Etodolac osmotic pump type controlled-release preparation and preparation thereof
A technology of etodolac and controlled-release preparations, applied in the directions of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of increased difficulty in the development of osmotic pump tablets, poor solubility, pH dependence, and large therapeutic doses, etc. The effect of achieving long-lasting blood drug concentration, reducing the number of medication times, and stabilizing blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
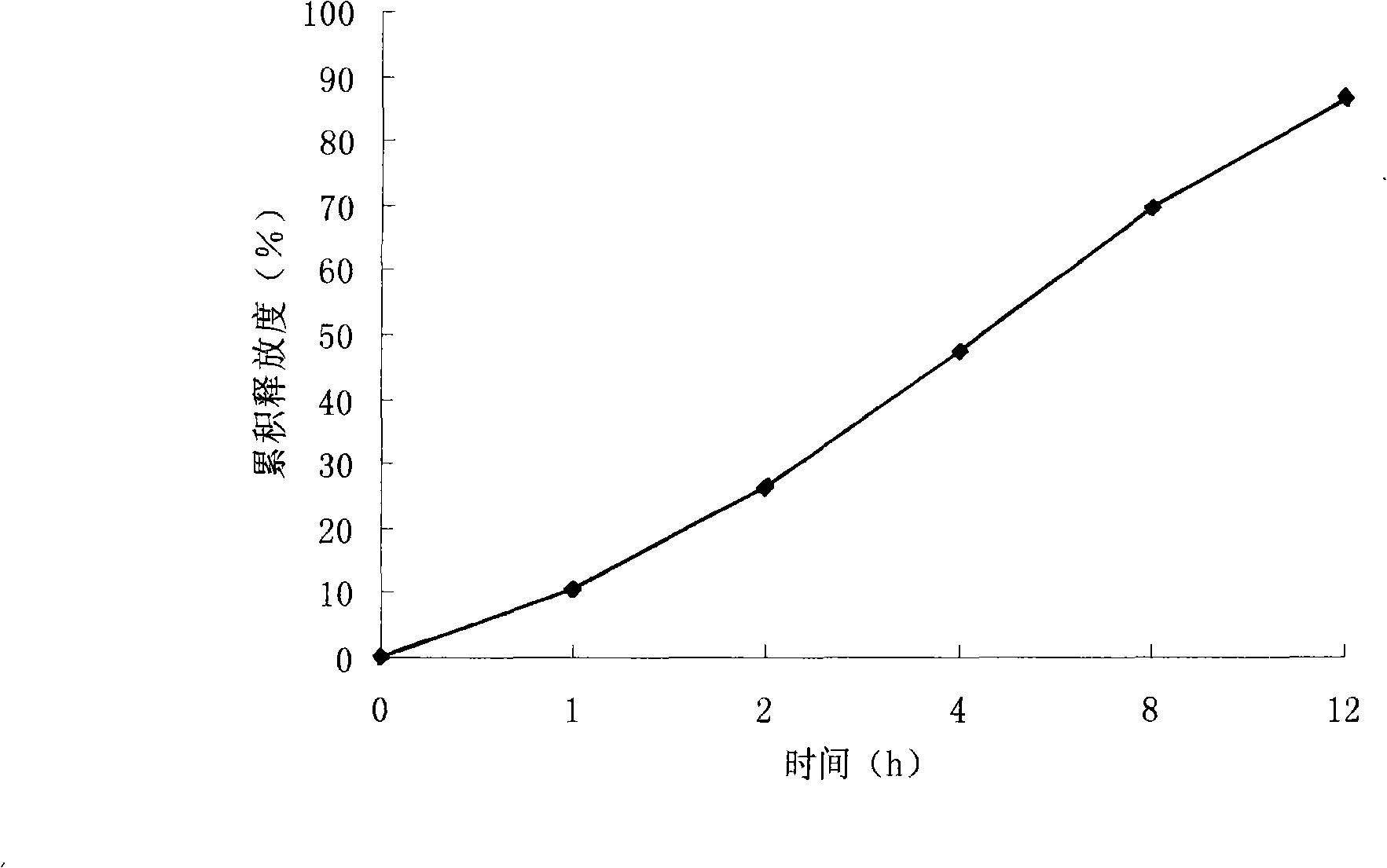
Examples
Embodiment 1
[0032] Tablet core prescription (weight percentage):
[0033] Etodolac 62.4%
[0034] NaCl 15.6%
[0035] Polyoxyethylene N80 15.6%
[0036] Na 2 CO 3 35.5%
[0037] 95% ethanol solution
[0038] Sodium fumarate stearate balance
[0039] Semi-permeable membrane prescription (weight percentage):
[0040] Cellulose acetate 71.4%
[0041] PEG4000 28.6%
[0042] Solvent prescription for dissolving coating film material (weight percentage):
[0043] Acetone 96.2%
[0044] Water 3.8%
[0045] Preparation Process:
[0046] Combine the prescribed amount of medicine with NaCl, N80, and Na 2 CO 3 Mix well, use 95% ethanol solution as binder to make soft material, granulate with 20-mesh sieve, after the wet granules are dried, use 18-mesh sieve to sizing, dry granules and lubricant sodium stearate fumarate, mix and press The tablet is the core. Dissolve cellulose acetate and pore-forming agent polyethylene glycol 4000 in a mixed solvent of acetone-water, and coat the tablet cores...
Embodiment 2
[0048] Tablet core prescription (weight percentage):
[0049] Etodolac 60.9%
[0050] NaCl 15.2%
[0051] Polyoxyethylene N80 15.2%
[0052] Na 2 CO 3 7.6%
[0053] 95% ethanol solution
[0054] Sodium fumarate stearate balance
[0055] Semi-permeable membrane prescription (weight percentage):
[0056] Cellulose acetate 71.4%
[0057] PEG4000 28.6%
[0058] Solvent prescription for dissolving coating film material (weight percentage):
[0059] Acetone 96.2%
[0060] Water 3.8%
[0061] Preparation Process:
[0062] Combine the prescribed amount of medicine with NaCl, N80, and Na 2 CO 3 Mix well, use 95% ethanol solution as binder to make soft material, granulate with 20-mesh sieve, after the wet granules are dried, use 18-mesh sieve to sizing, dry granules and lubricant sodium stearate fumarate, mix and press The tablet is the core. Dissolve cellulose acetate and pore-forming agent polyethylene glycol 4000 in a mixed solvent of acetone-water, and coat the tablet core with ...
Embodiment 3
[0064] Tablet core prescription (weight percentage):
[0065] Etodolac 65.5%
[0066] NaCl 19.6%
[0067] Polyoxyethylene N80 8.2%
[0068] Na 2 CO 3 5.7%
[0069] 95% ethanol solution
[0070] Sodium fumarate stearate balance
[0071] Semi-permeable membrane prescription (weight percentage):
[0072] Cellulose acetate 76.9%
[0073] PEG4000 23.1%
[0074] Solvent prescription for dissolving coating film material (weight percentage):
[0075] Acetone 97.1%
[0076] Water 2.9%
[0077] Preparation Process:
[0078] Combine the prescribed amount of medicine with NaCl, N80, and Na 2 CO 3 Mix well, use 95% ethanol solution as binder to make soft material, granulate with 20-mesh sieve, after the wet granules are dried, use 18-mesh sieve to sizing, dry granules and lubricant sodium stearate fumarate, mix and press The tablet is the core. Dissolve cellulose acetate and pore-forming agent polyethylene glycol 4000 in a mixed solvent of acetone-water, coat the tablet core with a co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com