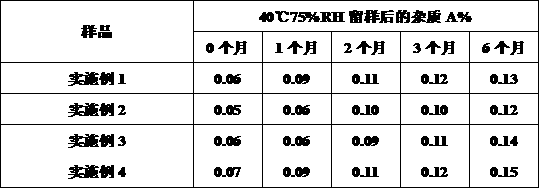
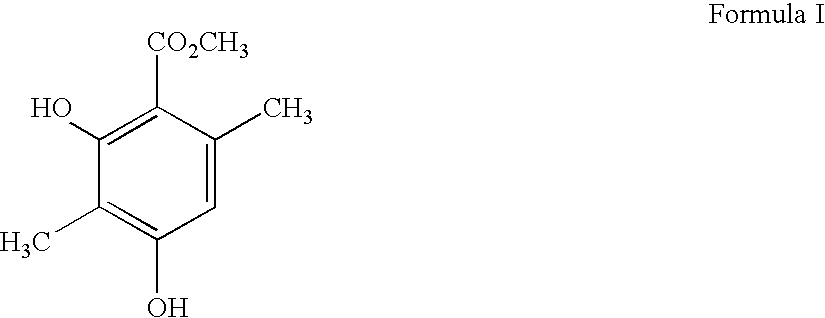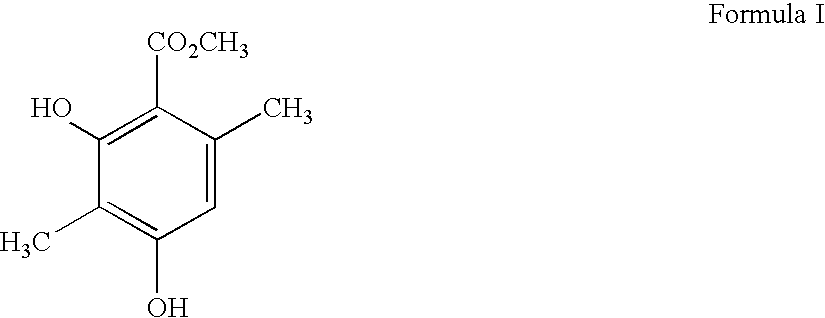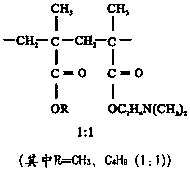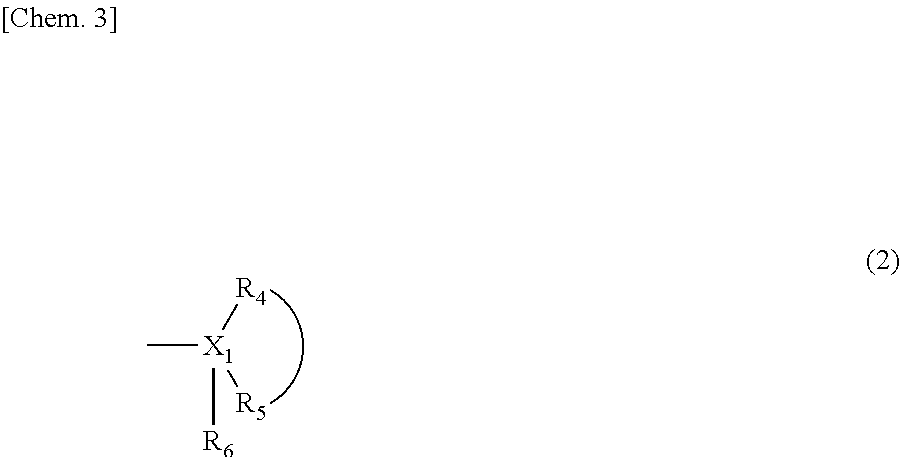Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86 results about "Clotrimazole Vaginal Tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Topically, clotrimazole is used for vulvovaginal candidiasis (yeast infection) or yeast infections of the skin. For vulvovaginal candidiasis (yeast infection), clotrimazole tablets and creams are inserted into the vagina.
Topical antifungal treatment
A topical mycological treatment composition for medical, veterinarian, or dental use contains as active ingredients clotrimazole, ketoconazole, micanazole, nystatin, tolnaftate, propionic acid, sodium propionate, undecelynic acid, and zinc undecelynate. These ingredients are contained in a natural cream base, and the base may also contain an anti-inflammatory agent and an antibacterial agent.
Owner:AYON COVARRUBIAS BLAS
Pharmaceutical composition
InactiveUS6537970B1Avoid infectionReduce financial costsBiocidePharmaceutical delivery mechanismClotrimazole Vaginal TabletVaginal infections
The invention relates to a novel pharmaceutical combination comprising clindamycin and clotrimazole for use for vaginal infections.
Owner:HEXAL AG
Method of treatment of otitis externa
This invention relates to a method of treating otitis externa, and in particular otitis externa of fungal etiology, using topical medication, including antifungal agents such as, for example fluconazole, voriconazole, itraconazole, clotrimazole, amphotericin B, caspofungin (Cancidas®), micafungin (Mycamine®), terbinafine, naftifine, butenafine, amorolfine, ravuconazole, posaconazole, flucytosine, econazole, enilaconazole, miconazole, oxiconazole, saperconazole, sulconazole, terconazole, tioconazole, nikkomycin Z, anidulafungin (LY303366), nystatin, pimaricin, griseofulvin, ciclopirox, haloprogin, tolnaftate, and undecylenate.
