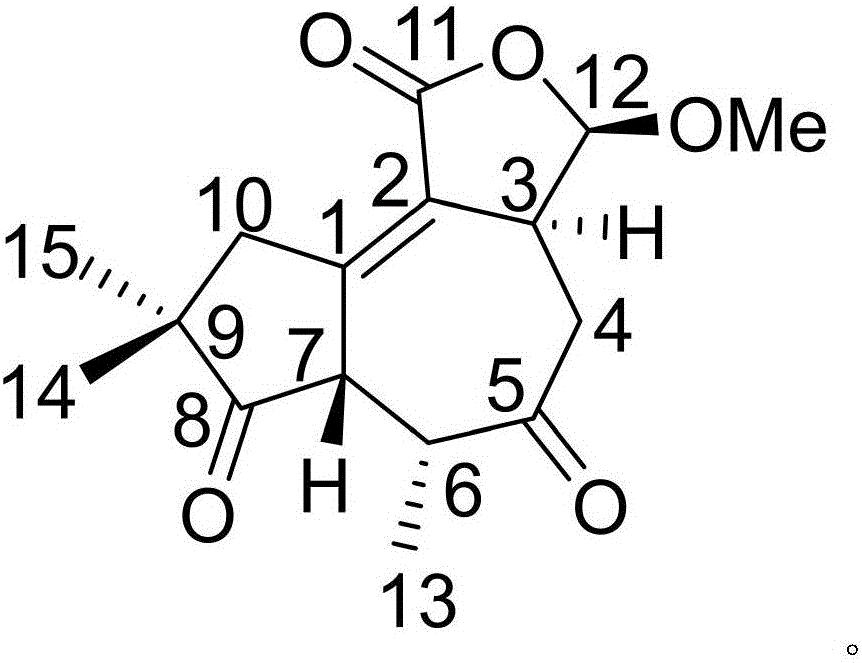
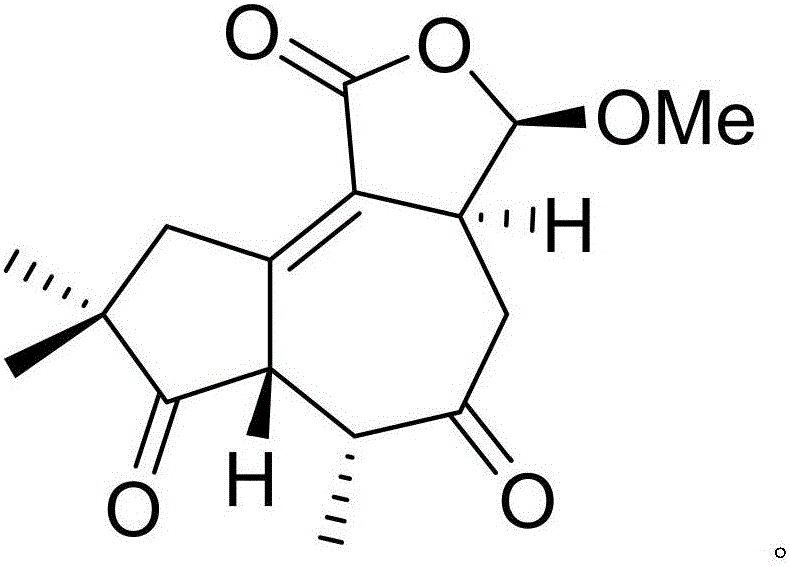
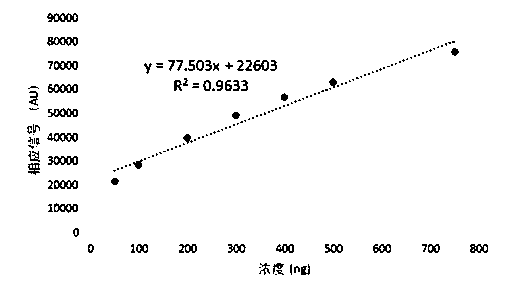
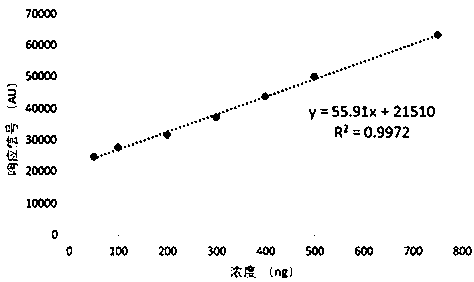
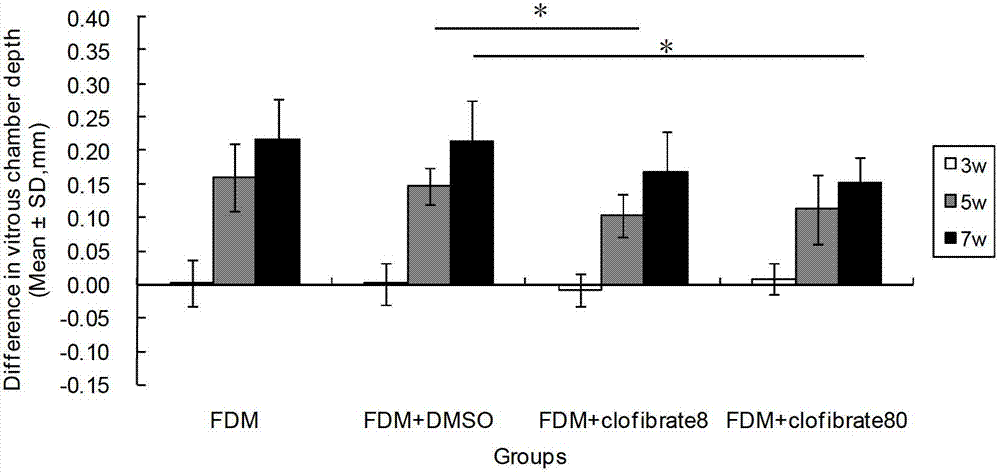
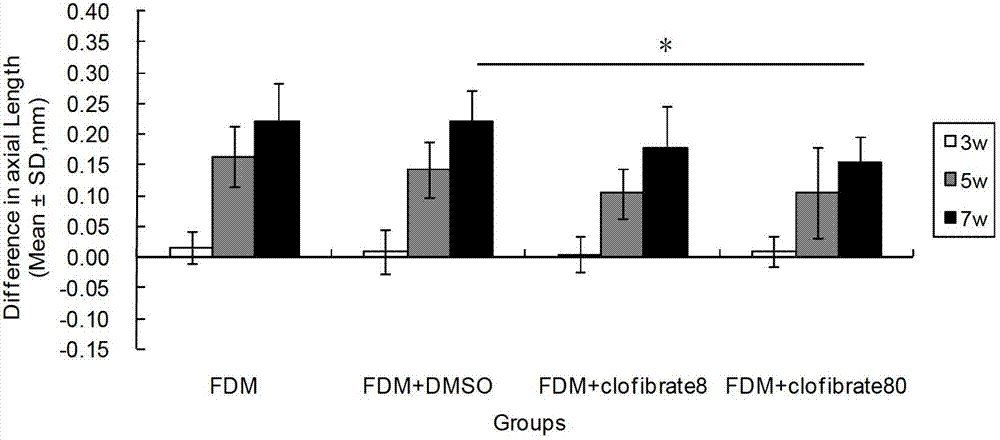
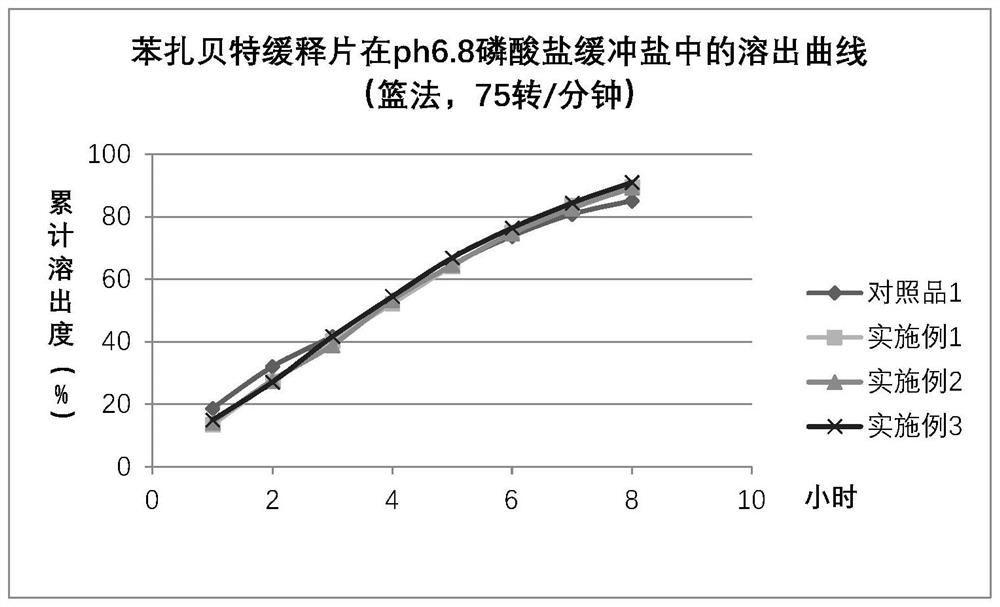
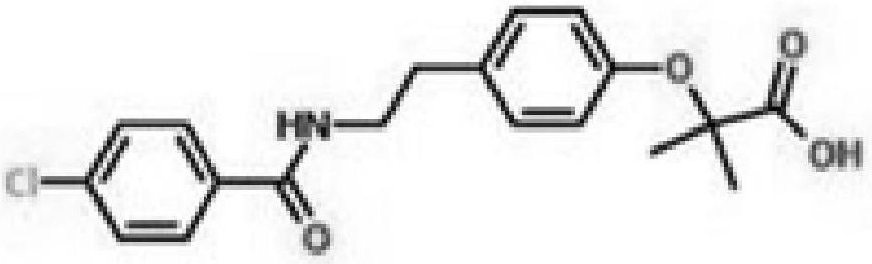
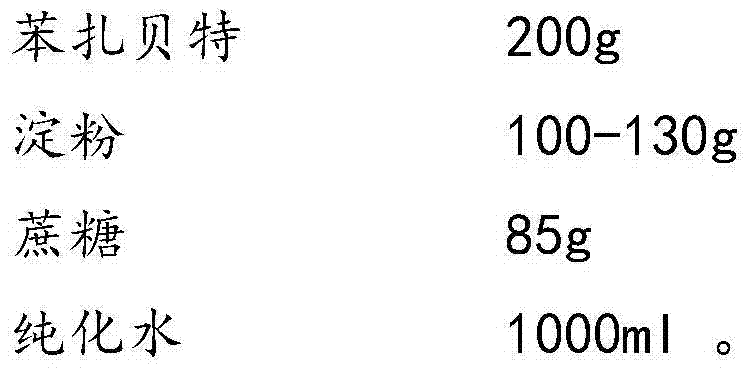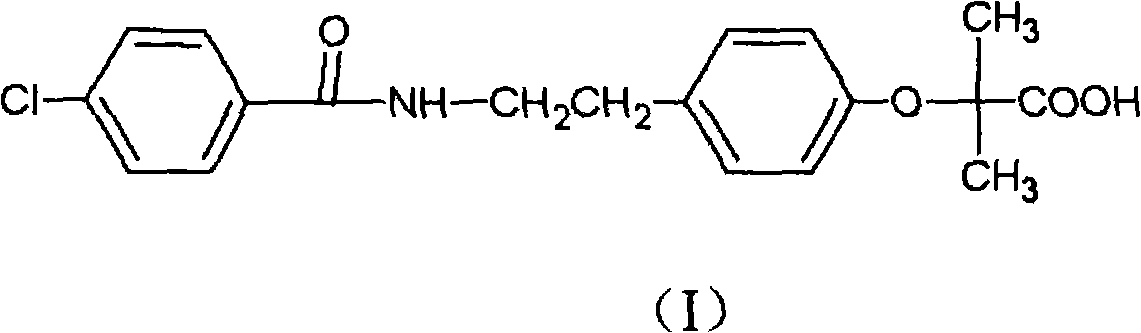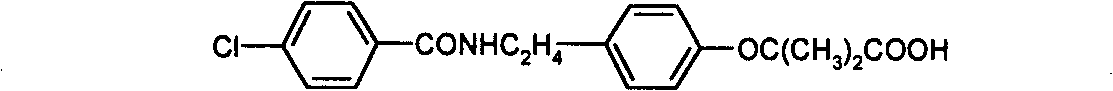Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Bezafibrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
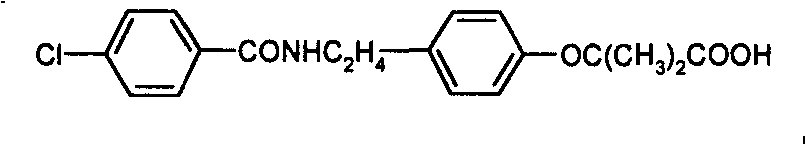
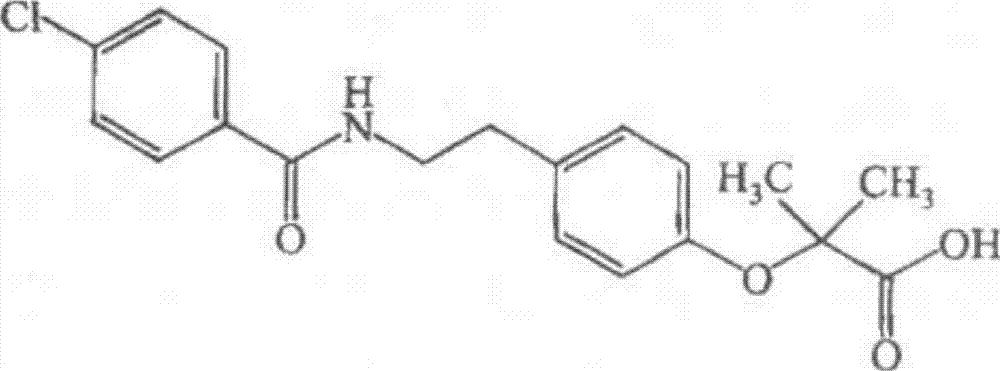
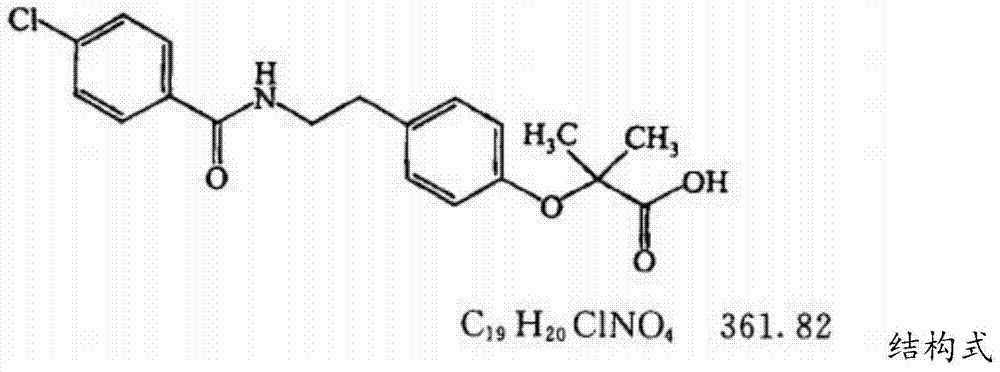
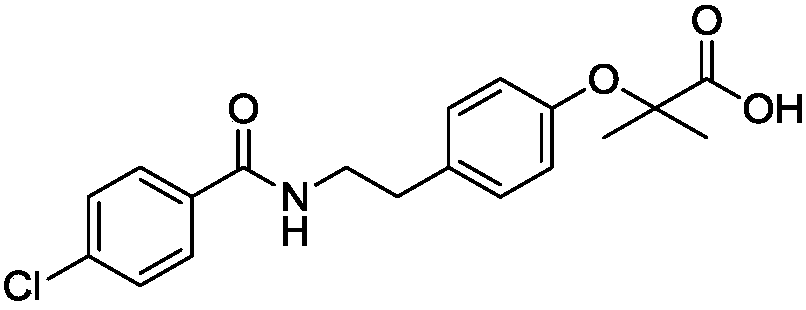
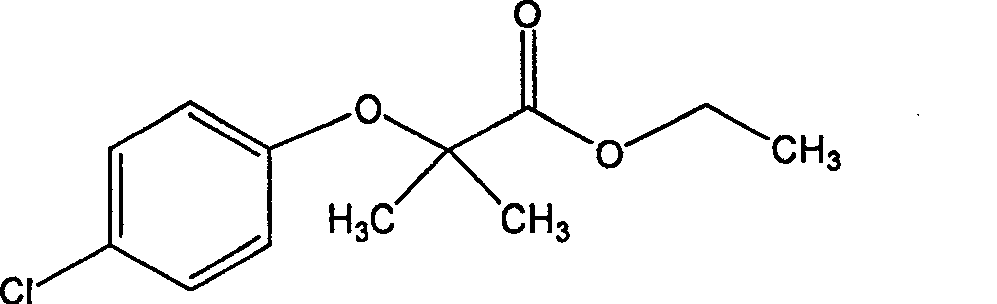
Bezafibrate (marketed as Bezalip and various other brand names) is a fibrate drug used as a lipid-lowering agent to treat hyperlipidaemia. It helps to lower LDL cholesterol and triglyceride in the blood, and increase HDL.
