Bezafibrate sustained release preparation and its preparation method
A sustained-release preparation, bezafibrate technology, applied in the field of bezafibrate sustained-release preparations and its preparation, can solve problems such as difficult to achieve treatment, low plasma peak concentration, and slow peak time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
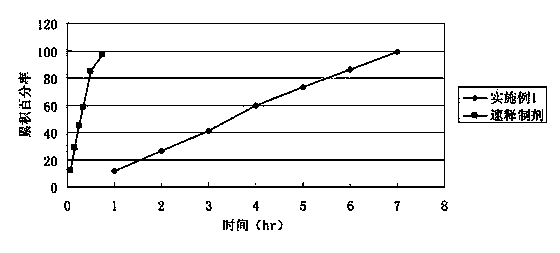
Embodiment 1
[0047] Adopt the method known in the pharmaceutical industry to make this embodiment 1 tablet core to be made up of the raw and auxiliary materials of following weight ratio:
[0048] Bezafibrate 400g Polyoxyethylene 85g Macrogol 4000 15g
[0050] Wetting agent: 95% ethanol
[0051] Coating material: gastric soluble film coating premix 15g
[0052] The core preparation process is as follows:
[0053] Polyoxyethylene and polyvinylpyrrolidone are mixed uniformly, bezafibrate is added in equal increments, mixed uniformly, 95% ethanol is added to granulate, dried, granulated, lubricant is added and mixed uniformly, compressed into tablets to obtain tablet cores.
[0054] Preparation of coating solution:
[0055] Use ethanol solution to prepare a coating solution of gastric-soluble film coating premix with a concentration of 12%. Stir the coating liquid to dissolve until it is uniform and fine without clumping before use.
[0056] Coating metho...
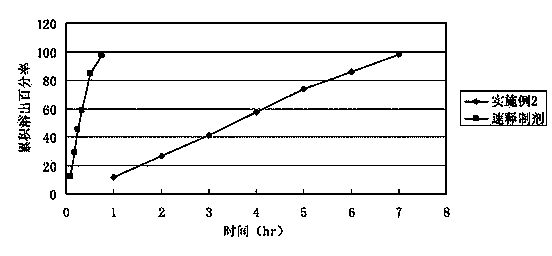
Embodiment 2
[0059] Adopt the known method of pharmaceutical industry to make this implementation 2 tablet cores to be made up of the raw and auxiliary materials of following weight ratio:
[0060] Bezafibrate 400g Polyoxyethylene N10 70g Polyoxyethylene N12K 30g Magnesium Stearate 7.5g
[0061] Wetting agent: 95% ethanol
[0062] The coating material used is a commercially available gastric-soluble film coating premix, and its main components are talcum powder, hypromellose, polyethylene glycol and titanium dioxide. The film coating material has the characteristics of moisture-proof and light-proof, and can improve the stability of the tablet.
[0063]We used the hydrophilic gel matrix technology to prepare bezafibrate sustained-release tablets with polyoxyethylene as the hydrophilic gel matrix sustained-release material. Usually high-molecular-weight polyoxyethylene is used as a release retardant in tablets. When high-molecular-weight polyoxyethylene is used as a skeleton material alon...
Embodiment 3
[0069] Adopt the method known in the pharmaceutical industry to make this embodiment 3 cores to be made up of the raw and auxiliary materials of following weight ratio:
[0070] Bezafibrate 400g Microcrystalline Cellulose 100g Hypromellose 4g Magnesium Stearate 6g
[0071] The binder used is a 4% hypromellose solution
[0072] The coating agent used is the coating material: polyacrylic acid resin 50g polyvinylpyrrolidone 3g absolute ethanol
[0073] The solution of polyacrylic acid resin and polyvinylpyrrolidone dissolved in absolute ethanol is coated to achieve sustained release. Polyacrylic acid resin is the coating material, and a small amount of polyvinylpyrrolidone solution is added to the coating solution as a porogen, which can be dissolved in the digestive juice, so that there are micropores on the coating film, and the drug is released from the micropores to play a slow-release effect. .
[0074] The core preparation process is as follows:
[0075] Mix bezafibrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com