Owner:FAIRFIELD CLINICAL TRIALS
Vagina swelling metronidazole clotrimazole suppository and preparation method and detection method thereof
ActiveCN103284996AImprove bioavailabilityPrevent outflowOrganic active ingredientsSuppositories deliveryChlorhexidine AcetateActive component
The invention relates to a vagina swelling metronidazole clotrimazole suppository which comprises an active component consisting of clotrimazole, metronidazole and chlorhexidine acetate, a fat-soluble matrix and a swelling carrier. The metronidazole clotrimazole is fully swelled, so that a medicine-containing layer can be in full contact with the inner wall of the vagina to prevent medicine liquid from flowing out; the swelling metronidazole clotrimazole suppository is an inner and outer layer swelling suppository; and the active component is mixed with different matrixes to prepare an inner layer and an outer layer, so that double effects of quick release and slow release can be achieved. According to the vagina swelling metronidazole clotrimazole suppository provided by the invention, seven unique leading technologies are adopted; and furthermore, the vagina swelling metronidazole clotrimazole suppository has the beneficial effects of preventing the medicine liquid from flowing out, quickly making a response and preventing secondary infection along with long medicine working time and the like.
Owner:哈尔滨田美药业股份有限公司
Method of treatment of otitis externa
This invention relates to a method of treating otitis externa using a topical combination medication, including one or more antifungal agent such as, for example fluconazole, voriconazole, itraconazole, clotrimazole, amphotericin B, caspofungin, micafungin, terbinafine, naftifine, butenafine, amorolfine, ravuconazole, posaconazole, flucytosine, econazole, enilaconazole, miconazole, oxiconazole, saperconazole, sulconazole, terconazole, tioconazole, nikkomycin Z, anidulafungin (LY303366), nystatin, pimaricin, griseofulvin, ciclopirox, haloprogin, tolnaftate, and undecylenate and one or more antibacterial agent such as neomycin sulfate, polymyxin B sulfate, colistin sulfate, gentamycin, tobramycin, chloramphenicol, Ciprofloxacin, Ofloxacin, a penicillin compound, a cephalosporin compound, a macrolide compound, a fluoroquinolone compound, streptomycin, or kanamycin.
Owner:FAIRFIELD CLINICAL TRIALS
Compsns-and methods for trapping and inactivating pathogenic microbes and spermatozoa
Antimicrobial and contraceptive compositions and methods which prevent and / or reduce the risk of transmission of sexually transmitted diseases through sexual activity as well as prevent and / or reduce the risk of pregnancy are provided. The compositions contain (1) a matrix-forming agent, (2) a bio-adhesive agent, (3) a buffering agent, (4) optionally a humectant, (5) optionally a preservative, and (6) water; wherein the composition is suitable for application within the vagina; wherein the compositions form a semisolid matrix on contact with ejaculate (thereby trapping ejaculated microbes and spermatozoa); wherein the composition causes hardening of cervical mucus (thereby decreasing the probability of sperm entry); wherein the composition forms a bio-adhesive layer over vaginal surfaces (thereby preventing or reducing the risk of contact of STD-causing microbes with the vaginal surfaces); wherein the composition maintains an acidic vaginal pH of less than about 5 in the presence of semen ejaculated from the male; and wherein the composition does not significantly impair the natural microbiological balance within the vagina. The antimicrobial and contraceptive compositions may also contain additional antimicrobial and / or contraceptive agents (e.g., nonoxynol-9, octoxynol-9, benzalkonium chloride, phosphorylated hesperidins, sulfonated hesperidins, polystyrene sulfonates, substituted benzenesulfonic acid formaldehyde co-polymers, H2SO4-modified mandelic acids, povidone iodine, itraconazole, ketoconazole, metronidazole, clotrimazole, fluconazole, teraconazole, miconazole, tinidazole, iconazole, chloramphenicol, nystatin, cyclopiroxolamine, and the like).
Owner:RUSH UNIV MEDICAL CENT
Antimicrobial and anticancer properties of methyl-beta-orcinolcarboxylate from lichen (Everniastrum cirrhatum)
InactiveUS20040198815A1Growth inhibitionDisruption of membrane integrityBiocideOrganic active ingredientsNystatin GAntibiotic Y
The present invention relates to the new use of an already known biomolecule methyl-beta-orcinol carboxylate of formula 1 isolated from a lichen (Everniastrum cirrhatum), for treating pathogenic fungal infections of humans that are resistant to polyene and azole antibiotics such as amphotericin B, nystatin, clotrimazole etc.
Owner:COUNCIL OF SCI & IND RES
Specific beriberi medicament
InactiveCN101601750AGood treatment effectReduce foot sweatAntimycoticsTetracycline active ingredientsVitamin CNystatin G
The invention provides a specific beriberi medicament, which is mainly prepared from the following raw materials in portion by weight: 3 to 12 portions of calcined alum, 1 to 4 portions of almond, 0.25 to 1 portion of metronidazole tablet, 0.15 to 1 portion of nystatin tablet, 0.15 to 1 portion of furazolidone tablet, 0.25 to 1 portion of oxytetracycline tablet, 0.15 to 1 portion of vitamin B1 tablet, 0.25 to 1 portion of vitamin B2 tablet, and 10 to 30 portions of clotrimazole cream. The medicament takes antibacterial medicaments as main ingredients, and can kill bacteria and fungi which cause the beriberi; the adding of vitamin B complex and vitamin C aims to repair wound surfaces, promote the healing, and ensure that the skin restores to normal more quickly; the calcined alum ensures that medicament application positions keep relatively dry; the almond relieves internal heat; various components supplement each other, can quickly and effectively treat the beriberi, and enables patients to recover; and at the same time, the medicament also has better efficacies in treating sole sweating and foot odor.