Method for preventing or treating metabolic syndrome
InactiveUS20070088088A1Enhanced inhibitory effectHigh expressionBiocideAnimal repellantsBezafibrateMetabolic syndrome
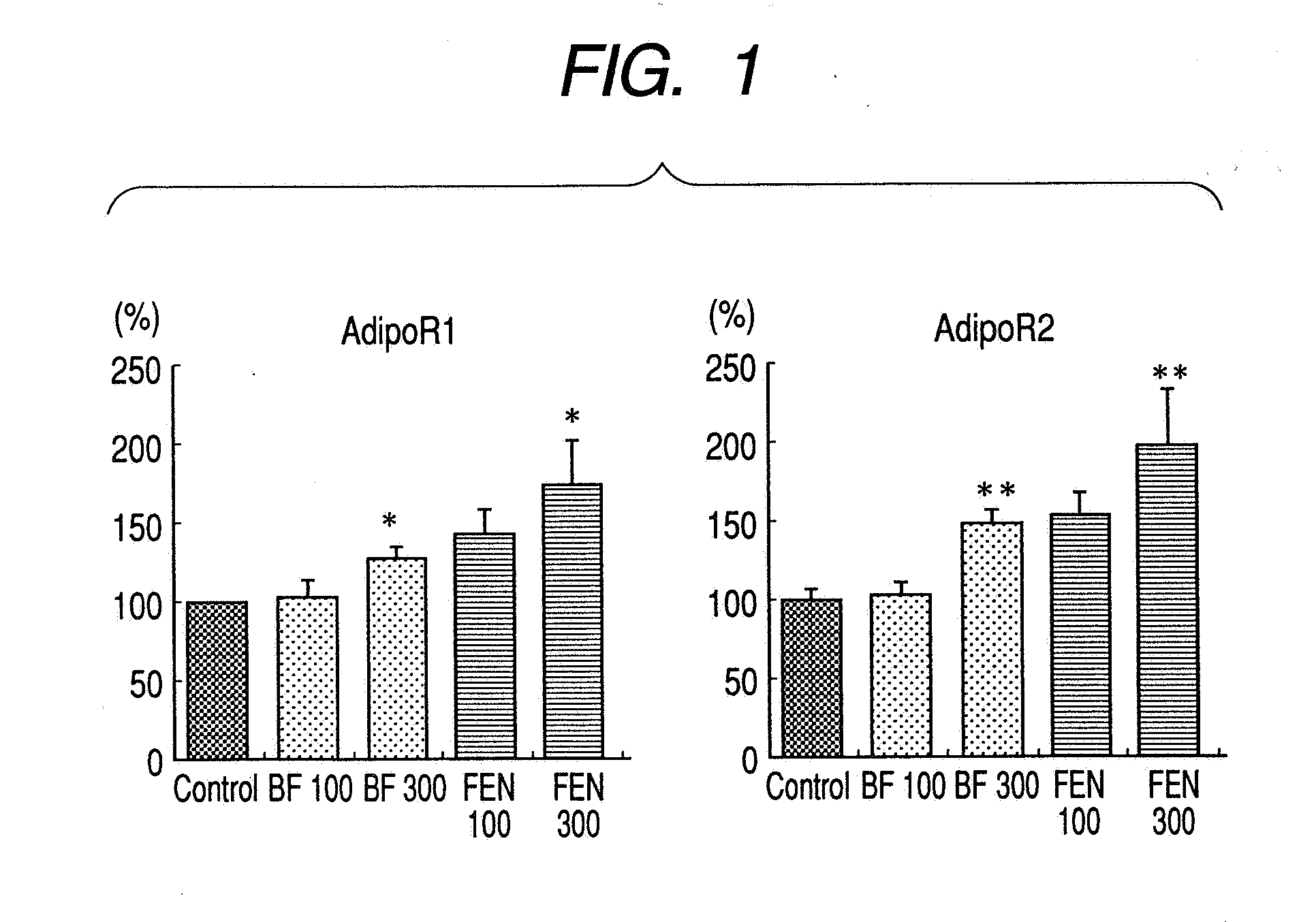
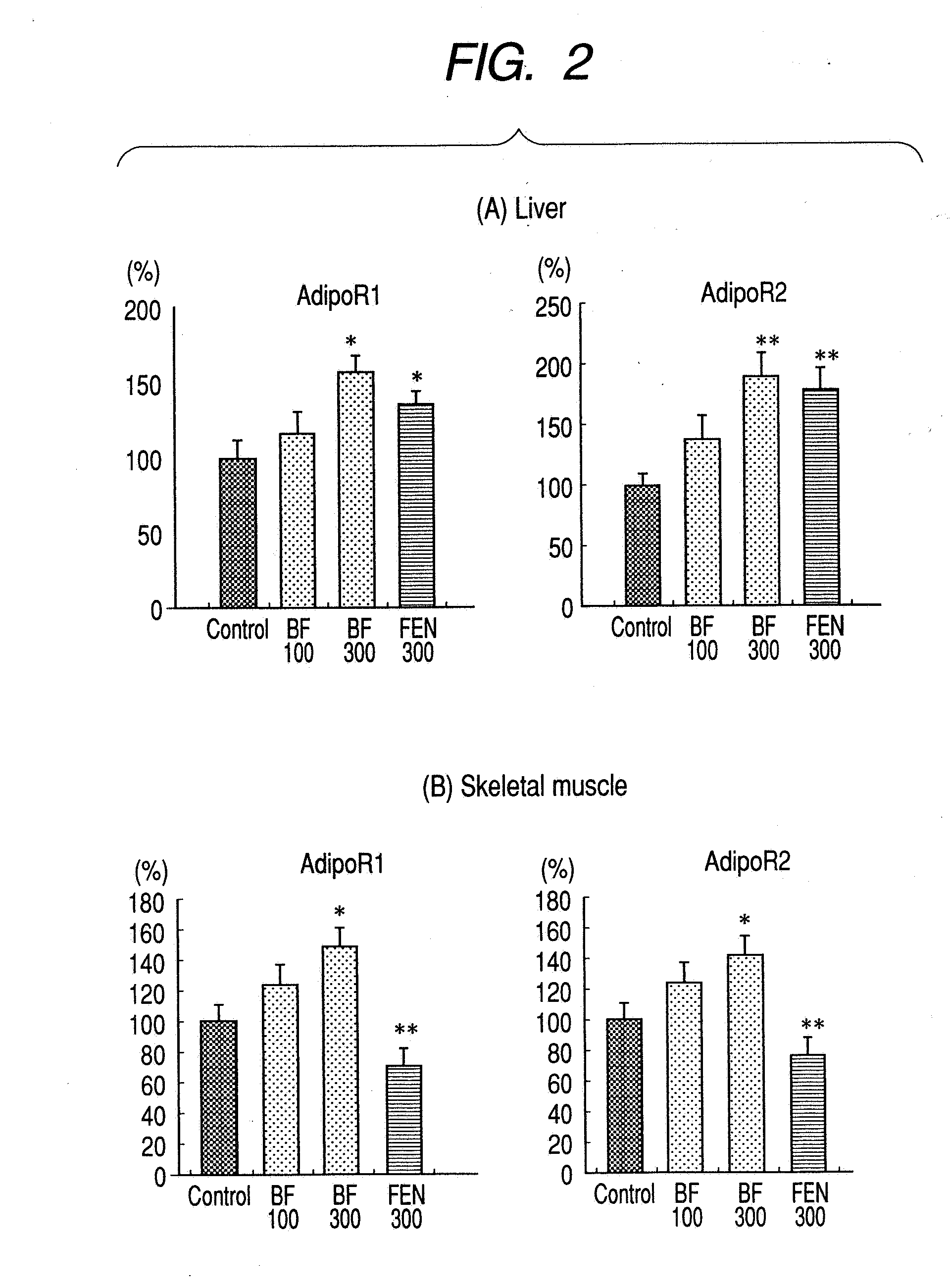
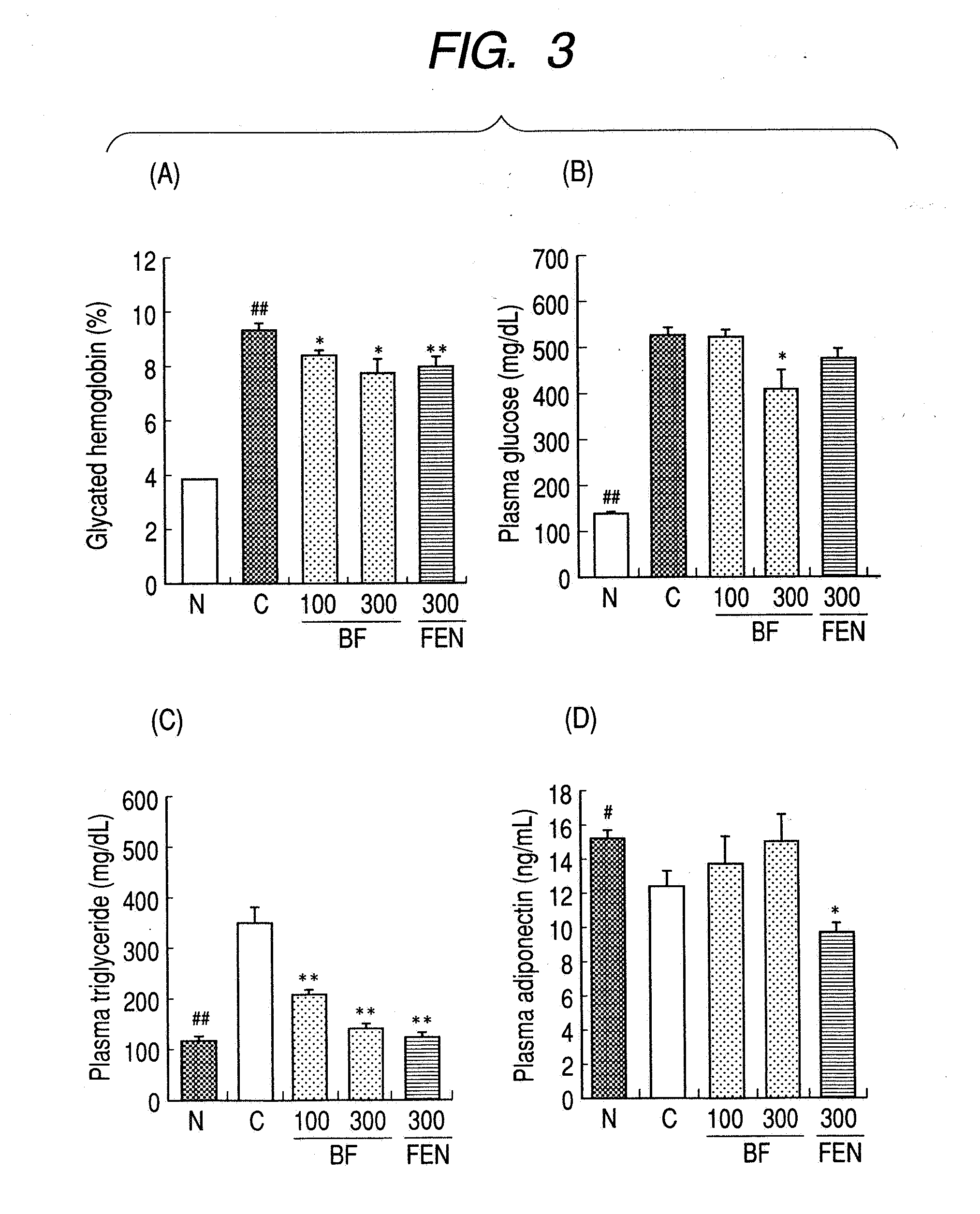
A method for preventing or treating metabolic syndrome by administering bezafibrate. Since bezafibrate suppresses the action of 11β-hydroxysteroid dehydrogenase type 1 and also accelerates expression of adiponectin receptor, it is used as an agent for preventing or treating metabolic syndrome.
