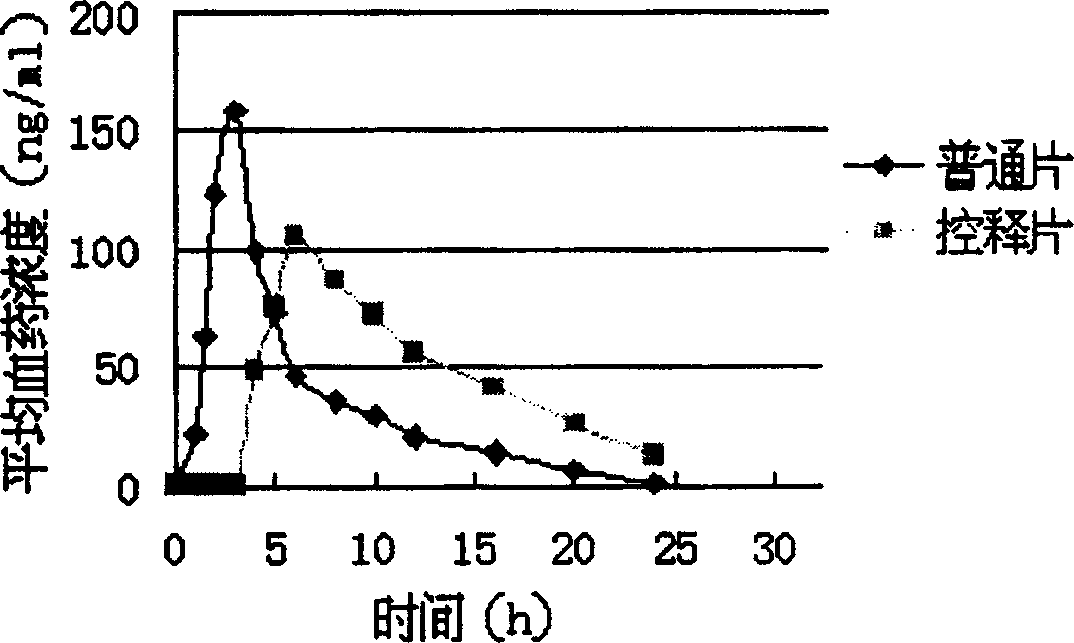
Albuterol time controlling pulse slow release oral preparation and its preparation method
A time-controlled pulse, albuterol technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, bulk delivery, etc., can solve the day-night change, the best administration time is 12:00 in the morning, easy to produce resistance. Receptivity, drug waste, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Slow-release microspheres:
[0055] Salbutamol sulfate 10g, acrylic resin RS 22g, acrylic resin RL 2g, triethyl citrate 2.4g, magnesium stearate 0.5g, absolute ethanol 90ml, liquid paraffin 270ml;
[0056] (2) Quick-release layer:
[0057] Salbutamol sulfate 10g, hypromellose 20g, water 400g;
[0058] (4) Expansion layer:
[0059] 42g of hydroxypropyl cellulose with a substitution degree of 10%, 13g of Tween 80, 7g of hydroxypropyl cellulose, and 400g of water;
[0060] (3) Isolation layer:
[0061] Hypromellose 2g, polyethylene glycol 4000 0.5g, water 100g;
[0062] (5) Retardation layer:
[0063] Ethyl cellulose 11g, acrylic resin L100 1g, tributyl citrate 1g, ethanol 120ml, talcum powder 1g.
[0064] time (h)
Embodiment 2
[0066] (1) Slow-release microspheres:
[0067] L-albuterol hydrochloride 3g, ethyl cellulose 80g, polyethylene glycol 8g, acetone 90ml, liquid paraffin 270ml;
[0068] (2) Quick-release layer:
[0069] L-albuterol hydrochloride 2.5g, polyvinylpyrrolidone 4g, water 80g;
[0070] (3) swelling layer:
[0071] Sodium carboxymethyl starch 12.6g, sodium lauryl sulfate 6g, hypromellose 1.5g, water 150g;
[0072] (4) Isolation layer:
[0073] Hypromellose 2g, dibutyl sebacate 0.4g, water 40g;
[0074] (5) Retardation layer:
[0075] time (h)
[0076] The process is the same as before. The technical parameters for the preparation of slow-release microspheres are stirring speed: 150 rpm, stirring time: 5 hours
Embodiment 3
[0078] (1) Slow-release microspheres:
[0079] Salbutamol sulfate 8g, ethyl cellulose 24g, polyethylene glycol 2.4g, magnesium stearate 0.63g, absolute ethanol 180ml, liquid paraffin 550ml;
[0080] (2) Quick-release layer:
[0081] Salbutamol sulfate 4g, polyvinylpyrrolidone 8g, water 150g;
[0082] (3) swelling layer:
[0083] Crospovidone 8g, hypromellose 1g, water 100g;
[0084] (4) Isolation layer:
[0085] Hypromellose 1g, polyethylene glycol 0.1g, water 30g;
[0086] (5) Retardation layer:
[0087] time (h)
[0088] The process is the same as before. The process parameters for the preparation of slow-release microspheres are stirring speed: 200 rpm, stirring time: 1 hour
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com