Bezafibrate sustained release tablet and preparation method thereof
A technology of bezafibrate and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of large doses and low solubility of bezafibrate, Achieve the effect of reducing side effects, lasting blood concentration and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Tablet formula:
[0069] Bezafibrate API 400mg Silicon dioxide 5mg Sodium lauryl sulfate 30mg Propylmethylcellulose 100mg Spray-dried lactose 45mg Magnesium stearate 6mg
[0070] Coating film coating material:
[0071] 15% Opadry II in water.
[0072] Preparation method: first mix 400mg of bezafibrate raw material with 5mg of silicon dioxide and 30mg of sodium lauryl sulfate for 5 minutes, then add 100mg of direct pressure grade hydroxypropylmethylcellulose and 45mg of spray-dried lactose, and mix well Finally, after adding 6 mg of magnesium stearate and mixing for 2 minutes, tableting was performed to obtain tablet cores. Place the obtained tablet cores in the coating pan, spray 15% Opadry aqueous solution at a uniform speed at a spray speed of 5ml / min, tablet bed temperature of 40°C, and coating pan rotation speed of 45r / min until the coating weight increases 2%.
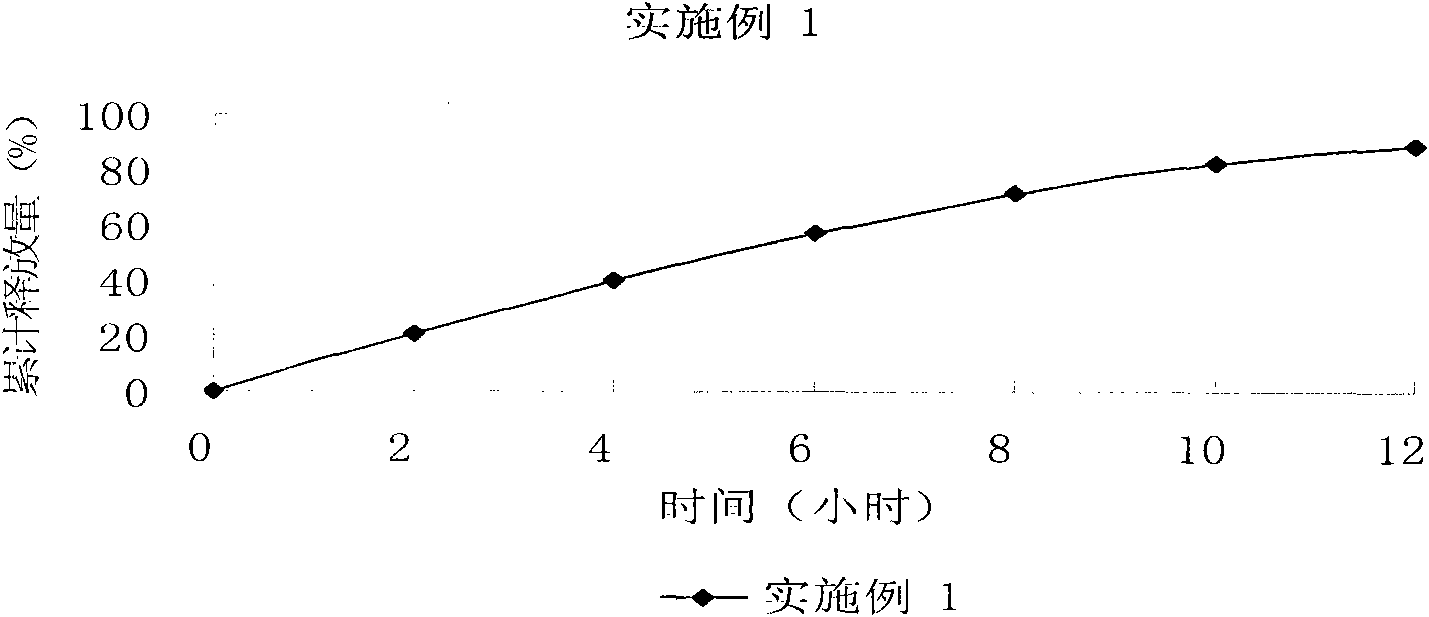
[0073] Technical effect: cumulative release of 23%, 45%, 63%, 77%, 87%, and 93% in 2, 4, 6, 8, 10, a...