Owner:KISSEI PHARMA
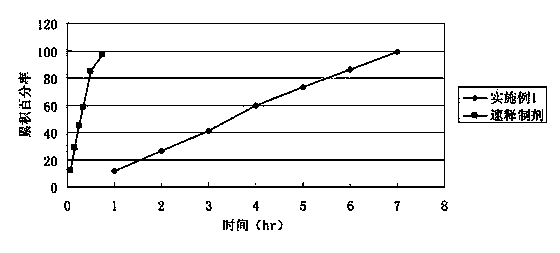
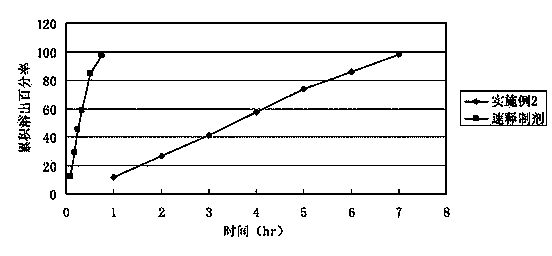
Bezafibrate controlled release formulation and preparation method thereof
ActiveCN101342164AIncrease production capacityImprove standardizationPeptide/protein ingredientsMetabolism disorderSide effectPolyvinyl alcohol
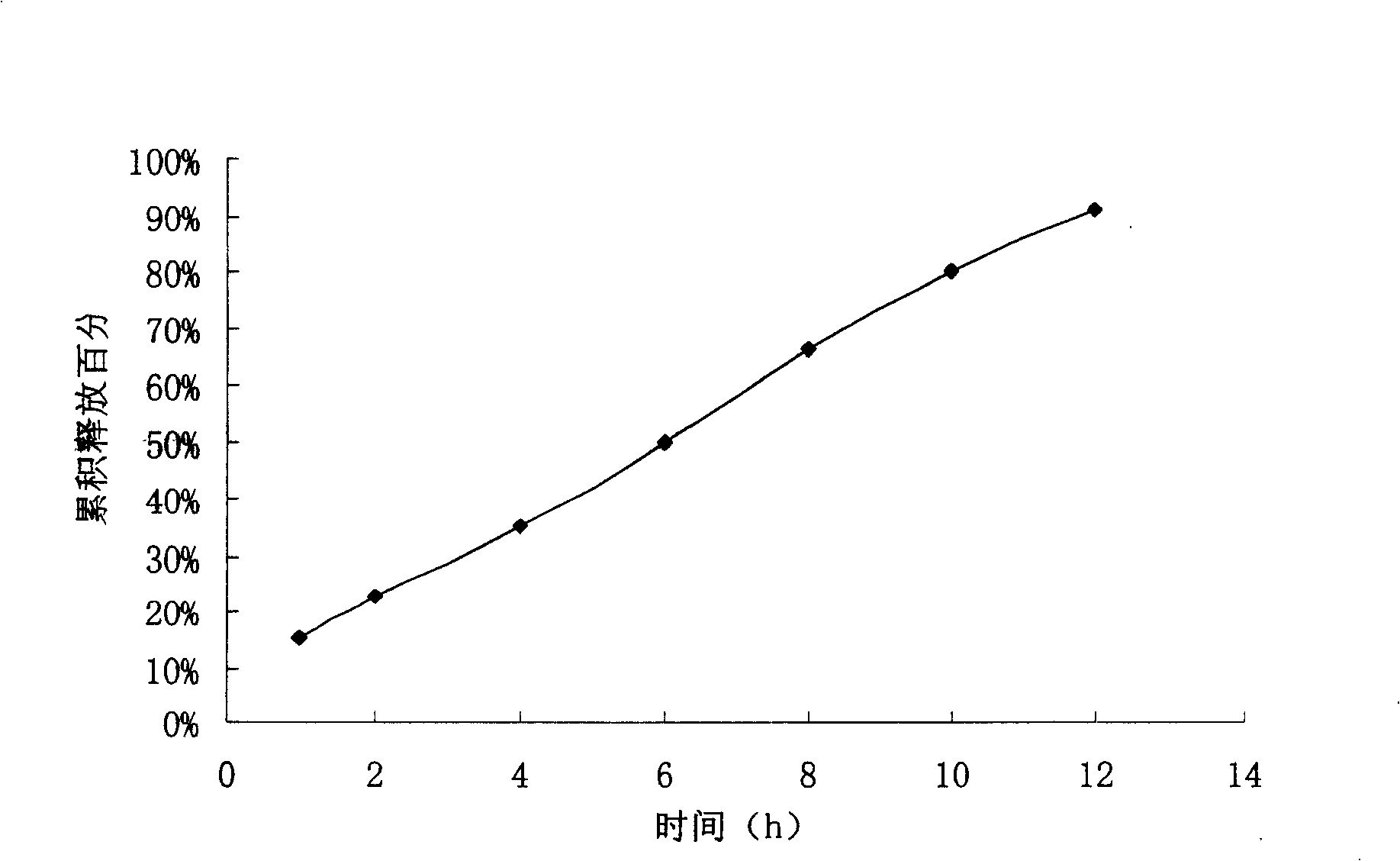
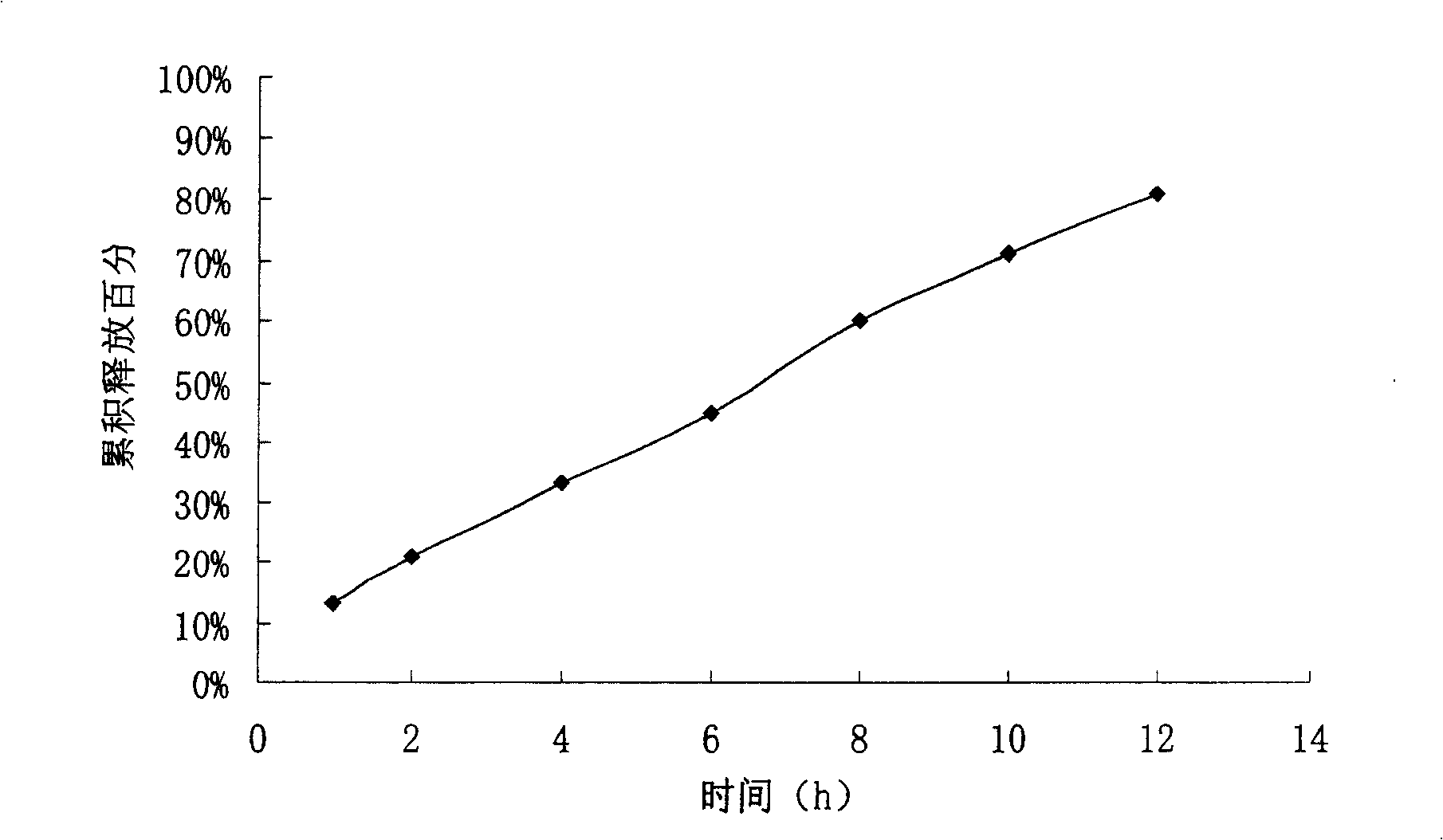
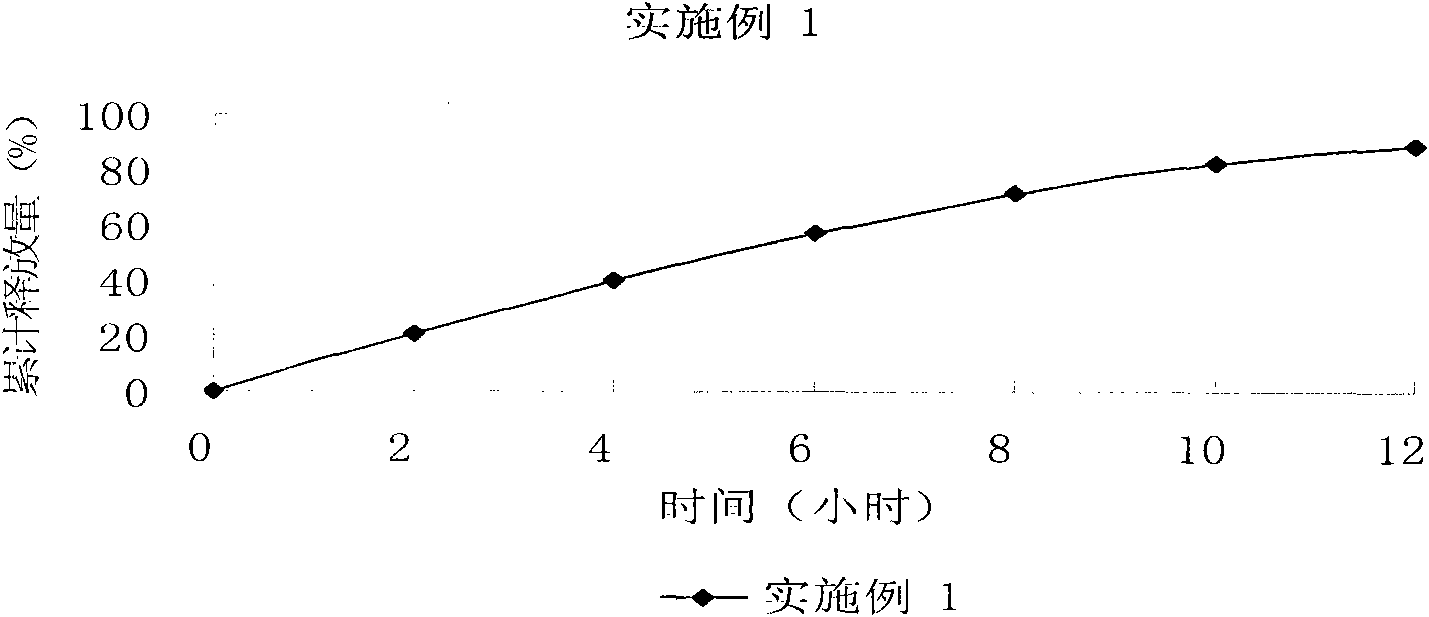
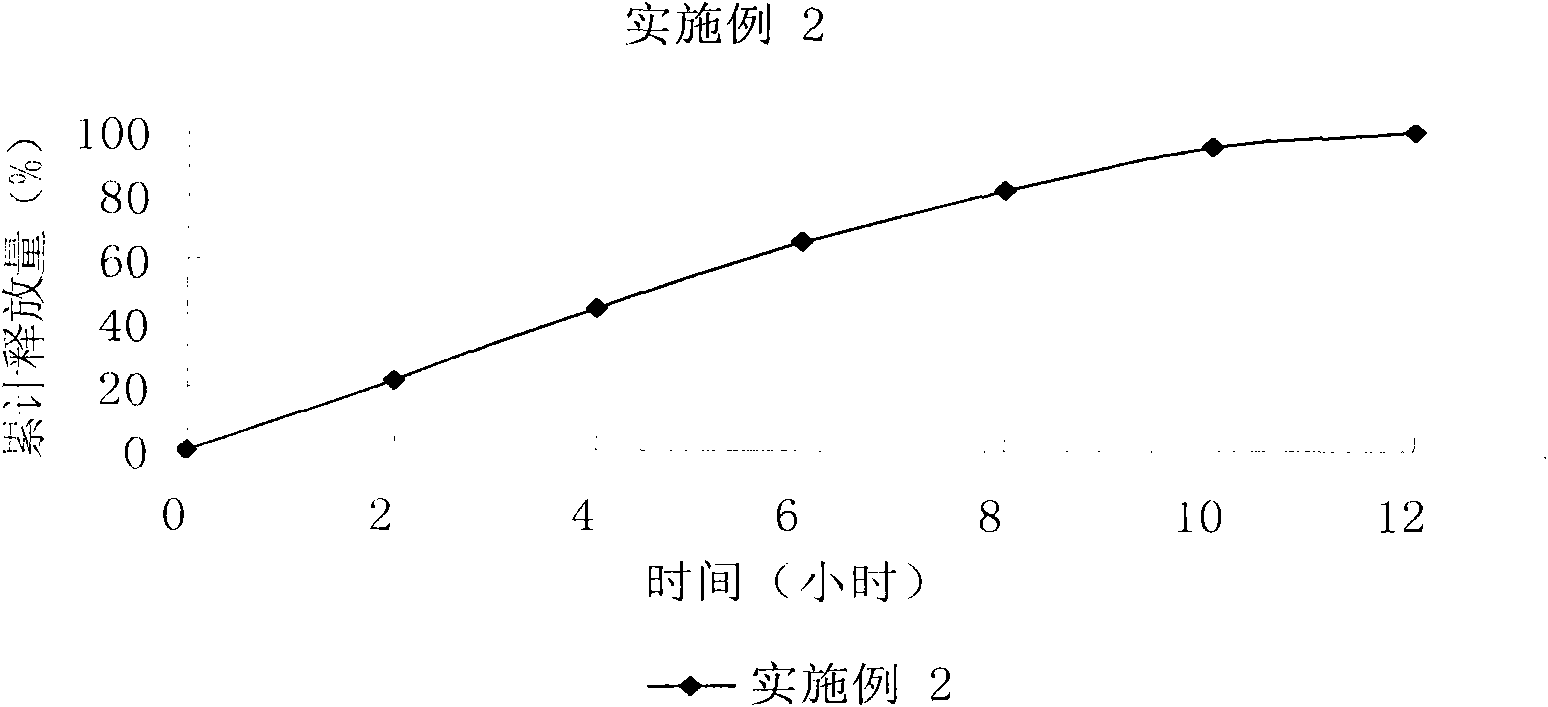
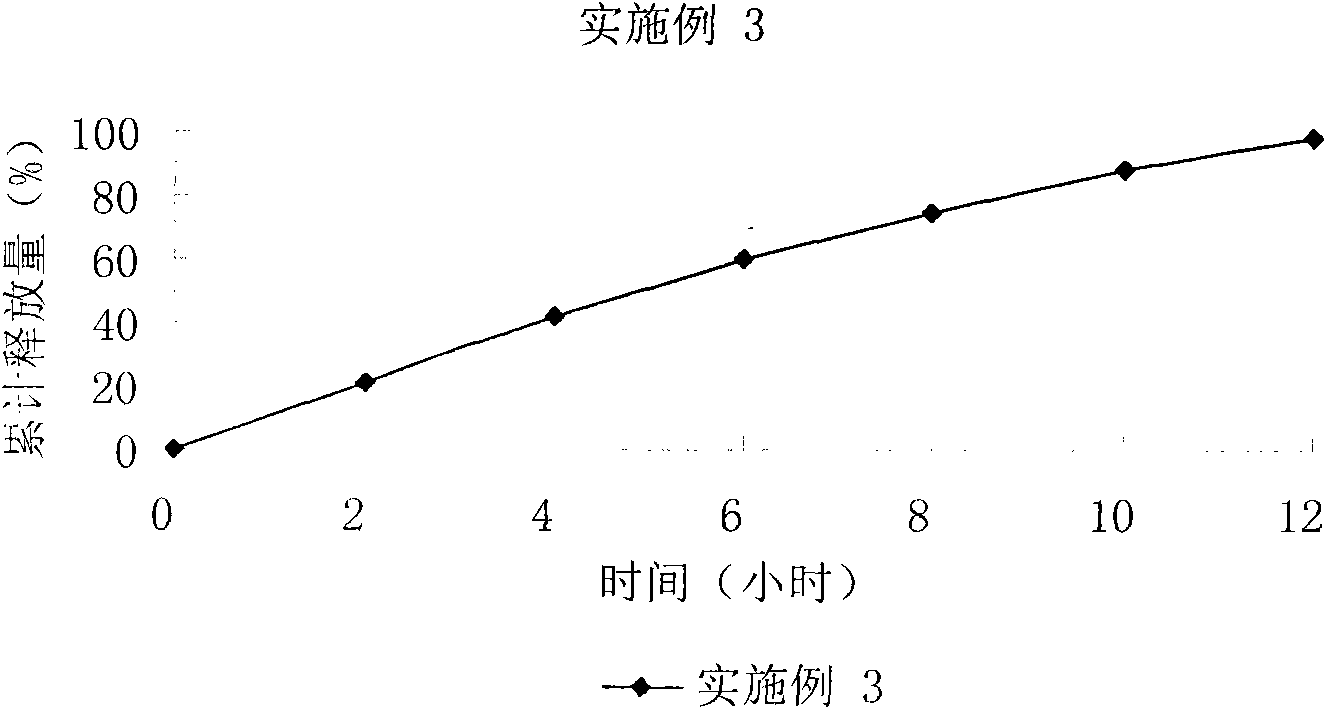
The invention relates to a controlled release preparation of bezafibrate. The controlled release preparation of bezafibrate contains a bezafibrate core and a semi-permeability film coating. The outer layer of the semi-permeability film coating is provided with a pore. The semi-permeability film coating is made of following components by mass proportion: 60 to 80 percent of polymer materials of the semi-permeability film coating and 20 to 40 percent of plasticizer. The polymer materials of the semi-permeability film coating are made by the mixture of one or more than one of cellulose acetate, ethyl cellulose, cellulose propionate, polycarbonate, polythene and PVA. The controlled release preparation of bezafibrate overcomes the 'peak valley' phenomenon of blood concentration existing in a common preparation and only needs to be taken once per day with the little adverse effect of a gastrointestinal tract, so a curative effect is ensured and patient compliance is also improved. The invention also relates to a preparation method of the controlled release preparation of bezafibrate.
Owner:TIANJIN TASLY LIAONING PHARMA
Bezafibrate sustained release tablet and preparation method thereof
ActiveCN102462675AAdjust the rate of constant releaseImprove complianceOrganic active ingredientsMetabolism disorderSustained Release TabletDisease
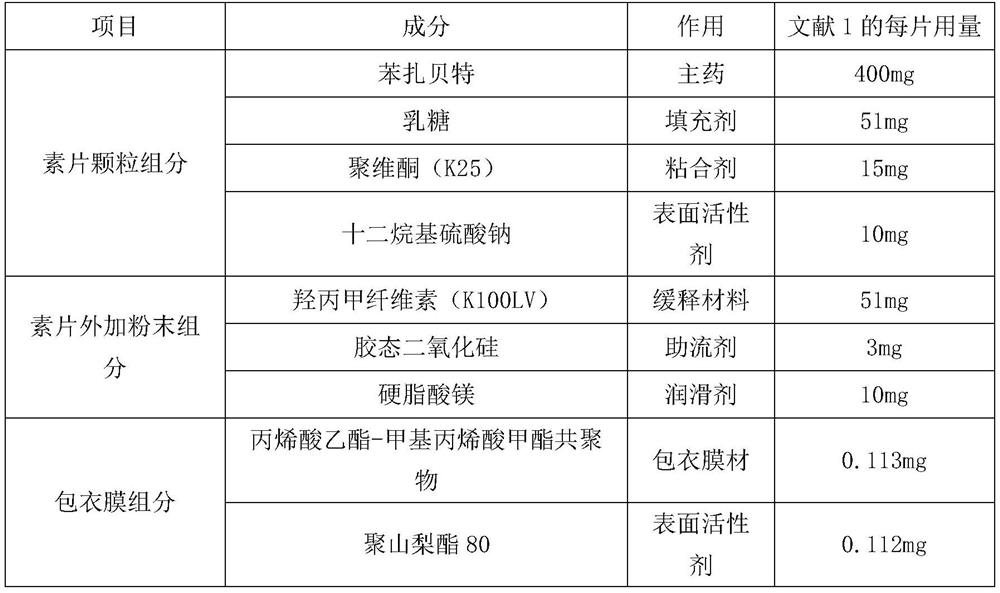
The invention provides a bezafibrate sustained release tablet, comprising a sustained release tablet core and a film coated on the tablet core. The bezafibrate sustained release tablet comprises, by weight, 400 parts of bezafibrate, 60-120 parts of skeletal material and 50-150 parts of accessory facilitating release of drug; and the coating film accounts for 1-5wt% of the tablet. The bezafibrate sustained release tablet of the invention can effectively adjust release rate of the medicament and obtain stationary and lasting effective plasma concentration, so as to reduce side-effect and drug administration frequency; and absorption of medicament is not influenced by food intake and internal environment, so as to increase compliance of a patient. Therefore, the bezafibrate sustained release tablet can be widely applied to prevention and treatment of disease like hyperlipidemia.
Owner:BEIJING YILING BIOENG
Dispersant tablet containing hypolipidemic component and its prepn process
The present invention relates to orally taken medicine preparation containing benzafibrate, and is especially dispersant tablet of benzafibrate with enhanced effect. The dispersant tablet of benzafibrate has fast acting, enhanced active component and easy taking.
Owner:江苏万高药业股份有限公司
Bezafibrate sustained-release composition
InactiveCN101120931AReduce releaseSmall fluctuations in blood concentrationPeptide/protein ingredientsMetabolism disorderSustained release pelletsSide effect
A slow-release compound of benzafibrate is a slow-release capsule, the main part of which are the slow-release pellets. The slow-release pellets comprise the benzafibrate served as the active component and the slow-release layer with slow-release property covering outer layer of the medical pellet. The other excipient comprises the excipient material with pharmaceutical approval after mixing with benzafibrate and the slow-release material with slow-release property. The ratio among the benzafibrate, the excipient material and the slow-release material (weight ratio) is 1 to 0.01 till 1 to 0.01 till 1. The compound can prolong residence time of the medicine in the stomach. The compound is also capable of controlling the drug release, reducing the administration times, reducing toxic and side effects and improving the treatment effect.