Owner:郭静 +2
Method for preparing metronidazole, clotrimazole and chlorhexidime vaginal acetate effervescent tablet and quality control method
InactiveCN101406463AEasy to useUse cleanOrganic active ingredientsPharmaceutical delivery mechanismDiseasePolyethylene glycol
The invention relates to a method for preparing a new gynecological anti-bacterial anti-inflammatory drug, namely dithiazole vaginal effervescent tablet and a method for quality control. The dithiazole vaginal effervescent tablet is used for treating gynecopathy, anorectal diseases and skin commonly-encountered diseases such as pelvic inflammatory disease, cervical erosion, vaginitis and the like caused by anaerobic bacteria, aerobic bacteria, trichomoniasis, pure or mixed fungal infections. The dithiazole vaginal effervescent tablet is characterized in that lactic acid and a calcium lactate buffer system are added to the formulation so that the pH value of the drug is between 3.5 and 4.0 similar to the vaginal physiological pH and is advantageous to remove pathogen and infusorian, provide living conditions suitable for vaginal beneficial bacterium group of lactic acid bacteria, and promote the illness rehabilitation. Besides, the dithiazole vaginal effervescent tablet uses pharmaceutical acceptable water-soluble polymer materials such as water-soluble polymer polyethylene glycol, polyvidone K30 and the like to enwrap acidic or alkaline components of an effervescent disintegrant so as to avoid the occurrence of hidden spots or even collapsing phenomenon on the surface of the tablet caused by gas production reaction resulted from small amount of water in the storage process, and uses a ethanol solution with adhesives and surface-active agents for granulation so that the hydrolysis of clotrimazole can be prevented, the infiltration of moisture is promoted in use, the uniform collapse is accelerated, and gas bubbles are homogeneous and stable.
Owner:DAKANG JUNBISHA XIANYANG SHAANXI PROV
Expansion suppository of clotrimazole or salt thereof, preparation method and detection method
InactiveCN103284941AGuaranteed effective concentrationPrevent outflowOrganic active ingredientsAntimycoticsSide effectSuppository
The invention relates to an expansion suppository of clotrimazole or a salt thereof. The expansion suppository consists of the clotrimazole or the salt thereof, a fat-soluble matrix and an expandable expansion carrier. The invention also relates to a preparation method and a detection method for the expansion suppository. The expansion suppository of the clotrimazole or the salt thereof provided by the invention adopts seven original leading technologies, wherein the addition of the expansion carrier applies six technologies including a suppository disposable filling integration technology, an inner core expansion coefficient control technology, an ergonomic suppository type technology, a treating and cleaning integrated preparation formulation, a vagina-attached administration technology and a side leakage prevention suppository body design; and poloxamer and a sustained-release material in the expansion suppository apply a medicament long-acting layered release technology. The expansion suppository of the clotrimazole or the salt thereof disclosed by the invention has the beneficial effects of having high efficiency, long acting, safety and few side effects, preventing the overflow of medicament liquid, cleaning the vagina, preventing secondary infection and the like.
Owner:HARBIN OT PHARMA +1
Oral sticking tablet and method of preparing the same
InactiveCN101108172APaste time is longGood flexibilityOrganic active ingredientsAntimycoticsDrug concentrationClotrimazole Vaginal Tablet
The invention relates to an oral cavity plaster, which has effective dosage of active drug ingredient, 1 per cent to 40 per cent bioadhesion ingredient, 0 per cent to 85 per cent (w / w) diluted filling ingredient, 0 per cent to 5 per cent (w / w) lubricant glidant ingredient and 0.5 per cent to 89 per cent (w / w) water absorption carrier ingredient. Wherein, the active drug ingredient comprises fluconazole, itraconazole, clotrimazole or Nystatin. Therefore, the oral cavity plaster in the invention can be adhered to the medication position, is able to continuously and stably release drug in during a long time (within 9 hours) as well as maintain effective and safe drug concentration and prevent the impact on medication resulted from eating, drinking and swallow action of oral cavity.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Clotrimazole gel, and its prepn. method
ActiveCN1903174AGood curative effectImprove transdermal absorption rateOrganic active ingredientsAntimycoticsClotrimazole Vaginal TabletChemistry
A mycosporin gel with high percutaneous absorptivity and stability and its preparing process are disclosed.
Owner:北京科信聚润医药科技有限公司
Metronidazole effervescence patch and technique of preparing the same
ActiveCN101152176AImprove the bactericidal effectGood synergyOrganic active ingredientsAntimycoticsChlorhexidine AcetateTalc
The invention discloses a Metronidazole, Clotrimazole and Chlorhexidine Acetate Effervescent Tablet and the preparation method. Each tablet has the following proportion of contents: metronidazole 0.2 gram, clotrimazole 0.16 gram, acetic acid chlorhexidine acetate 0.008 gram, fumaric acid 0.108 to 0.162 gram, tartaric acid 0.012 to 0.018 gram, solium bicarbonate 0.12 to 0.18 gram, starch 0.02 to 0.04 gram, dextrin 0.003 to 0.007 gram, hydroxypropyl cellulose 0.007 to 0.013 gram, talc 0.01 to 0.017 gram, sodium dodecyl sulfate 0.01to 0.02 gram, and proper proportion of povidone K30 water solution. According to the preparation method, the components are mixed to produce granule (1) and granule (2); the talc, sodium dodecyl sulfate and the granule (1) and granule (2) are mixed uniformly; granule content is tested and according to the granule content, the tablet weight is identified for tabletting. The invention overcomes the shortages with prior art, enables the acetic acid chlorhexidine acetate to perform functions normally, performs synergistic effects of the three drugs, and greatly enhances the cure rate. Besides, the frothing volume is high, and within storage period, the quality is stable and the drug effects are not affected.