Embodiment 2
[0075] Tablet core formula: bezafibrate 400mg polyoxyethylene 80mg ethyl cellulose 40mg polyethylene glycol 6000 30mg magnesium stearate 5mg
[0076] Coating film coating material:
[0077] Hydroxypropyl Methyl Cellulose 20% Titanium Dioxide 1% Triacetyl Glyceride 3% Ethanol 5% Water 71%.
[0078] Preparation:
[0079] Mix the prescribed amount of medicine with polyoxyethylene, ethyl cellulose, and polyethylene glycol evenly, granulate with 95% ethanol, granulate after drying, add the prescribed amount of magnesium stearate, mix evenly, compress into tablets, and obtain tablet cores . Place the obtained tablet cores in the coating pan, spray the above coating solution at a uniform speed at a spray speed of 5ml / min, a tablet bed temperature of 40°C, and a coating pan rotation speed of 45r / min until the coating weight gain is 3 %.
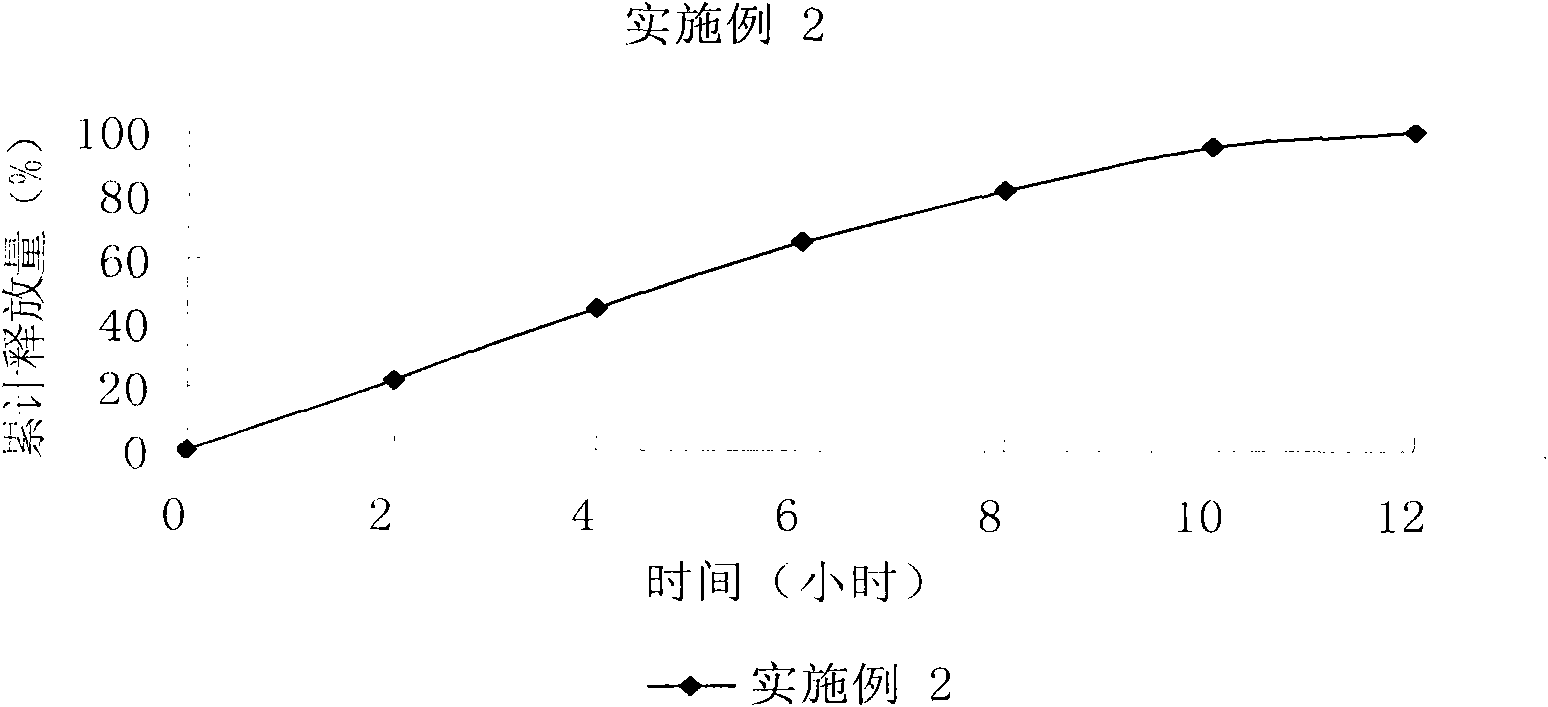
[0080] Technical effect: cumulative release of 24%, 45%, 64%, 82%, 92%, and 93% in 2, 4, 6, 8, 10, and 12 hours respectively, see the in vitro r...