Owner:珠海天翼医药技术开发有限公司
Pharmaceutical composition of bezafibrate and application thereof to rheumatoid arthritis
InactiveCN106109461ANovel structureHas the effect of treating rheumatoid arthritisOrganic active ingredientsOrganic chemistryNatural productBezafibrate
The invention discloses a pharmaceutical composition of bezafibrate and application thereof to rheumatoid arthritis. The pharmaceutical composition of bezafibrate provided by the invention contains bezafibrate and a natural compound (I) with novel structure and isolated from the dried clove buds; when bezafibrate and the compound (I) are combined for action, the synovial cells arrest in a G1 phase of a cell cycle, thereby inhibiting the proliferation of synovial cells for the treatment of rheumatoid arthritis. The treatment effect of the pharmaceutical composition is better than the individual effect of bezafibrate or compound (I). The composition of bezafibrate and compound (I) can be developed into drugs for the treatment of rheumatoid arthritis. Compared with the prior art, the invention has prominent substantive features and obvious progress.
Owner:赵吉永
Adulteration detection method to fibrates lipid-lowering chemicals of tea by high-performance thin-layer chromatograph(HPTLC)-bioluminescent method
The invention discloses an adulteration detection method to fibrates lipid-lowering chemicals of tea by the high-performance thin-layer chromatograph(HPTLC)-bioluminescent method and belongs to the technical field of food detection. The adulteration detection method includes, firstly, preparing standard product solution of bezafibrate and ciprofibrate and a tea sample; secondly, pre-washing thin-layer plates and performing HPTLC sample application; thirdly, performing HPTLC separation to move target objects mixed originally to different positions of the thin-layer plates according to difference of molecular structures so as to form physical isolation; finally, simultaneously detecting multiple targets of the samples conveniently through luminous strains coupled with the thin-layer plate byan impregnation method. The adulteration detection method refers to a method capable of detecting lipid-lowering chemicals of tea rapidly quantitatively by the HPTCL-bioluminescent method, and has the advantages of being economic, rapid, simple and convenient.
Owner:JIANGNAN UNIV
Modulating inflammation with cytochrome P-450 activators and inhibitors
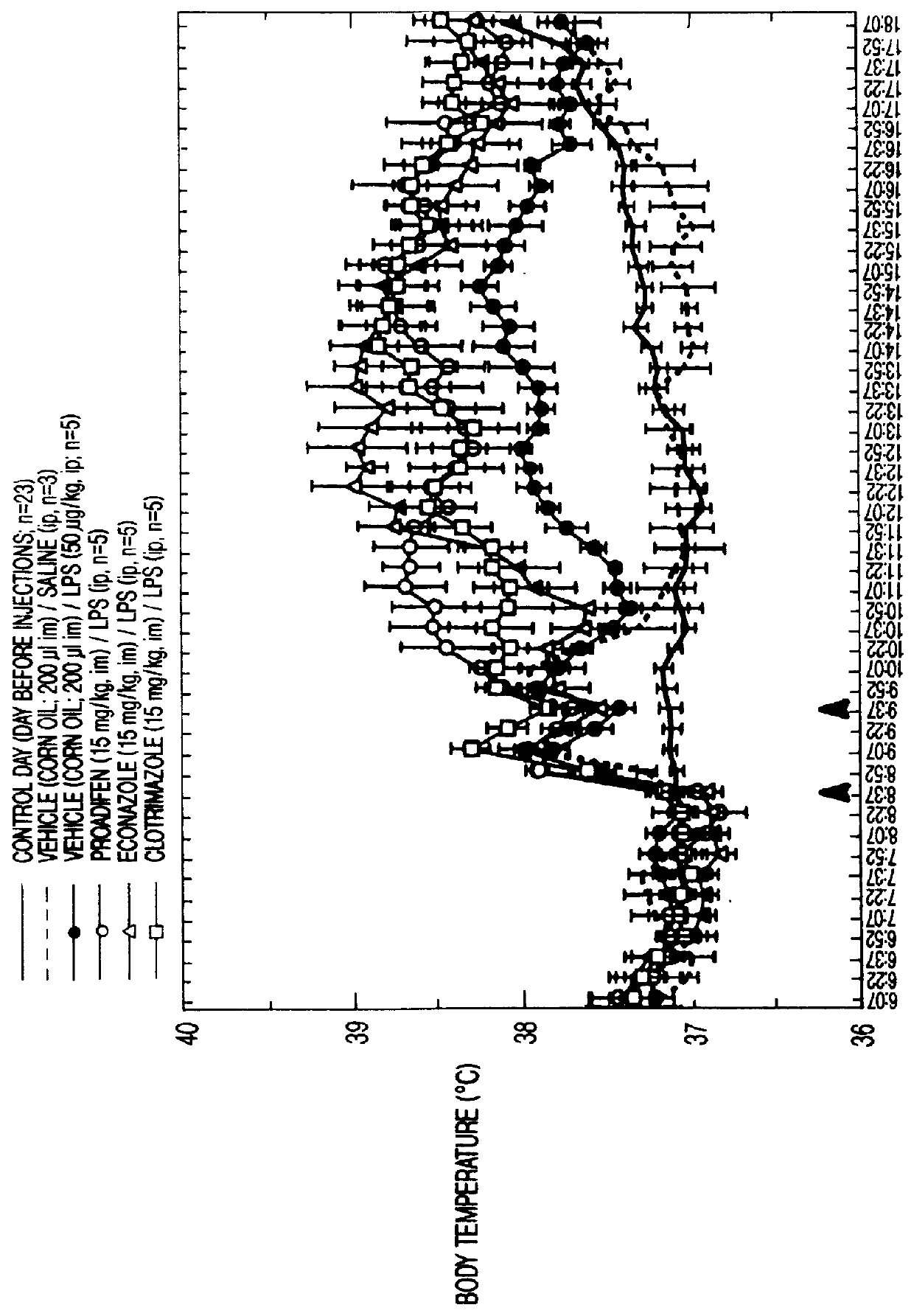
This invention relates to methods of modulating inflammation in mammals. Inflammation is modulated by regulating the cytochrome P-450 pathway. Inflammation is reduced by treating the subject with substances, such as bezafibrate and clofibrate, which induce the P-450 pathway. Inflammation is promoted by treating the subject with substances, such as proadifen, econazole, and clotrimazole, which inhibit the cytochrome P-450 pathway.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Dissolution determination method of hawthorn extract lipid-lowering dispersion tablet
ActiveCN101008610AImprove solubilityStrong specificityColor/spectral properties measurementsTesting medicinal preparationsSolubilityTriterpene
This invention relates to haw bezafibrate discrete slice fuse concentration test method, which considers chromocor compound in ultraviolet area, wherein, the slices bezafibrate compound is of large volume and the alcohol isopropylicum-water the main component is as triterpene acids and chromocor compound of good solution property to establish the fuse concentration method by lighting ultraviolet method.