Owner:SHANDONG SBOND PHARMA
Nanometer clotrimazole emulsion medicine and its prepn process
InactiveCN1931163AEnhanced inhibitory effectHigh thermodynamic stabilityOrganic active ingredientsAntimycoticsSkin permeabilityDistilled water
The nanometer clotrimazole emulsion medicine consists of surfactant, oil, clotrimazole and distilled water. Its preparation process includes the following steps: weighing surfactant with or without co-surfactant; calculating HLB value and selecting oil for reaching emulsifying HLB value near that of the surfactant phase; dissolving clotrimazole into surfactant; changing the ratio between the surfactant phase and the oil phase regularly; adding distilled water slowly at 20-25 deg.c to form clear and flowing O / W type stable nanometer emulsion liquid. The nanometer clotrimazole emulsion has high skin permeability, no contamination to clothing, high dissolubility of clotrimazole, raised bioavailability of clotrimazole, delayed metabolism time and wide medicine market foreground.
Owner:NORTHWEST A & F UNIV
Medicine composition for preventing and treating fungal disease of pet rabbit
InactiveCN103285032AEnsure normal growth and developmentInhibitionOrganic active ingredientsAntimycoticsFungal diseaseEuropean rabbit
The invention provides a medicine composition for preventing and treating the fungal disease of a pet rabbit. The medicine composition is prepared from the following components in parts by mass: 3 parts of talcum powder, 2 parts of sulphur powder, 1 part of diaveridine and 0.5 part of clotrimazole. The medicine composition can be used for preventing and treating the fungal disease of the pet rabbit, and inhibiting, preventing and treating the occurrence and the infection of diseases caused by the fungus of the pet rabbit, so that the normal growth and development of the pet rabbit are ensured. Therefore, the medicine composition is suitable for the prevention and the treatment of fungal disease of the pet rabbits and other susceptible animals.
Owner:鲍成杰
Preparation method of clotrimazole vaginal tablets
ActiveCN109528673AIsolated from direct contactInhibit migrationOrganic active ingredientsAntimycoticsPrillPharmaceutical formulation
The invention discloses a preparation method of clotrimazole vaginal tablets, and belongs to the field of pharmaceutical preparations. The method comprises the following steps: 1, preparation of an adhesive solution by wet granulation; 2, preparation of a granule coating liquid; 3, preparation and coating of lactic acid and calcium lactate granules; 4, total mixing of materials; and 5, tableting and packaging. The method separately granulates lactic acid, calcium lactate and starch, and coats granules, and can effectively isolate the direct contact between raw materials and acidic components and prevent the migration of the acidic components during the tablet placing process, thereby effectively avoiding the curative effect reduction caused by the degradation of active components, and theprepared sample has good stability. The preparation process of the invention uses common equipment, can realize industrial production, and is worthy of application and promotion.
Owner:南京泽恒医药技术开发有限公司
Melanin production inhibitor
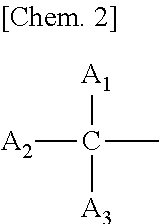
ActiveUS20110243865A1Enhanced inhibitory effectImprove securityCosmetic preparationsHair cosmeticsArylHydrogen
Disclosed is a melanin production inhibitor which has an excellent inhibitory activity on the production of melanin and is highly safe. The melanin production inhibitor comprises a compound represented by general formula (1) (excluding clotrimazole), and / or a pharmacologically acceptable salt thereof. In the formula, A1, A2 and A3 are independently selected from a hydrogen atom, an aryl group which may have a substituent, and an aromatic heterocyclic group which may have a substituent, wherein at least one of A1, A2 and A3 is selected from the aryl group and the aromatic heterocyclic group, the total number of carbon atoms contained in A1, A2 and A3 is 6 to 50 and, when at least two of A1, A2 and A3 represent the aryl groups or the aromatic heterocyclic groups, the adjacent two aryl or aromatic heterocyclic groups may be bound to each other via an alkyl chain or an alkenyl chain to form a ring; m represents an integer of 0 to 2; X represents a hetero atom, a hydrogen atom, or a carbon atom; R1 and R2 are independently selected from a hydrogen atom and an oxo group, wherein when one of R1 and R2 is an oxo group, the other is not present; and R3 is selected from a hydrogen atom, and a C1-8 hydrocarbon group in which one or some of hydrogen atoms or carbon atoms may be substituted by a hetero atom or hetero atoms, wherein the number of R3's present in the compound corresponds to the number of X's and, when two or more R3's are present, the R3's are independently present and the adjacent two R3's may be bound to each other to form, together with X, a ring, and the terminal of R3 may be bound to a carbon atom to which A1, A2 and A3 are bound, thereby forming a ring.
