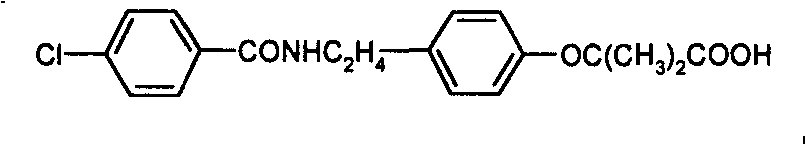
Preparation of bezafibrate
A technology of bezafibrate and chlorobenzamide, which is applied in the field of preparation of bezafibrate, can solve problems such as difficult operation, strong corrosion of personnel and equipment, and long time consumption, so as to achieve short reaction cycle, reduce operation cost, The effect of convenient and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 20g (72.6mmol) of N-p-hydroxyphenethyl-4-chlorobenzamide, 300ml of methylene chloride, 50ml of acetone and 0.5g (2mmol) of triethylbenzyl ammonium chloride into the reaction flask. Install a reflux condenser, a mechanical stirring device, a dropping funnel and a thermometer on the bottle, stir at room temperature for 0.5 hour, add 100ml of 50% sodium hydroxide solution, heat up to 35°C, add 26ml of chloroform (323mmol) dropwise, and React for 1 hour; cool to room temperature, add 200ml of water to the reactant, let stand to separate the layers, wash the organic layer twice with 100ml of water, combine the water layer, cool to below 20°C, add 15% hydrochloric acid dropwise under stirring to adjust the pH To 3.5-4.5, a beige solid was precipitated, continued to stir at this temperature for 0.5 hours, then suction filtered, and the filter cake was dried to obtain 24.9 g of beige powder, with a yield of 95%. The crude product was analyzed by high performance liquid chrom...
Embodiment 2
[0024] N-p-hydroxyphenethyl-4-chlorobenzamide 100g (363mmol), 1,1-dichloroethane 1500ml, acetone (200ml) and hexadecyltrimethylammonium chloride 0.57g (1.8mmol ) was added to the reaction flask, a reflux condenser, a mechanical stirring device, a dropping funnel and a thermometer were installed on the reaction flask, stirred at room temperature for 0.5 hour, 485ml of 30% sodium hydroxide solution was added, the temperature was raised to 40°C, and 29.3ml was added dropwise Chloroform (363mmol), heat up, control the reaction temperature to be 50~55 ℃ for 3 hours, cool to room temperature, add 800ml water to the reactant, let stand to separate the layers, wash the organic layer twice with 200ml water, combine the water layer, Cool to below 20°C, add 15% hydrochloric acid dropwise under stirring to adjust the pH to 3.5-4.5, a beige solid precipitates, continue to stir at this temperature for 1 hour, filter with suction, and dry the filter cake to obtain 128.6 g of beige powder with...
Embodiment 3
[0026] Add 25g (90.8mmol) of N-p-hydroxyphenethyl-4-chlorobenzamide, 750ml of toluene, acetone (100ml) and 1.4g (4.3mmol) of tetrabutylammonium bromide to the reaction flask, and in the reaction flask Install a reflux condenser, a mechanical stirring device, a dropping funnel and a thermometer on top, stir at room temperature for 0.5 hours, add 130ml of 70% sodium hydroxide solution, heat up to 40°C, add dropwise 58.5ml of chloroform (0.73mol), heat up, and control the reaction React at 55-60°C for 2 hours, cool to room temperature, add 300ml of water to the reactant, let stand to separate the layers, wash the organic layer twice with 100ml of water, combine the water layer, cool to below 20°C, stir and drop Add 15% hydrochloric acid to adjust the pH to 3.5-4.5, a beige solid precipitates, continue to stir at this temperature for 1 hour, then filter with suction, and dry the filter cake to obtain 30.5 g of beige powder with a yield of 93%. The purity of the crude product is 92....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com