Embodiment 3
[0082] Tablet core formula: bezafibrate 400mg hydroxypropyl methylcellulose 60mg pregelatinized starch 22mg lactose 30mg sodium lauryl sulfate 26mg silicon dioxide 6mg talcum powder 3mg.
[0083] Coating film coating material:
[0084] none
[0085] Preparation:
[0086] Mix the prescription amount of bezafibrate raw material with hydroxypropyl methylcellulose, pregelatinized starch, lactose, and sodium lauryl sulfate evenly, then granulate with 5% hydroxypropylmethylcellulose E5 aqueous solution, After drying, granulate, add the prescribed amount of talcum powder and silicon dioxide, mix evenly, and press into tablets to obtain tablet cores.
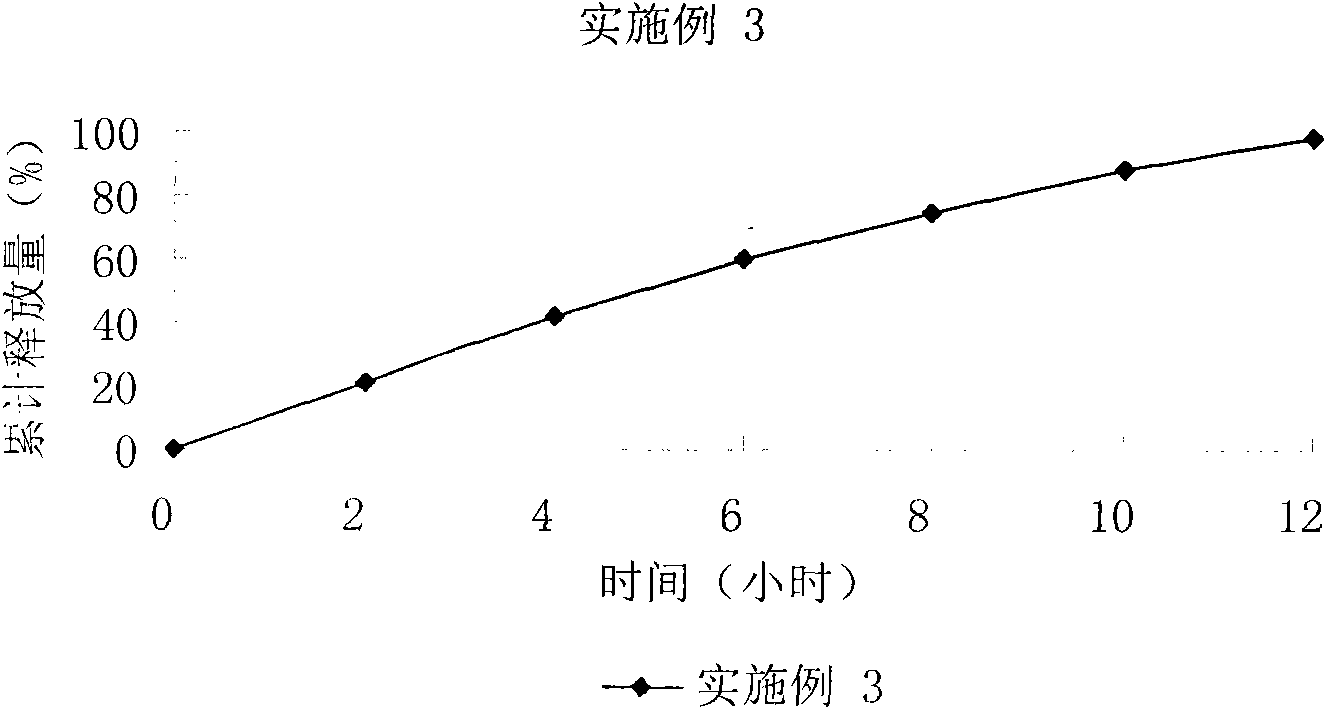
[0087] Technical effect: Cumulative release of 21%, 42%, 60%, 75%, 88%, and 98% in 2, 4, 6, 8, 10, and 12 hours respectively, see the in vitro release curve image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com