Owner:POLA CHEM INDS
Modulating inflammation with cytochrome P-450 activators and inhibitors
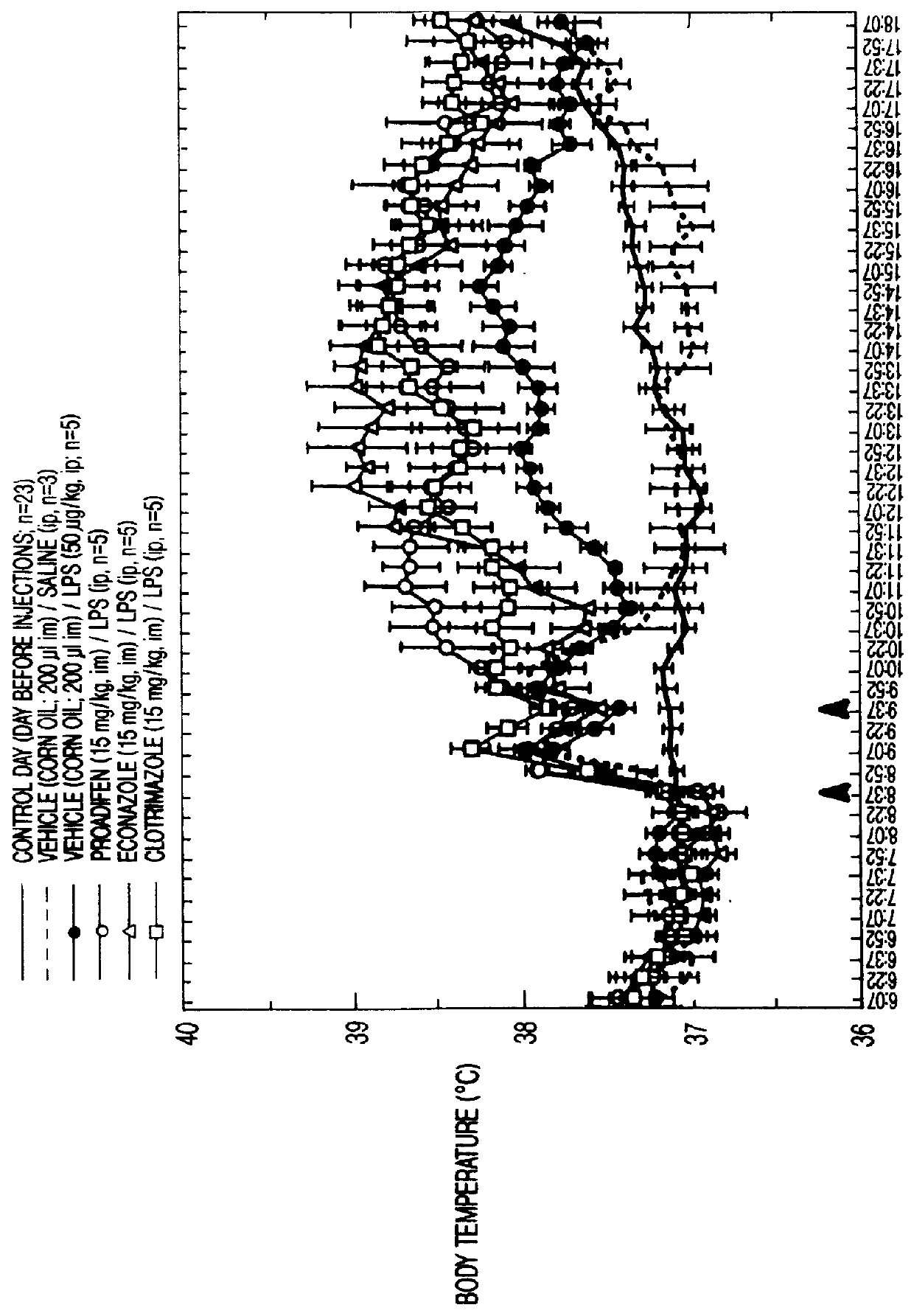
This invention relates to methods of modulating inflammation in mammals. Inflammation is modulated by regulating the cytochrome P-450 pathway. Inflammation is reduced by treating the subject with substances, such as bezafibrate and clofibrate, which induce the P-450 pathway. Inflammation is promoted by treating the subject with substances, such as proadifen, econazole, and clotrimazole, which inhibit the cytochrome P-450 pathway.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Preparation method of metronidazole clotrimazole and chlorhexidine acetate vaginal effervescent tablet
ActiveCN102379870AAntibacterial agentsOrganic active ingredientsEffervescent tabletChlorhexidine Acetate
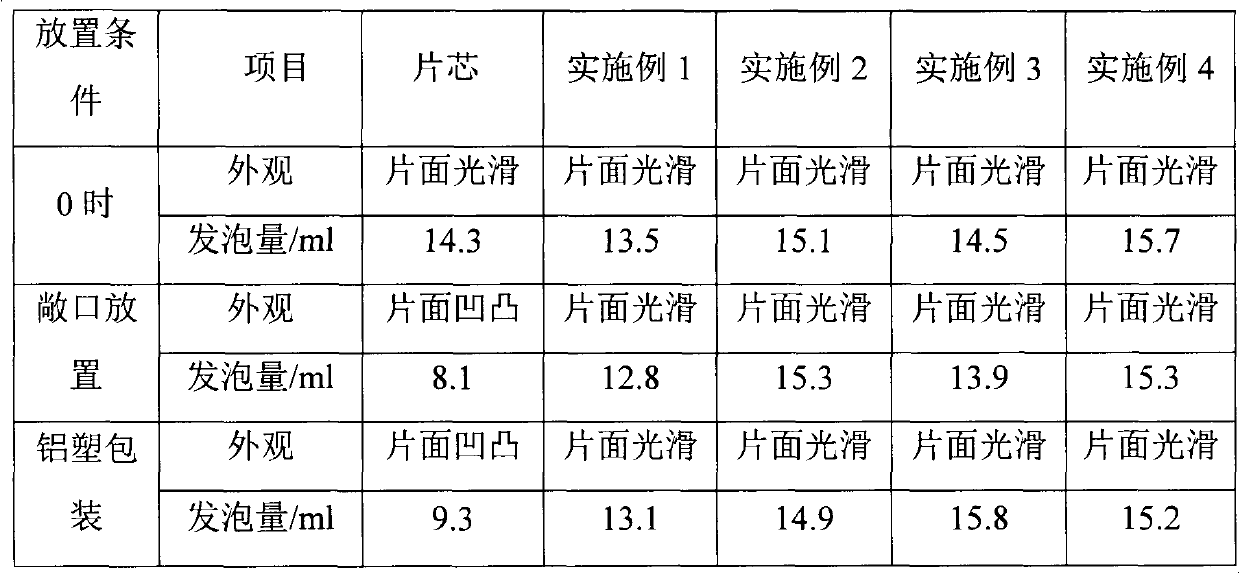
The invention relates to a metronidazole clotrimazole and chlorhexidine acetate vaginal effervescent tablet, which consists of a tablet core, an isolating layer and a filmcoating layer, wherein the tablet core of the metronidazole clotrimazole and chlorhexidine acetate vaginal effervescent tablet is firstly prepared, and then ethyl cellulose and polyethylene glycol are wrapped by the isolating layer and are finally wrapped by filmcoating. The appearance, foaming amount and other indicators of the vaginal effervescent tablet are stable during the storage process, and the storage requirement islow, so the use and curative effect of the medicine are ensured.
Owner:南京海鲸药业股份有限公司
Clotrimazole vaginal tablets
InactiveCN106420726AFine grainGood sustained releaseOrganic active ingredientsAntimycoticsPrillYeast vaginitis
The invention discloses clotrimazole vaginal tablets and a production method thereof. Raw materials and process parameters are optimized comprehensively, and it is unexpectedly found that the clotrimazole vaginal tablets have a good slow release effect, are not required to be combined with oral medication and have a good control effect on recurrence of recurrent vulvovaginal candidiasis under the optimized parameters. The method comprises steps as follows: (1) material preparation in parts by weight: (2) pretreatment: all raw and auxiliary materials, except kushenin powder and nano tourmaline powder, are sieved by an 80-mesh sieve for later use; the kushenin powder is sieved by a 200-mesh powder for later use; the particle size of the nano tourmaline powder is selected to range between 8 nm and 12 nm; (3) granulation: the raw and auxiliary materials are weighed according to a prescription, placed in a high-speed mixing granulator for mixing and subjected to wet granulation with a lactic acid aqueous solution; (4) drying: prepared wet granules are dried at 60 DEG C for 4 h; (5) granulation and whole mixing: dried granules are placed in a 3D motion mixer to be uniformly mixed as a batch; (6) tableting: tableting can be performed after the granules pass inspection, and each table contains 500 mg of clotrimazole.