Owner:宁夏启元国药有限公司
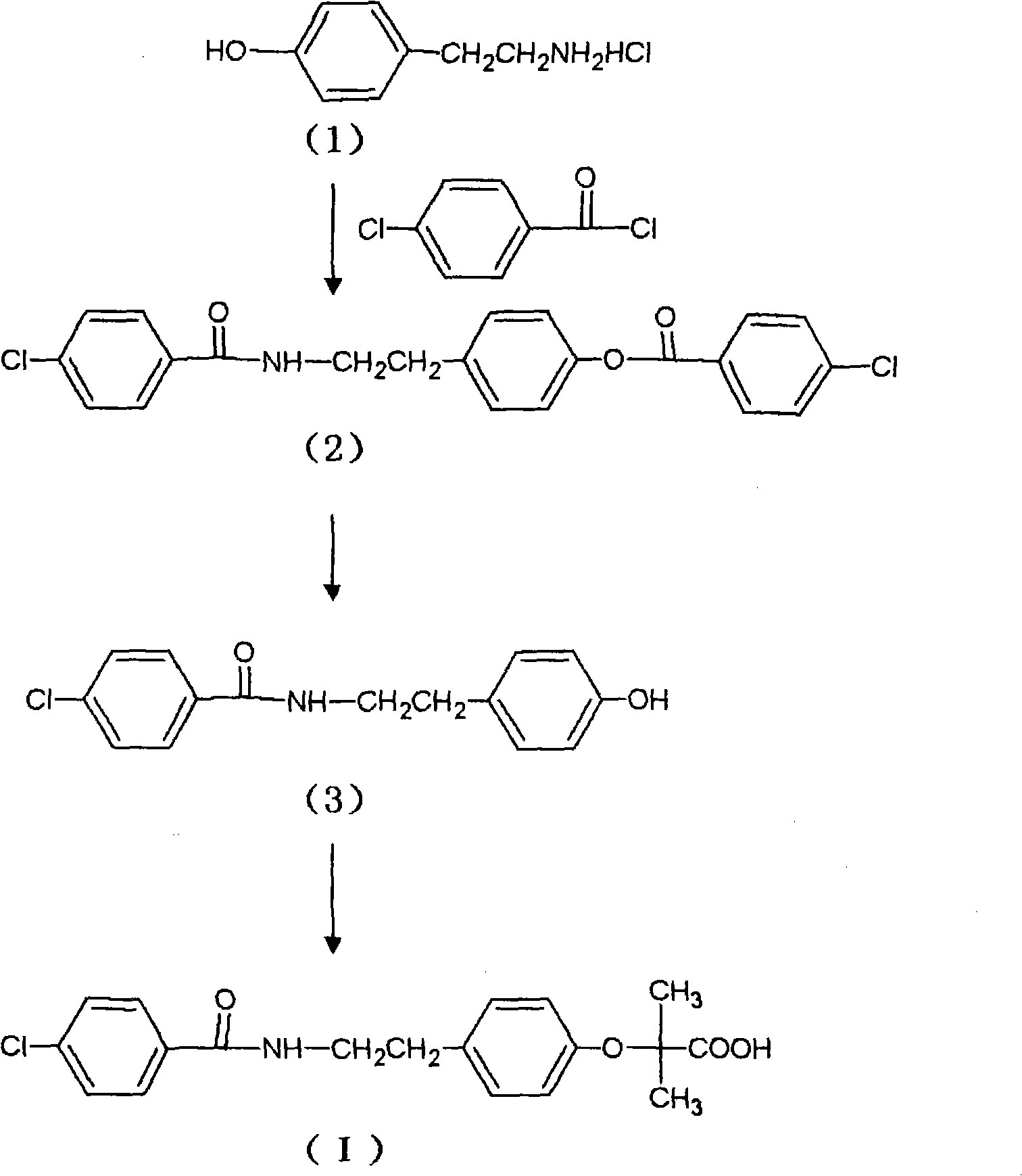
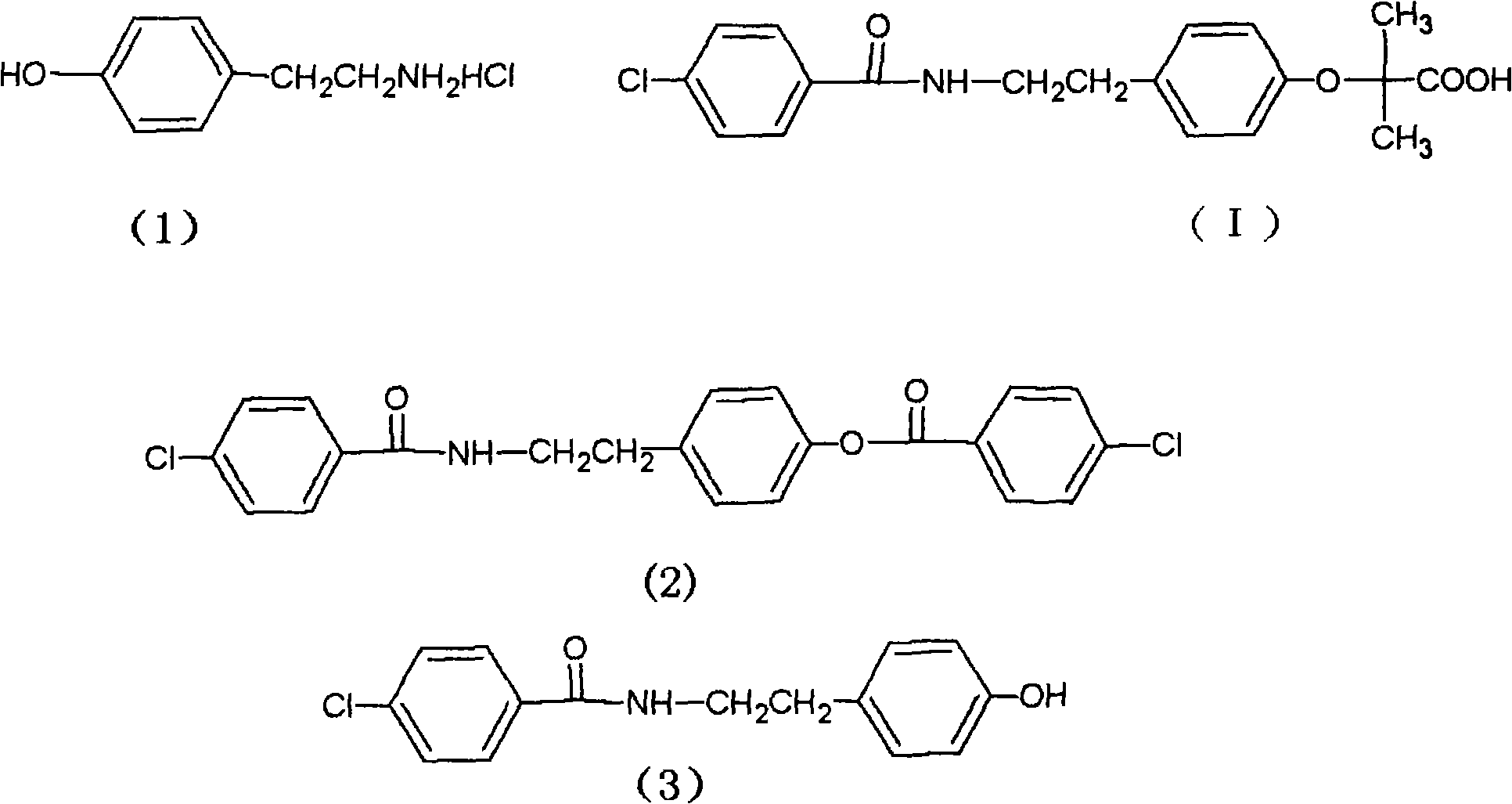
Preparation of bezafibrate
InactiveCN101353315AAvoid distillationShort reaction cycleOrganic compound preparationCarboxylic acid amides preparationDistillationOperational safety
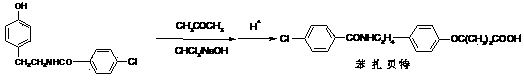
The invention discloses a preparation method of bezafibrate. The preparation method is characterized in that N-p-hydroxybenzylethyl-4-chlorobenzamide, sodium hydroxide aqueous solution, acetone and a phase transfer catalyst are added to an organic solvent, chloroform is added dropwise to react, after the reaction is completed, water is added to the system to extract and stratify, the water layer is acidified, and the bezafibrate is obtained. In the preparation method, an organic solvent incompatible with water is added in the reaction, and the reaction is carried out in water condition, thus avoiding solvent distillation, rectification dehydration and the crushing of solid sodium hydroxide; and the preparation method shortens production cycle, reduces operation cost and improves operational safety, thus being favorable for industrialized production.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
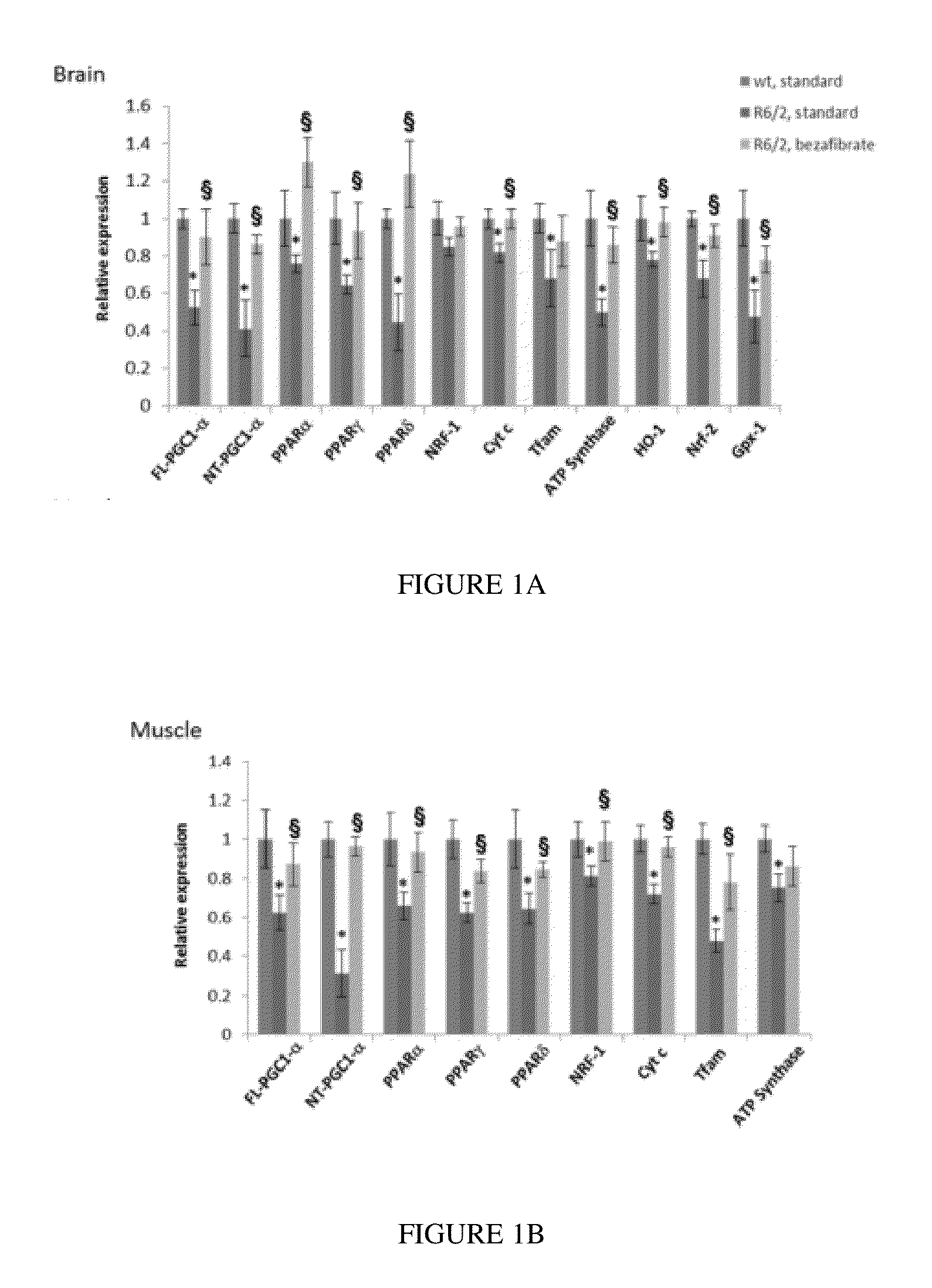
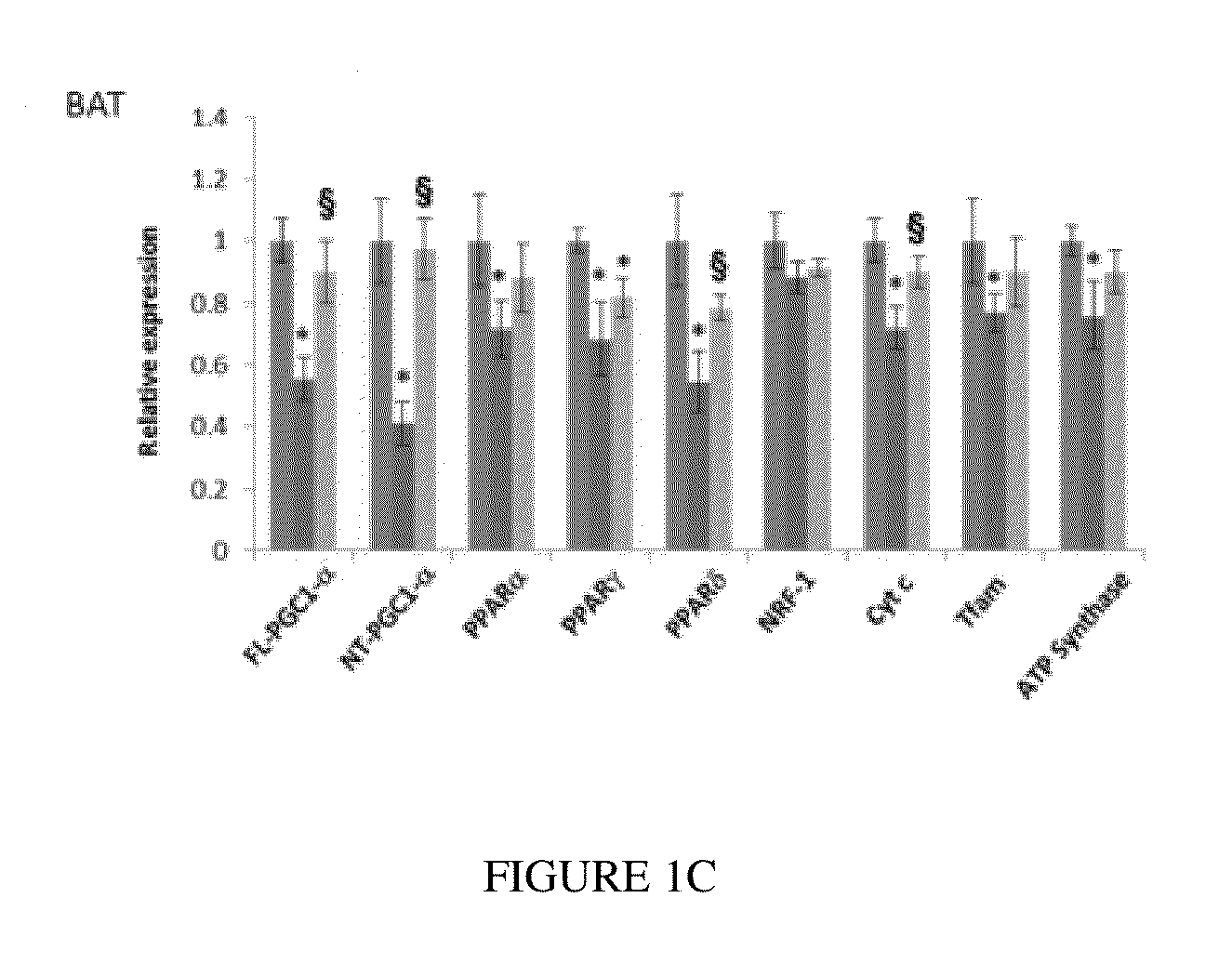
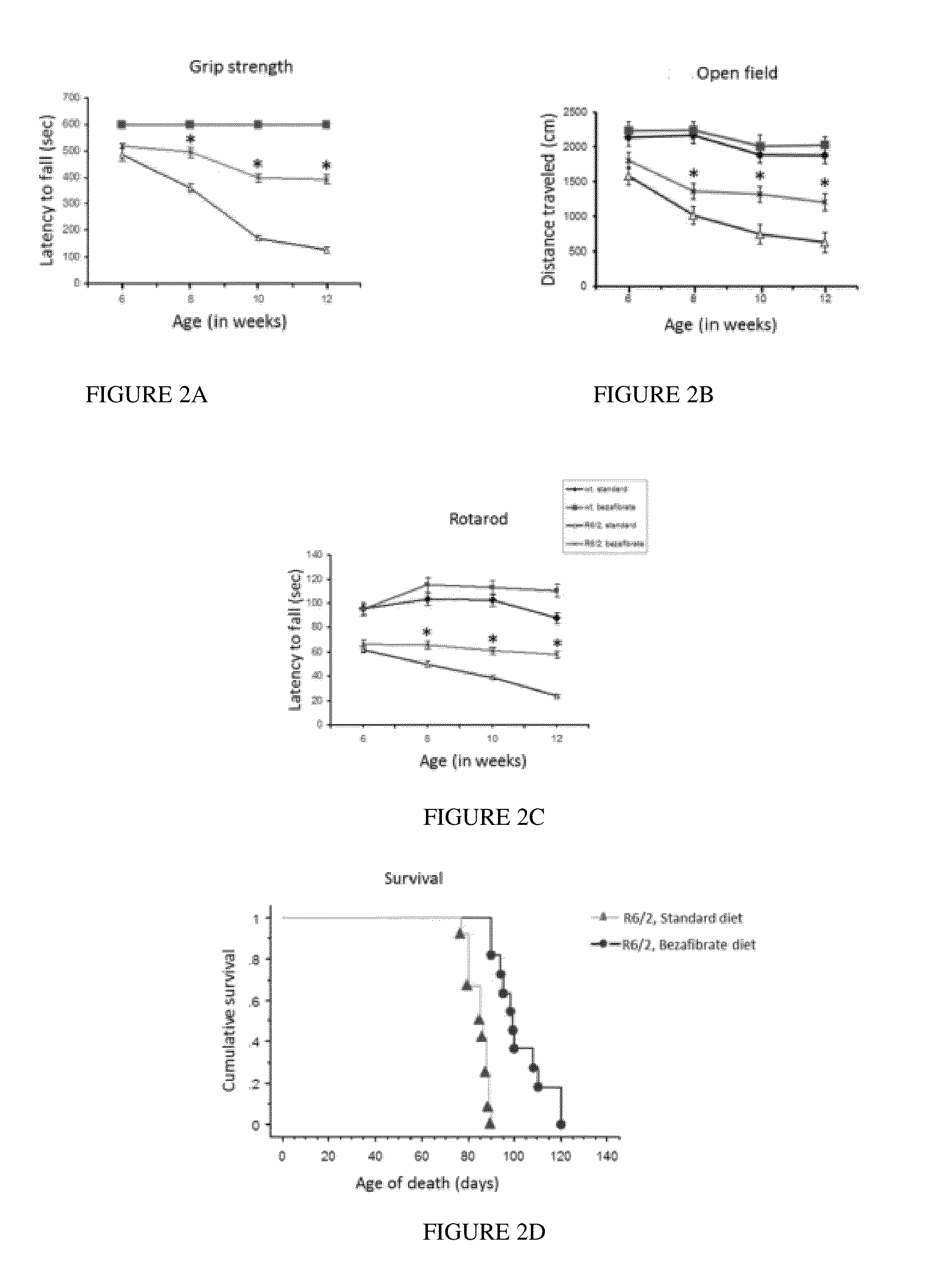
Use of Pan-PPAR Agonists for Prevention and Treatment of Huntington's Disease and Tauopathies
ActiveUS20140350107A1Improve pathologyImprove survivalOrganic active ingredientsBiocideHuntingtons choreaBezafibrate
The present invention provides a therapeutic treatment for a neurodegenerative disease with a pan-PPAR agonist, such as bezafibrate. In particular, the present invention provides that pan-PPAR agonists enhance PPAR related responses in both the central nervous system and peripheral tissues in Huntington's Disease (HD) and tauopathy. Therapeutic compositions comprising one or more pan-PPAR agonist(s), and kit thereof, for treating a neurodegenerative disease or disorder are also provided.
Owner:CORNELL UNIVERSITY
Electrocatalytic particle electrode for efficiently degrading bezafibrate in wastewater and preparation method thereof
The invention relates to an electrocatalytic particle electrode for efficiently degrading bezafibrate in wastewater and a preparation method thereof, belonging to the technical field of wastewater treatment. The preparation method comprises the following steps: uniformly mixing and stirring treated nonferrous metal slag, clay, a pore forming agent and an activator used as raw materials according to a certain weight ratio to prepare a mixed crude material, impregnating the mixed crude material in a carrier sol, drying, cooling to room temperature, extruding to obtain fresh pellets, drying, heating at specific temperature for activation and roasting for some time, taking out, and cooling to room temperature to obtain the finished product. The electrocatalytic particle electrode for efficiently degrading bezafibrate is porous, has large specific area, high adsorbability and favorable electrocatalytic property, and can degrade bezafibrate efficiently. The electrocatalytic particle electrode and preparation method thereof fully utilize the nonferrous metal slag, thereby changing the waste into valuable substances, reducing the environmental pollution and solving the problems of land occupation and the like.
