Bezafibrate controlled release formulation and preparation method thereof
A technology of bezafibrate and controlled-release preparations, which is applied in the field of pharmacy, can solve the problems that the drug dissolution rate cannot reach the expected effect, the drug effect is not long-lasting, and it is not convenient for patients to take it. It is easy to produce industrialization and standardization, Fewer gastrointestinal side effects and long-lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
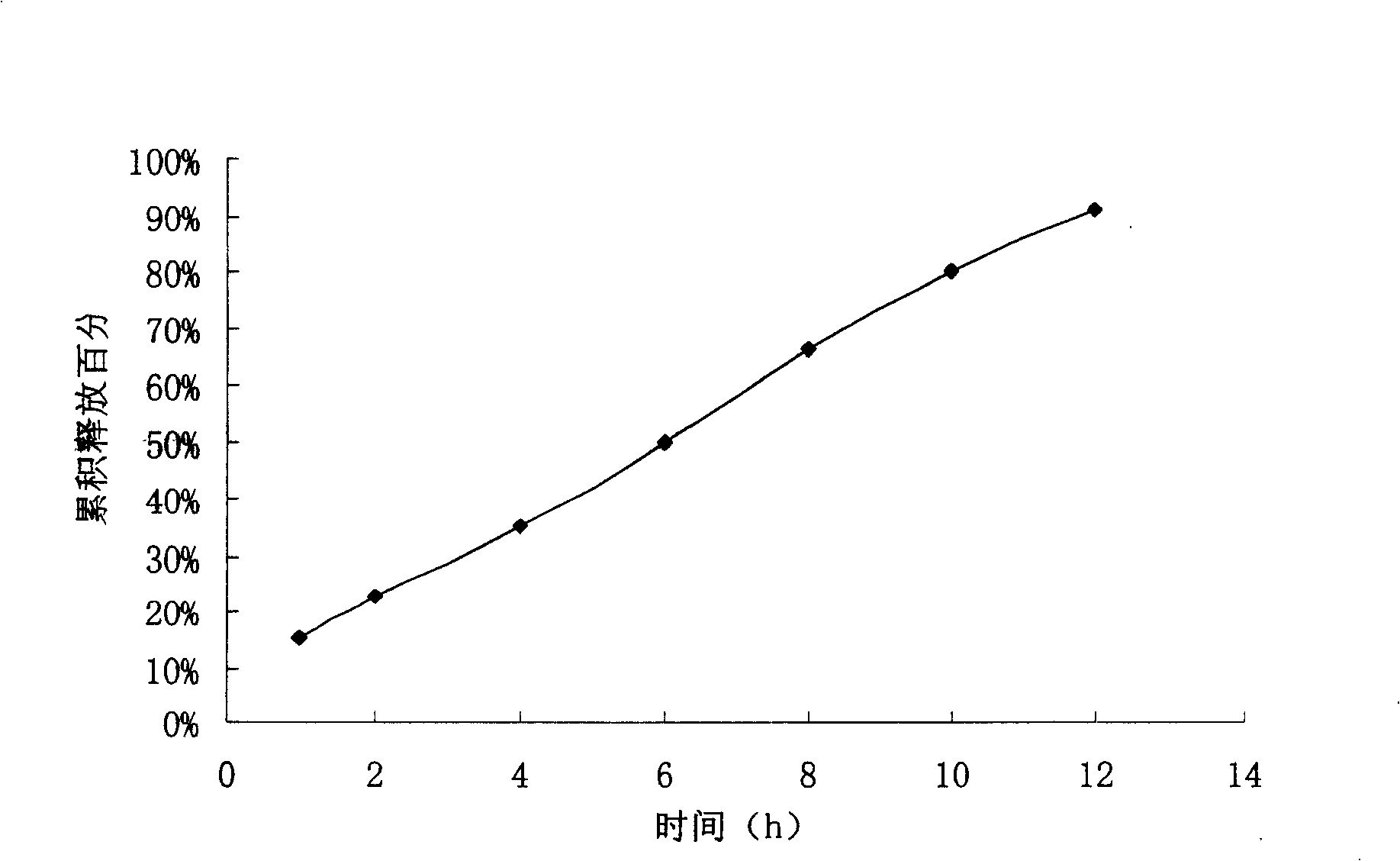
experiment example 1
[0043] Tablet core formula:
[0044] Benzafib 40g
[0046] Sodium lauryl sulfate 5g
[0047] Polyoxyethylene N80 8g
[0049] Magnesium stearate 0.6g
[0050] Coating liquid formula:
[0051] Cellulose acetate 15g
[0052] Polyethylene glycol 4000 5g
[0053] Acetone 500ml
[0054] Water 30ml
[0055] Preparation process: fully mix the bezafibrate, sodium carbonate, sodium lauryl sulfate, polyoxyethylene N80 and sodium chloride in the formula, add 95% ethanol to make soft material, and granulate on a 20-mesh sieve. Dry at ℃, size with 16 mesh sieve, add proper amount of magnesium stearate, mix well, and compress. The tablet cores are coated with the coating liquid, and the weight of each tablet is about 35mg. Place the coated tablet in a drying box at 40°C for 8 hours and then punch a 0.8μm hole on each side of the tablet.