Owner:ZHEJIANG SHENGBOKANG PHARMA CO LTD
Compound betamethasone 17-valerate otic gel used for pet and preparation method of compound betamethasone 17-valerate otic gel
InactiveCN107773565AAnti-inflammatoryAntipruriticAntibacterial agentsOrganic active ingredientsChronic otitis externaIrritation
The invention relates to a compound betamethasone 17-valerate otic gel used for a pet and a preparation method of the compound betamethasone 17-valerate otic gel, and belongs to the technical field ofpet medicines. Betamethasone 17-valerate, florfenicol sodium succinate and clotrimazole are combined in use and can treat acute and chronic otitis externa caused by bacteria and fungus. The gel has agood anti-inflammatory effect, is wide in antibacterial spectrum, and achieves the effect of in-depth treatment, and the irritation is low.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Compound suspended ear drops for treating auditory canal inflammation of dogs
InactiveCN104739850ASimple processSuitable for industrial productionOrganic active ingredientsSenses disorderMedicineAdditive ingredient
The invention relates to a preparation method for compound clotrimazole, betamethasone valerate and gentamicin sulphate suspended ear drops for treating ear inflammation of dogs. According to the preparation method, the above several medicines are stably and uniformly stored in oily ear drops and not precipitated in several hours, with stable effective ingredient contents through a special process treatment. The production process disclosed by the invention is simpler and more convenient to operate, easily available in raw materials, beneficial to industrialized production, good in stability, convenient to store and transport, and remarkable in clinic treatment effect, as well has a large-scale production and clinic application advantage.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Method of treating neurological disorders using clotrimazole and derivatives thereof
Methods and pharmaceutical compositions are disclosed for treating neurological disorders, such as Huntington's disease or Alzheimer's disease. The methods involve the administration of a triarylmethane compound, such as clotrimazole, or a salt thereof.