Owner:UNIV OF JINAN
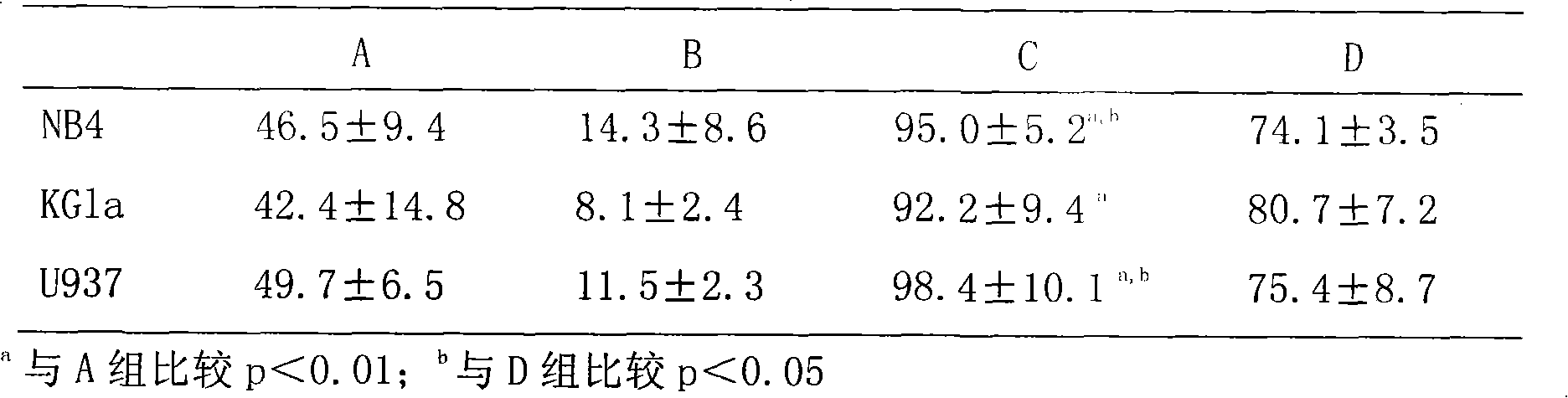
Medicine compound against acute myeloid leukemia
InactiveCN101856359AGood curative effectReduce healingHydroxy compound active ingredientsPharmaceutical non-active ingredientsBezafibrateMyeloid leukemia
The invention discloses a medicine compound against acute myeloid leukemia; each unit dose of the medicine compound contains 2mg of all-trans-retinoic acid, 1 to 2mg of megestrol acetate, 100 to 200mg of bezafibrate and a carrier acceptable of medical science, and the mass ratio of megestrol acetate to bezafibrate is 1:100. The medicine compound can significantly improve the survival rate of patients with acute myeloid leukemia, and can open up a new way for clinical treatment.
Owner:SHANDONG UNIV
Bezafibrate dual-release slow-release capsule medicinal composition
The invention relates to a bezafibrate dual-release slow-release medicinal composition. The above dual-release capsule includes an immediate-release bezafibrate pellet and a slow-release bezafibrate pellet. The bezafibrate dual-release slow-release medicinal composition fills a gap in the market in China, and is convenient for clinical application.
Owner:天津梅花生物医药科技有限公司
Method for extracting high-efficiency bezafibrate degrading bacteria
InactiveCN101792724AProvenance is easy to getThe separation and purification method is simple and fastBacteriaMicroorganism based processesActivated sludgePurification methods
The invention relates to a method for extracting high-efficiency bezafibrate degrading bacteria, which comprises the following steps: (1) inoculating activated sludge from a secondary sedimentation tank of an urban sewage treatment plant to an inorganic salt culture medium in an inoculation amount of 5 weight percent and domesticating for 2 months in a gradient pressure domestication way; and (2) standing a sample domesticated for 2 months for 30 minutes, taking 1mL of supernatant water sample for gradient dilution, inoculating 1mL of bacterial solution with each dilution concentration respectively to a solid culture medium for enrichment culture to ensure that a pure single strain is grown, and inoculating the pure single strain to a slant culture medium and storing at the temperature of 4 DEG C. In the method, the bezafibrate degrading bacterial strains are extracted from the activated sludge in the secondary sedimentation tank of the urban sewage treatment plant, so the bezafibrate degrading bacterial strains have the advantages of readily available source, simple and convenient separation and purification method, low cost and environmental protection; and the extracted bacterial strains have a bezafibrate degrading rate of over 85 percent and have good application prospect.
