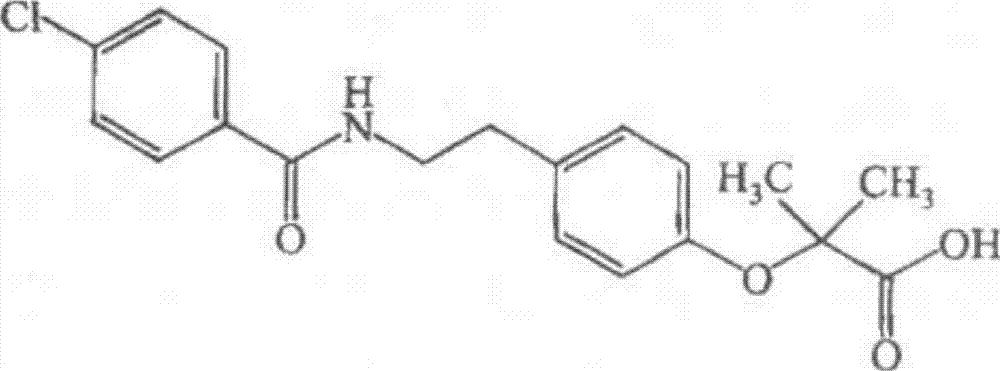
Bezafibrate dual-release slow-release capsule medicinal composition
A technology of bezafibrate and sustained-release capsules is applied in the field of dual-release sustained-release capsules and their preparation, and can solve the problems of uneven drug release, poor medication compliance, and low bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Every 1000 described bezafibrate pharmaceutical compositions, its formula consists of:
[0141] release immediately
[0142] Bezafibrate 100g
[0143] Polyvinylpyrrolidone K30 40g
[0144] Microcrystalline Cellulose 10g
[0145] slow release
[0146]
[0147]
[0148] Process steps:
[0149] 1) Preparation and processing of raw and auxiliary materials: prepare raw and auxiliary materials according to the prescription quantity, and meet the quality standards;
[0150] 2) Weighing: According to the prescription amount, double-checked to calculate the feeding amount, and weigh the above raw and auxiliary materials respectively;
[0151] 3) Mixing: mixing the prescribed amount of bezafibrate with each auxiliary material in the prescribed amount so as to fully mix them;
[0152] 4) Extrusion: Set the position of each head of the twin-screw extruder to 100°C-130°C-140°C-145°C, and the screw speed to 40r / min. Put the mixture into the hopper of the machine, and the ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com