Owner:ENVIVO PHARM INC
Poultry feed additive and preparation method and application thereof
InactiveCN101703157AChange permeabilityLower resistanceAnimal feeding stuffAccessory food factorsVitamin CMycotoxin
The invention discloses a poultry feed additive and a preparation method and application thereof, and aims to provide the poultry feed additive used for detoxicating mycotoxin and preventing and controlling fungal infection, and the preparation method and application thereof. The feed additive comprises the following components in percentage by weight: 1 to 4 percent of clotrimazole or 1 to 3 percent of nystatin, 5 to 20 percent of malto-oligosaccharides, 5 to 12 percent of glucurolactone, 10 to 20 percent of glucomannan polymer, 20 to 40 percent of sepiolite, 0.2 to 1 percent of vitamin C and the balance of conventional feed additive auxiliary material. Different adsorbents are mixed in a proper ratio so as to contribute to improving detoxication effect. Various active components are reasonably compatible, so the feed additive has synergism, and has the effects in various aspects such as treating the fungal infection, avoiding mould damaging livestock organisms, detoxicating organisms and adsorbing the mycotoxin, and has quick response, good treatment effect and safe use when used for detoxicating the mycotoxin and preventing and controlling the fungal infection.
Owner:TIANJIN SHENGJI GRP CO LTD
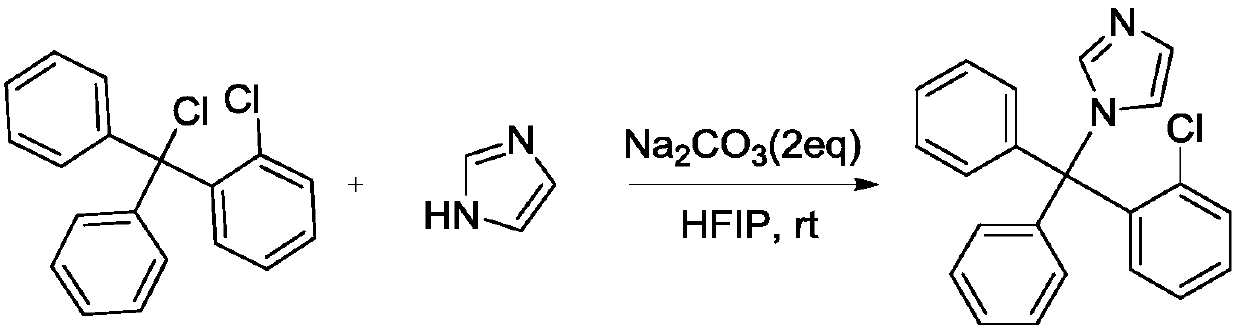
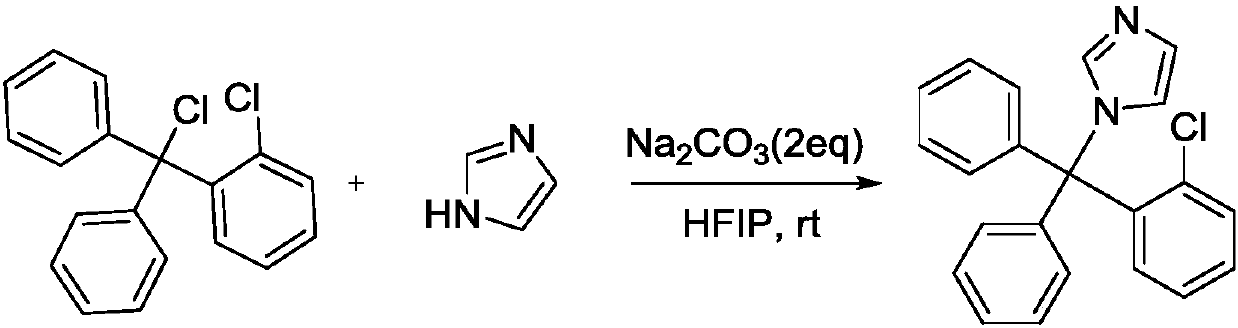
Synthesis method of clotrimazole
ActiveCN107629006AFully transformedPromote conversionOrganic chemistryChemical synthesisSynthesis methods
The invention discloses a synthesis method of clotrimazole, and belongs to the technical field of chemical synthesis. According to the method, 2-chlorotrityl chloride and imidazole are taken as raw materials, hexafluoroisopropanol is taken as a solvent, and reaction is performed in an alkali environment. The method has the advantages that the raw materials 2-chlorotrityl chloride and imidazole areeasy to get; the reaction can be performed at room temperature, and the reaction condition is mild; other than previous multistep reaction, the method adopts one-step synthesis and is simple to operate; the method is high in reaction activity, the yield reaches 92 percent, and the raw materials are completely converted; products are convenient to separate, and the reaction has green economical efficiency, and is environmentally friendly.
Drug for treatment of eczema skin disease
The invention relates to a drug for treatment of eczema skin disease. A preparation method of the drug comprises the following steps of weighting traditional Chinese medicine components according to weight ratio: 1-3 parts of scutellaria baicalensis, 0.5-1.5 parts of coptis chinensis, 1-3 parts of golden cypress, and 1-3 parts of rheum officinale; and after smashing, screening with a 20-mesh sieve, blending, taking one proportional quantity of sesame oil and heating till boiling, then adding the medicine powder, frying till withered and yellow, filtering and then adding two proportional quantity of beeswax till melting, dripping on deslagging material fried with sesame oil, taking 5-15 parts of the medicine and 0.5-1.5 parts of borneol, taking western medicines of 5-15 parts of cod-liver oil, 0.5-1.5 parts of erythrocin, and 0.5-1.5 parts of clotrimazole, grinding and dissolving the components, and then adding vaseline to 50-150 parts so as to prepare the drug. The drug is prepared by a Chinese and western medicine formula, has effects in clearing away heat and toxic materials, diminishing inflammation, relieving pain and itching, and promoting skin union, has good curative effect for refractory and relapsed diseases such as eczema, angular cheilitis, anal fissure and the like, and especially has good effect for skin disease caused by heat toxin.