Owner:DONGHUA UNIV
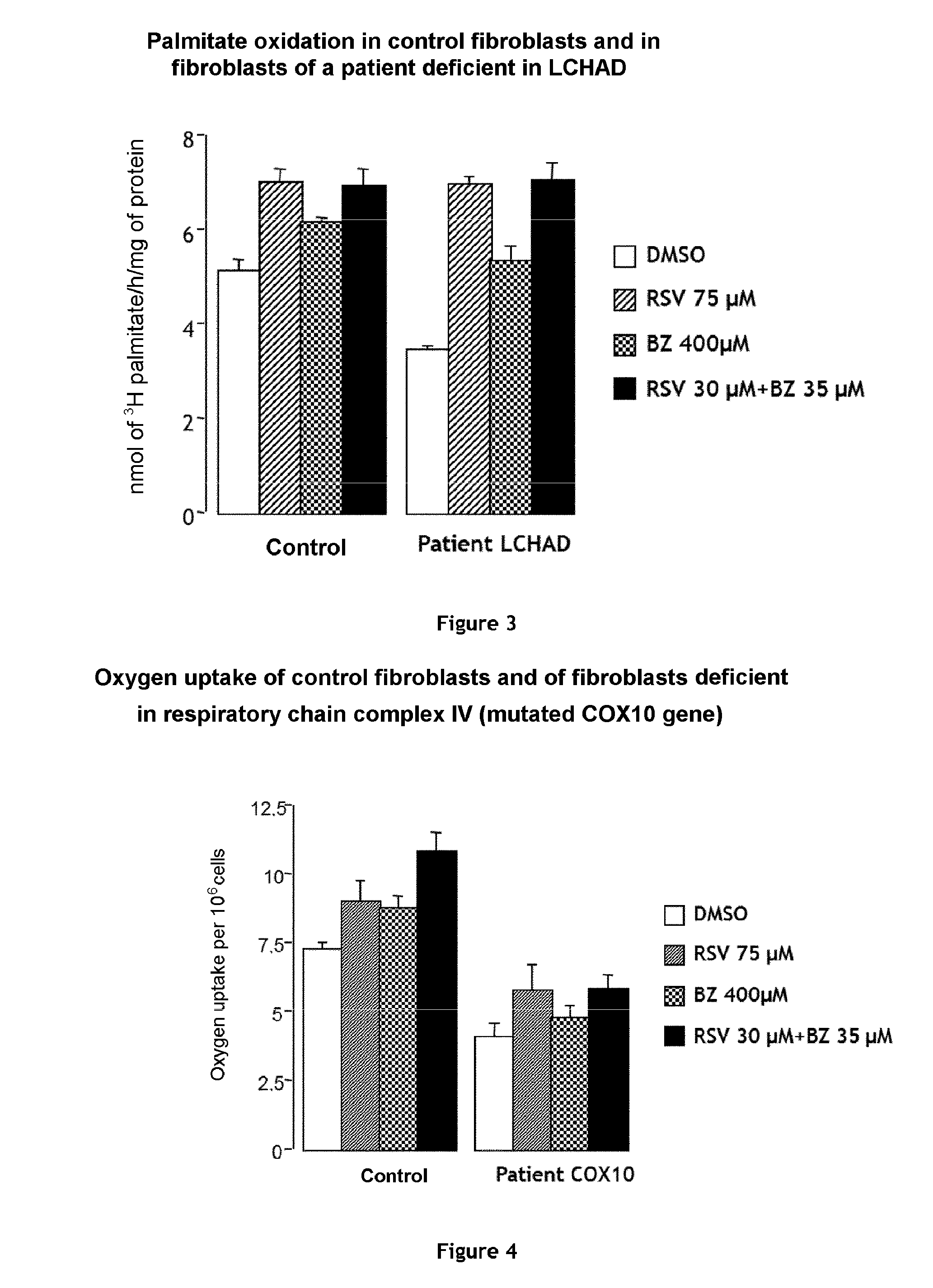
Combination of bezafibrate and of resveratrol or resveratrol derivatives for the treatment and prevention of diseases involving a mitochondrial energy dysfunction
ActiveUS20160317483A1Function increaseBetter correction of deficiencies of β-oxidationNervous disorderHydroxy compound active ingredientsDiseaseBezafibrate
The present invention relates to the combined use of bezafibrate and of resveratrol or resveratrol derivatives for the treatment of diseases involving a mitochondrial energy dysfunction, and also to a pharmaceutical kit comprising both bezafibrate and resveratrol or resveratrol derivatives. The combination is more particularly used in the treatment of moderate defects of β-oxidation of long-chain fatty acids or of the respiratory chain of mitochondria.
Owner:UNIV DE PARIS +1
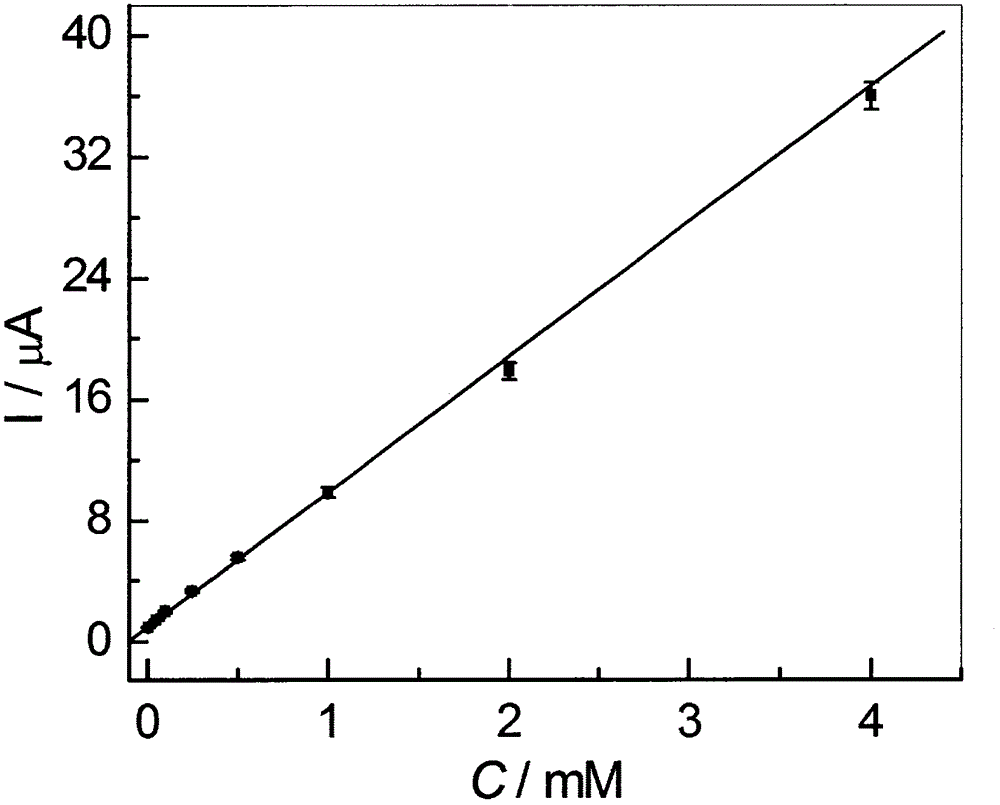
Method for preparing high-sensitivity bezafibrate molecular imprinting electrochemical sensor
InactiveCN106770557AHigh sensitivityEasy to manufactureMaterial electrochemical variablesFunctional monomerCross linker
The invention discloses a method for preparing a high-sensitivity bezafibrate molecular imprinting electrochemical sensor. According to the method, the high-sensitivity bezafibrate molecular imprinting electrochemical sensor is prepared from bezafibrate as template molecules, ceramide as a functional monomer, azodiisobutyronitrile as an initiator, a copper-zinc double-metal organic frame material as a doping agent and acetylshikonin as a cross-linking agent. The analysis method is simple and practical, and defects that a conventional analysis method is complex, expensive in equipment and low in sensitivity can be overcome.
Owner:GUANGXI UNIV FOR NATITIES
Bezafibrate sustained release preparation and its preparation method
The invention relates to a bezafibrate release preparation, which comprises a tablet core and a coating layer, wherein the tablet core comprises the following ingredients by weight percentage.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
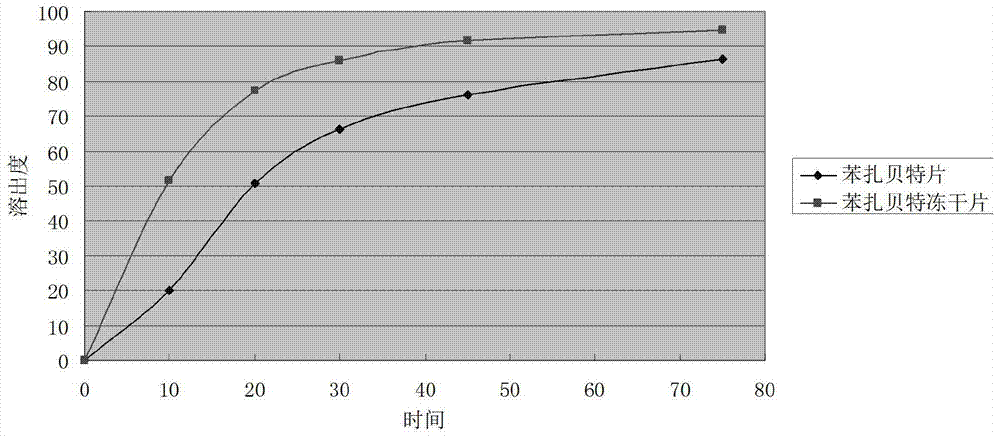
Bezafibrate composition freeze-dried tablet and preparation method thereof
InactiveCN104490830AReduce typesReduce dosageOrganic active ingredientsMetabolism disorderSucroseFreeze-drying
The invention provides a bezafibrate composition freeze-dried tablet and a preparation method thereof, and relates to the technical fields of medicines and medicine production. The bezafibrate composition freeze-dried tablet comprises bezafibrate, starch and sucrose; the starch and the sucrose are taken as auxiliary materials; and heating process treatment is carried out on ordinary corn starch, so that the bonding and disintegrating effects of the starch in the tablet can be improved; and the moldability of the tablet is improved. The bezafibrate composition freeze-dried tablet only requires two auxiliary materials namely the starch and the sucrose, and adopts a two-fall and two-lift freeze-drying process, so that the moldability of the tablet can be improved by cooling twice and heating twice; and the dissolution rate of the tablet is improved, thus the bioavailability of the tablet is improved; the defects of an ordinary bezafibrate tablet are overcome; the variety and the amount of auxiliary materials in the bezafibrate tablet are reduced; and the tablet is high in dissolution rate, and high in bioavailability; and the curative effect and the safety of clinical medication are ensured.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
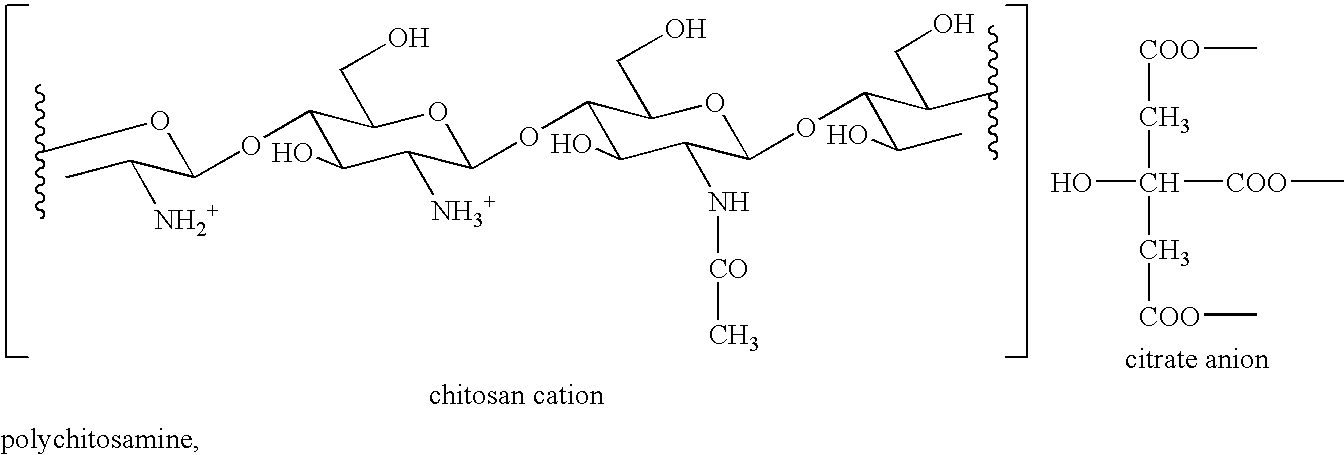
Combination of Polychitosamine and Fibrate for the Prevention and Treatment of Hyperlipidemia
InactiveUS20080293671A1Lowering blood cholesterolEliminate side effectsOrganic active ingredientsBiocideBezafibrateSecondary hyperlipidemia
The present invention relates to pharmaceutical compositions comprising a fibrate (e.g. ciprofibrate, gemfibrozil, benzafibrate, and fenofibrate) and a polychitosamine (e.g. chitosan) having molecular weights of less than 650 Kda and degrees of deacetylation of from 70% to 100%. Uses of said compositions for the treatment of hyperlipidemia and associated conditions (e.g. hypercholesterolemia, atherosclerosis, coronary heart disease and cardiovascular disease) or as functional foods are also disclosed.