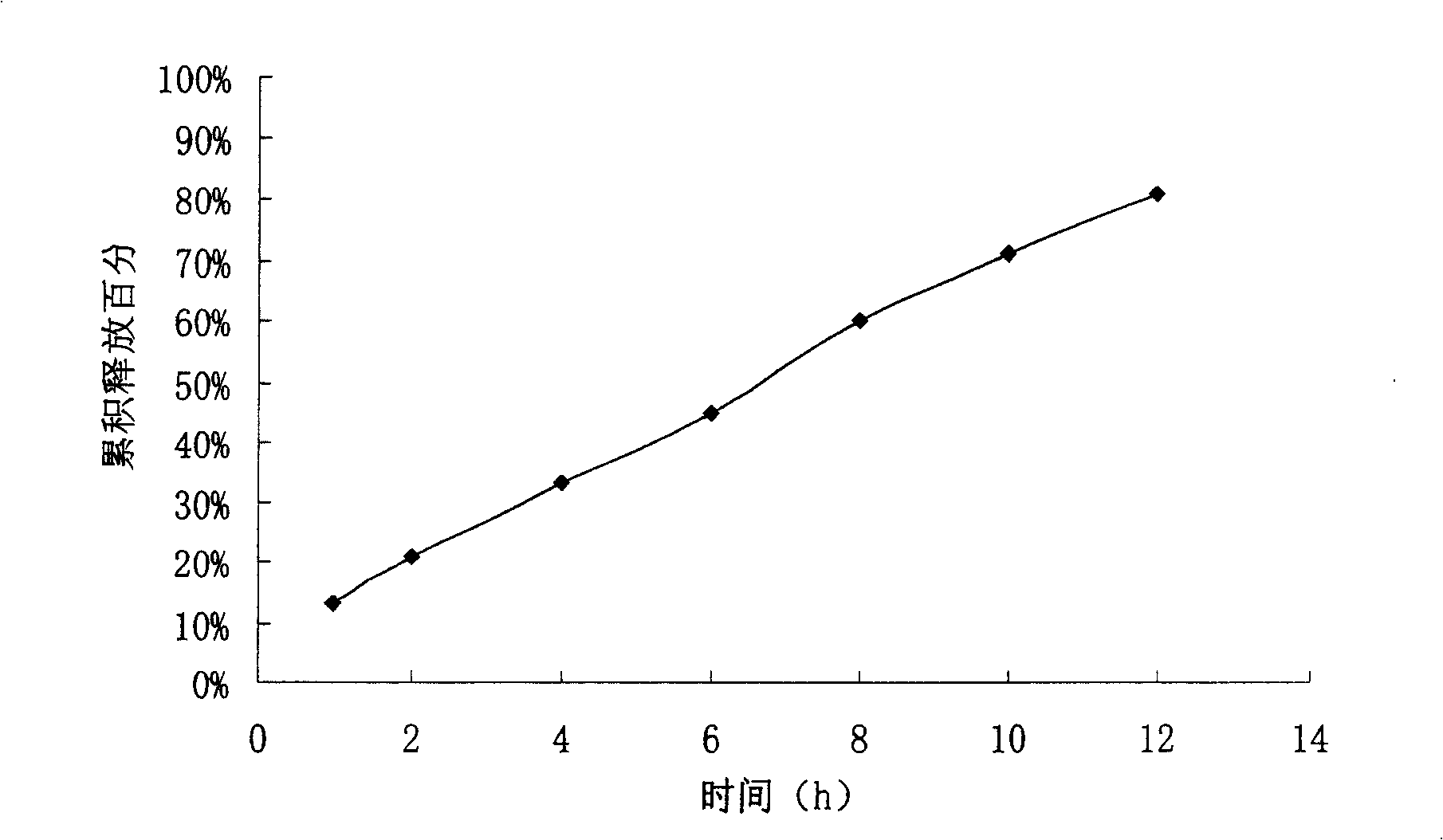
experiment example 2
[0057] Tablet core formula:
[0058] The first layer: bezafibrate 30g
[0059] Sodium carbonate 2g
[0060] Sodium lauryl sulfate 3g
[0061] Sodium chloride 8g
[0062] Hypromellose K4M amount
[0063] Magnesium stearate 0.4g
[0064] The second layer: bezafibrate 10g
[0065] Polyoxyethylene N80 8g
[0066] Sodium chloride 2g
[0067] Magnesium stearate 0.2g
[0068] Coating liquid formula:
[0069] Cellulose acetate 15g
[0070] Polyethylene glycol 4000 7g
[0071] Acetone 500ml
[0072] Water 30ml
[0073] Preparation process: Mix the bezafibrate, sodium carbonate, sodium lauryl sulfate and sodium chloride in the first layer of the formula fully and uniformly, add 2% hypromellose solution to make soft material, 20 Mesh granulation, drying at 45℃, 16 mesh granules, then add appropriate amount of magnesium stearate, mix well, and take the proportion of bezafibrate, polyoxyethylene N80 and sodium chloride in the second layer Mix well, add 95% ethanol to make soft material, granul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com