Owner:呼和浩特市第一医院
Clotrimazole cream and preparation method thereof
InactiveCN105012229ADoes not disrupt acid-base balanceEnsure safetyAntibacterial agentsOrganic active ingredientsAntibacterial activityBacterial vaginitis
The invention discloses clotrimazole cream and a preparation method thereof. The clotrimazole cream comprises components in percentage by mass as follows: 1% of clotrimazole, 17%-34% of oleaginous bases, 4%-6% of an emulsifying agent, 5%-10% of a moisturizer, 1.1%-3% of a preservative, 0.03%-0.05% of a complexing agent and the balance of water. According to the clotrimazole cream, the antibacterial drug clotrimazole with the concentration of 1% is reasonably proportioned with components such as the preservative with antibacterial activity and the like, so that the antibacterial drug plays the important role, can improve the trichomonas killing effect, can be used for treating bacterial vaginitis, trichomonas vaginitis, senile vaginitis, male balanitis, penis itching and the like clinically, and enlarges the treatment range.
Owner:GUANGDONG SHUNFENG PHARMA
Clotrimazole vaginal tablet composition and preparation method thereof
ActiveCN111789838AImprove complianceGood antibacterial effectAntimycoticsUnknown materialsBiotechnologyCALCIUM LACTOBIONATE
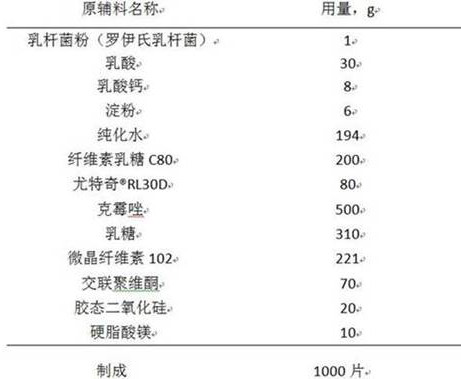
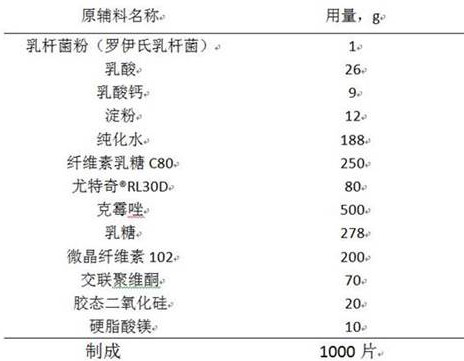
The invention relates to a clotrimazole live lactobacillus composition and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The clotrimazole live lactobacillus vaginal tablet disclosed by the invention comprises 500mg of clotrimazole; 1mg of lactobacillus powder; 20mg to 30mg of lactic acid; 8-12mg of calcium lactate; 6-12mg of starch; 200-300mg of cellulose lactose C80; 66mg to 180mg of eudragit-RL30D; 225mg to 314mg of lactose; 170mg to 225mg of microcrystalline cellulose; 70mg of crospovidone; 20mg of colloidal silicon dioxide; and 10 mg of magnesium stearate. The invention provides the clotrimazole live lactobacillus vaginal tablet.
Owner:DISHA PHARMA GRP
Clotrimazole ointment and preparation method thereof
InactiveCN107998072AImprove absorption efficiencyGood treatment effectOrganic active ingredientsAntimycoticsMedicineIrritation
The invention relates to the field of pharmacy, in particular to clotrimazole ointment and a preparation method thereof. The clotrimazole ointment is mainly prepared from various raw materials and auxiliary materials, wherein the various raw materials and auxiliary materials are prepared from 1 to 3 parts of clotrimazole, 4 to 5 parts of oil phase mixture, 7 to 9 parts of water phase mixture and 4to 6 parts of dispersing agent. The ointment is stable in characteristics, uniform and exquisite in matrix, low in irritation to the skin, good in tolerance, easy to clean, and capable of avoiding the secondary infection.
Owner:重庆希尔安药业有限公司
Externally-used medicine for treating ringworm of nails
InactiveCN108714162AReasonable ratioNo side effectsAntimycoticsInorganic boron active ingredientsBenzoic acidSide effect
The invention discloses an externally-used medicine for treating ringworm of the nails, containing an ointment component and an auxiliary liquid medicine component, wherein the ointment component is prepared from the following materials in parts by weight: 11-13 parts of salicylic acid, 7-9 parts of benzoic acid, 25-27 parts of boric acid, 13-14 parts of radix sophorae flavescentis, 6-7 parts of talcum powder, 0.1-0.3 part of erythrocin, 9-11 parts of liquid paraffin, 1-3 parts of lanolin, 4-5 parts of yellow vaseline and 16-18 pats of clotrimazole; the auxiliary liquid medicine component is prepared from the following materials in parts by weight: 120-130 parts of salicylic acid, 120-130 parts of benzoic acid, 120-130 parts of cortex pseudolaricis and 620-630 parts of 90% alcohol. The externally-used medicine for treating ringworm of the nails, by combination of multiple components, is reasonable in ratio, free from toxic and side effect, strong in osmotic force, short in treatment time, and fast to take effect, and has the efficacies of sterilizing and disinfecting, clearing away heat and drying dampness, astringing dampness and closing sores, and relieving itching and killing insects.
Owner:张笑笑
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com