Owner:DNP CANADA +1
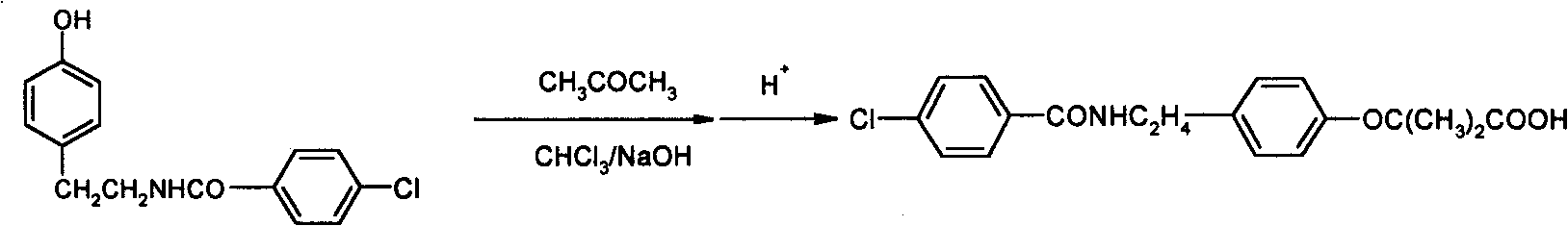
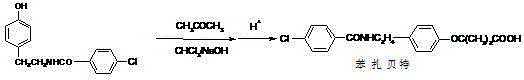
Synthesis method of bezafibrate for regulating blood fat
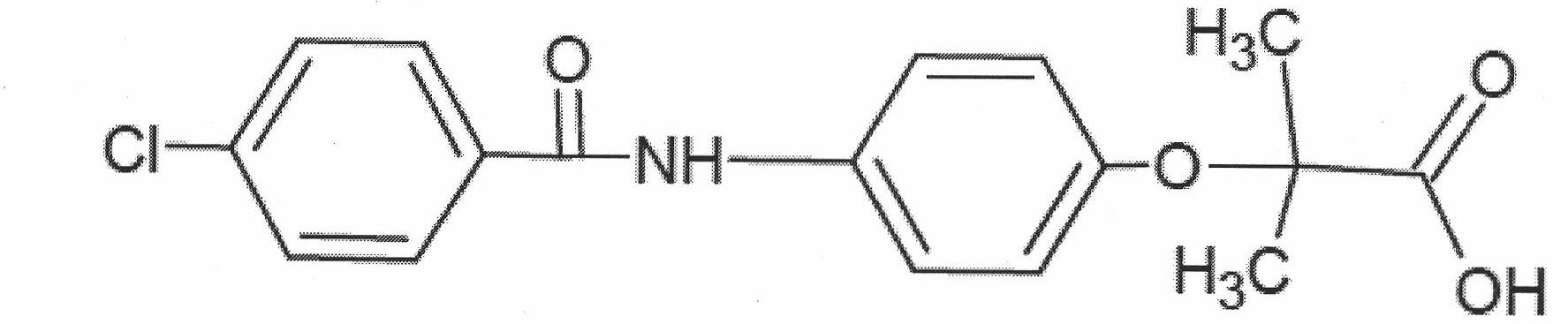
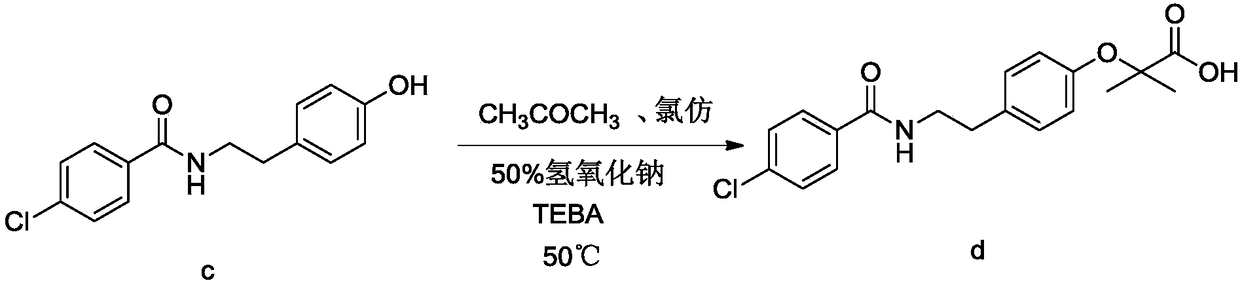
InactiveCN102030676AReduce difficultyIncrease optionalityOrganic compound preparationCarboxylic acid amides preparationBezafibrateSynthesis methods
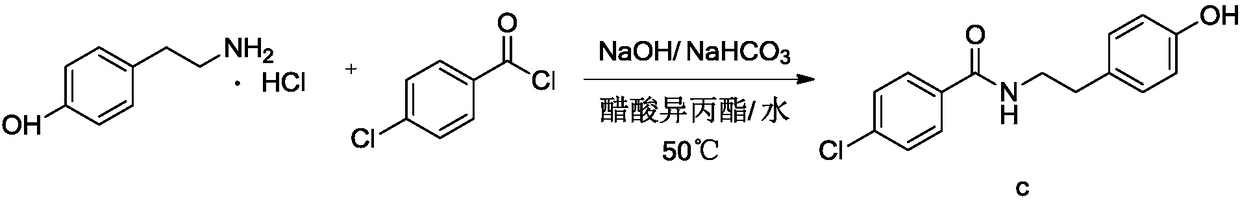
The invention relates to the field of medicinal chemistry and discloses a synthesis method of bezafibrate for regulating blood fat. In the synthesis method, stabilized p-hydroxybenzene ethylamine hydrochloride and p-chlorobenzene formyl halide are used as initial raw materials to be substituted, hydrolyzed and condensed to obtain a target compound. The synthesis method has the advantages of low cost, mild reaction conditions, controllable processes and stable quality and is beneficial to industrialized production, the raw materials can be easily obtained and are relatively stabilized, the purity reaches 99.5% by the detection of the high performance liquid chromatography (HPLC), and the total yield is 48.1%.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Preparation of bezafibrate
InactiveCN101353315BAvoid distillationShort reaction cycleOrganic compound preparationCarboxylic acid amides preparationDistillationOperational safety
The invention discloses a preparation method of bezafibrate. The preparation method is characterized in that N-p-hydroxybenzylethyl-4-chlorobenzamide, sodium hydroxide aqueous solution, acetone and a phase transfer catalyst are added to an organic solvent, chloroform is added dropwise to react, after the reaction is completed, water is added to the system to extract and stratify, the water layer is acidified, and the bezafibrate is obtained. In the preparation method, an organic solvent incompatible with water is added in the reaction, and the reaction is carried out in water condition, thus avoiding solvent distillation, rectification dehydration and the crushing of solid sodium hydroxide; and the preparation method shortens production cycle, reduces operation cost and improves operational safety, thus being favorable for industrialized production.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Method for recovering bezafibrate from bezafibrate synthesis waste liquid
InactiveCN107892659AEfficient recyclingHigh purityOrganic compound preparationCarboxylic acid amide separation/purificationBenzeneFlocculation
The invention discloses a method for recovering bezafibrate from bezafibrate synthetic waste liquid, wherein the synthetic waste liquid refers to N-p-hydroxyphenethyl-4-chlorobenzamide, acetone and chloroform in alkaline The mother liquor after condensation and precipitation of crude bezafibrate under conditions, and the remaining acetone in the mother liquor has been recovered, including dilution with water, flocculation adsorption and acidification crystallization; wherein, flocculation adsorption is carried out under weakly acidic conditions, flocculation adsorption material At least chitosan is included; preferably, chitosan / attapulgite complex is also included. In short, the method provided by the present invention not only uses a low-cost green material for separation, but also has a simple and easy operation method, which is suitable for large-scale industrial production, and is a green and low-cost method, which can effectively remove bezabe The high-purity crude bezafibrate is recovered from the specially synthesized waste liquid, thereby reducing the production cost.
Owner:JIANGSU HEALTH VOCATIONAL COLLEGE
Purification method of bezafibrate in preparation process
InactiveCN103804220BHigh product contentAvoid lostCarboxylic acid amide separation/purificationBezafibrateAfter treatment
The invention discloses a purification method of bezafibrate in a preparation process. The purification method comprises the steps of evaporating and recycling solvent acetone after a synthesis reaction of the bezafibrate is finished, adding water to residues until the residues are clarified, adding hydrochloric acid to regulate the pH to be 11, introducing carbon dioxide into the water solution until no precipitate is generated, filtering, acidizing filter liquid until the pH of the filter liquid is 3-4, separating a product out, and filtering and washing to obtain a bezafibrate product. According to the purification method, unreacted raw materials are separated in an after-treatment stage of the synthesis reaction by virtue of acidity difference between the raw materials and the product, so that the content of the product is effectively increased, and the loss of the product caused by recrystallization is avoided.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Preparation process of bezafibrate compound
InactiveCN108569979AHigh purityOvercome the problem of improving yieldOrganic active ingredientsOrganic compound preparationBezafibrateHalogen
The invention especially relates to preparation technology for a bezafibrate compound, belonging to the field of pharmaceutical synthesis. The invention provides a preparation process of the bezafibrate compound. The preparation process comprises the following steps: subjecting a compound with a structure as shown in a formula a or a' and a compound with a structure as shown in a formula b to an acylation reaction in an aqueous solution of inorganic base B so as to obtain a bezafibrate intermediate with a structure as shown in a formula c; and then subjecting the bezafibrate intermediate, R1COR2 and methyl halide to a condensation reaction in an aqueous solution of inorganic alkali A to obtain the bezafibrate compound with a structure as shown in a formula d, wherein process flow is as described in the specification, M is a water-soluble inorganic acid, X is a halogen or alkoxy group, and R1 and R2 are the same or different and are selected from a group consisting of H and C1-C3 alkylgroups.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Medicine compound against acute myeloid leukemia
InactiveCN101856359BGood curative effectReduce healingHydroxy compound active ingredientsPharmaceutical non-active ingredientsBezafibrateMyeloid leukemia
The invention discloses a medicine compound against acute myeloid leukemia; each unit dose of the medicine compound contains 2mg of all-trans-retinoic acid, 1 to 2mg of megestrol acetate, 100 to 200mg of bezafibrate and a carrier acceptable of medical science, and the mass ratio of megestrol acetate to bezafibrate is 1:100. The medicine compound can significantly improve the survival rate of patients with acute myeloid leukemia, and can open up a new way for clinical treatment.
Owner:SHANDONG UNIV
Application of fibrate drugs used as drugs for inhibiting myopia
InactiveCN102961749AReduce myopiaCurb myopiaOrganic active ingredientsSenses disorderBezafibrateClofibrate
The invention relates to a method for preparing drugs capable of delaying and inhibiting myopia. The fibrate lipid-lowering drugs, such as Clofibrate, Fenofibrate and Bezafibrate, are used as the myopia drugs for treating myopia by inhibiting the formation of the myopia.
Owner:WENZHOU MEDICAL UNIV
Bezafibrate sustained-release tablet and preparation method thereof
PendingCN113559077AImprove hydrophilicityFine grainOrganic active ingredientsMetabolism disorderBezafibrateProlonged-release tablet
The invention relates to a bezafibrate sustained-release tablet and a preparation method thereof. The bezafibrate sustained-release tablet comprises a tablet core and a coating layer, wherein the tablet core is prepared from the following raw materials in parts by mass: 400 parts of bezafibrate, 25-38 parts of lactose, 28-32 parts of povidone, 10-20 parts of sodium lauryl sulfate, 30-59 parts of hydroxypropyl methylcellulose, 1-5 parts of colloidal silicon dioxide and 8-12 parts of magnesium stearate. According to the preparation prescription provided by the invention, the prepared bezafibrate sustained-release tablet is uniform in weight and balanced in dissolution, and the problem that the dissolution is fast before and slow after is solved.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Purification method of bezafibrate in preparation process
InactiveCN103804220AHigh product contentAvoid lostCarboxylic acid amide separation/purificationBezafibrateAfter treatment
The invention discloses a purification method of bezafibrate in a preparation process. The purification method comprises the steps of evaporating and recycling solvent acetone after a synthesis reaction of the bezafibrate is finished, adding water to residues until the residues are clarified, adding hydrochloric acid to regulate the pH to be 11, introducing carbon dioxide into the water solution until no precipitate is generated, filtering, acidizing filter liquid until the pH of the filter liquid is 3-4, separating a product out, and filtering and washing to obtain a bezafibrate product. According to the purification method, unreacted raw materials are separated in an after-treatment stage of the synthesis reaction by virtue of acidity difference between the raw materials and the product, so that the content of the product is effectively increased, and the loss of the product caused by recrystallization is avoided.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
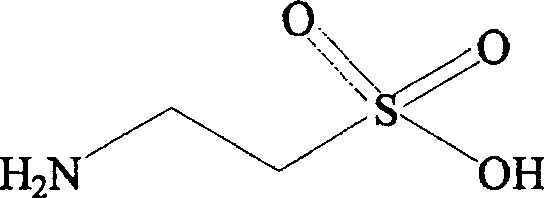
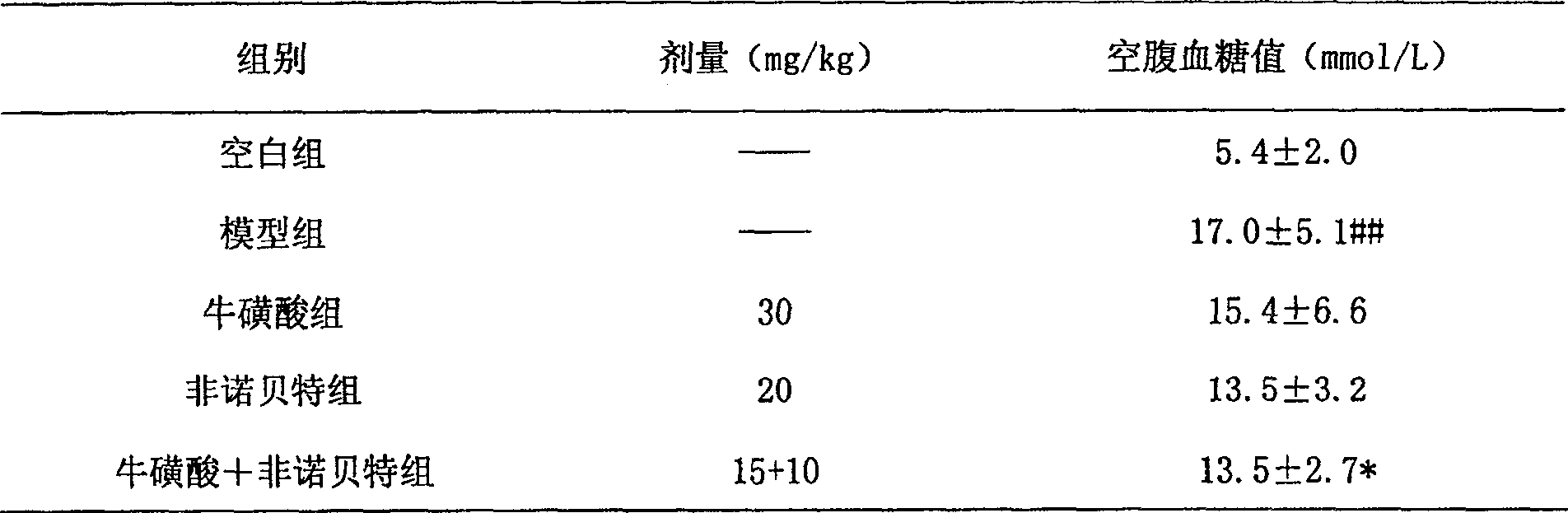
Composition containing fibrate drug and taurine
ActiveCN100531729CControlled resistance stateEffective prescriptionMetabolism disorderAnhydride/acid/halide active ingredientsDyslipidemiaLipid formation
The invention relates to a composition capable of comprehensively improving blood lipid and insulin resistance state of a body at the same time, and belongs to the field of pharmacy. The composition comprises pharmaceutically acceptable doses of phenoxyaromatic acid drugs (including fenofibrate, bezafibrate, gemfibrozil, etc.) or pharmaceutically acceptable salts thereof, pharmaceutically acceptable doses of taurine and pharmaceutically acceptable carriers or excipients. The present invention also relates to that the composition is used to comprehensively control blood lipids of patients, including reducing total cholesterol (TC), low-density lipoprotein (LDL) and triglyceride (TG), and increasing high-density lipoprotein (HDL); simultaneously the present invention The composition involved can be used to treat metabolic syndrome, synergistically improve the insulin resistance state of the body, and improve the insulin sensitivity index, and its effect is obviously better than that of a single medicine. The present invention also relates to the application of the composition in the preparation of medicines for the treatment of living bodies with dyslipidemia or metabolic syndrome.
Owner:SHENZHEN AUSA PHARM CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com