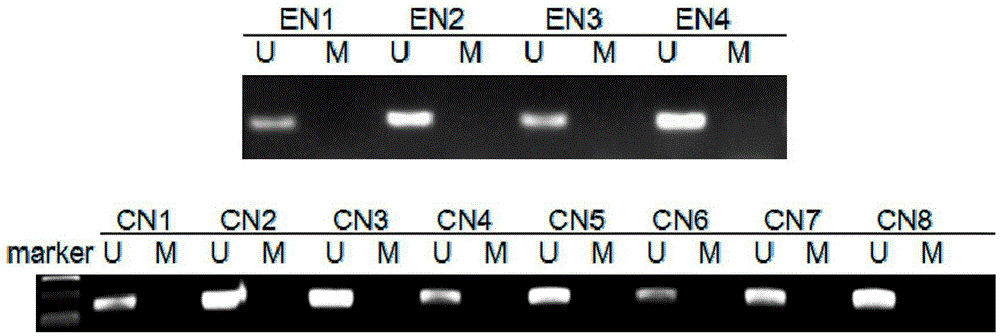
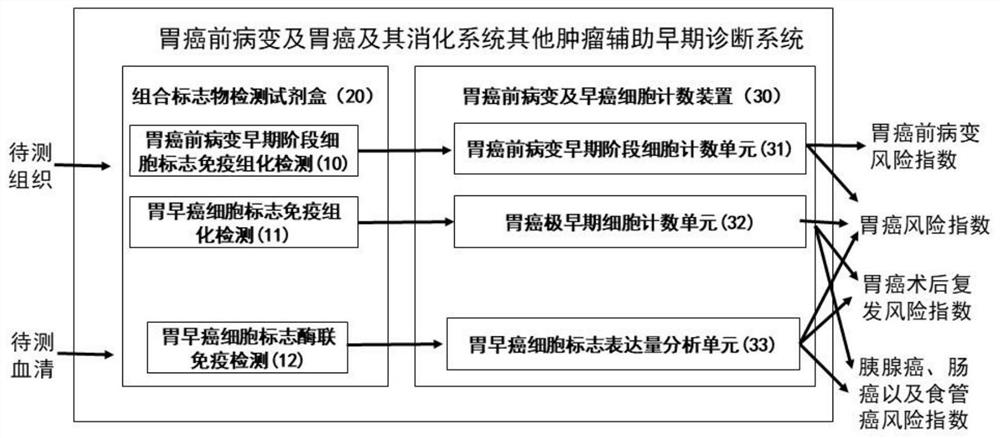
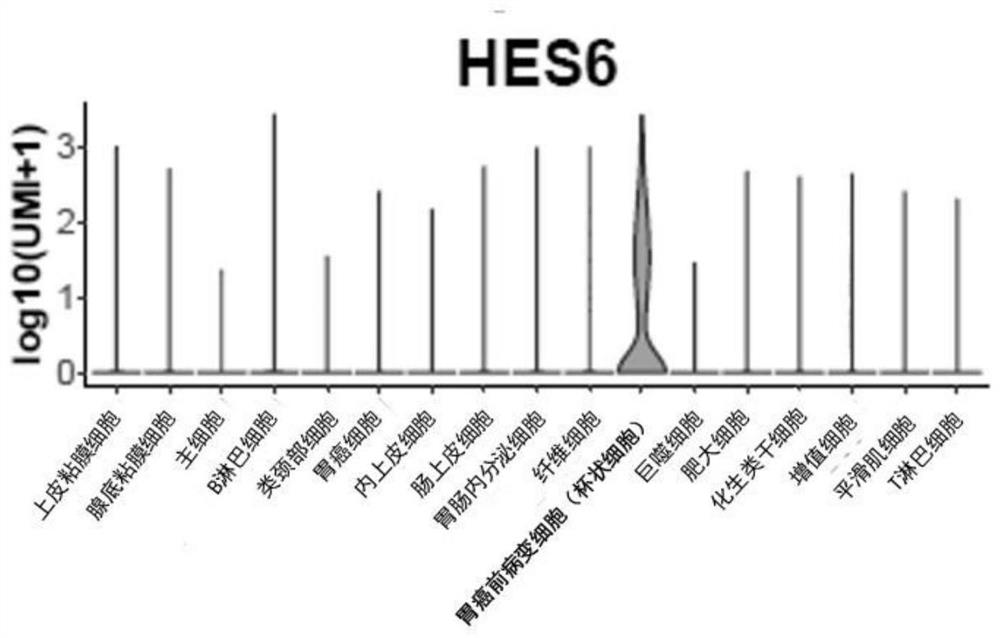
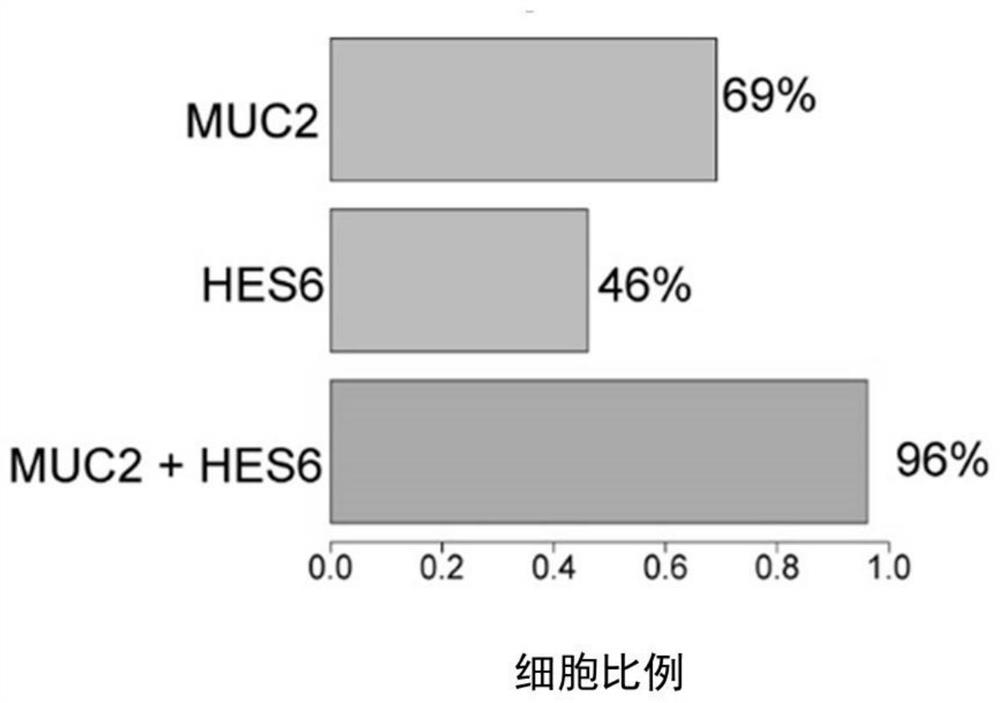
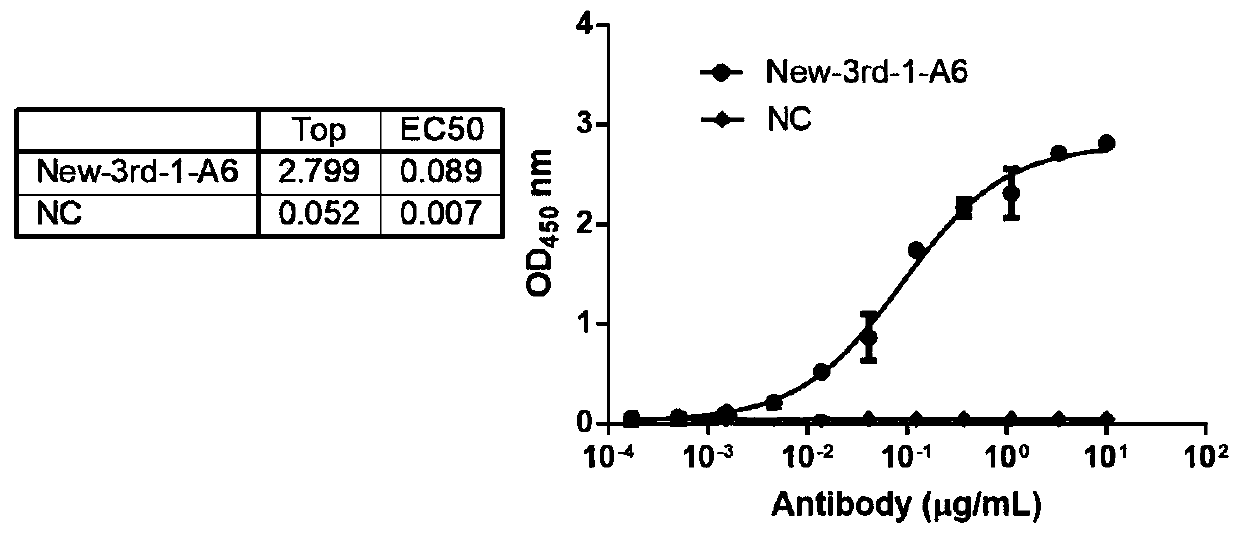
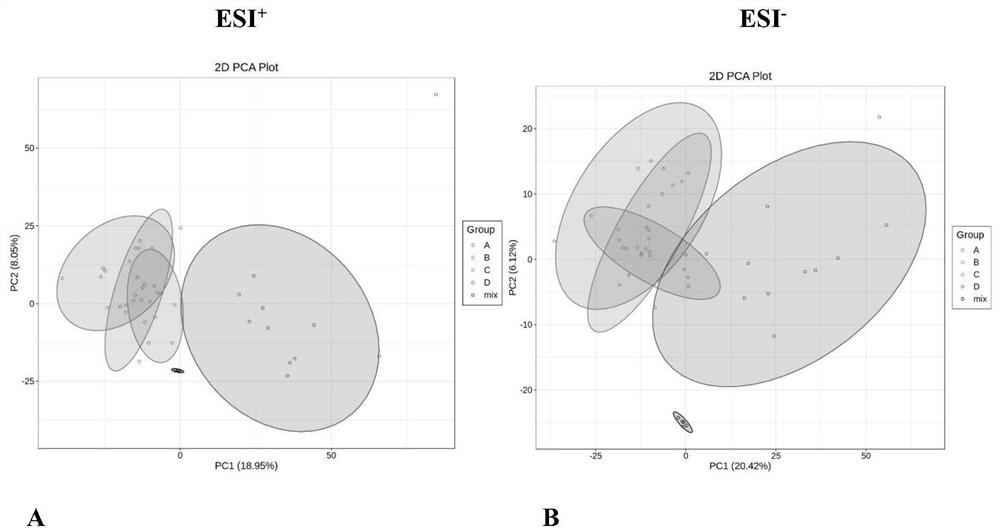
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Digestive Tumor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Capsules for treating respiratory tract and digestive tract cancers and preparation method thereof
InactiveCN102579916AReduced number of splitsSevere degenerationUnknown materialsAntineoplastic agentsRespiratory tract diseaseBud
The invention discloses capsules for treating respiratory tract and digestive tract cancers. The capsules are prepared by taking the following traditional Chinese medicines in parts by weight as active pharmaceutical ingredients: 40-60 parts of ginseng, 8-14 parts of calculus bovis factitious, 40-60 parts of pseudo-ginsheng, 10-15 parts of hairyvein agrimonia herb and bud, 8-10 parts of solanum lyratum, 25-45 parts of Chinese Paris rhizome, 25-45 parts of membranous milkvetch root, 25-45 parts of radix sophorae flavescentis, 8-15 parts of ganoderma, 10-30 parts of dried mushroom, 10-30 parts of glabrous greenbrier rhizome, 40-75 parts of longhairy antenoron herb, 8-12 parts of common dysosmatis rhizome and root, 25-45 parts of barbed skullcap herb, 15-21 parts of salvia chinensis, 18-25 parts of spreading hedyotis herb, 10-15 parts of baical skullcap root, 8-12 parts of licorice, 6-14 parts of Indian Iphigenia bulb, 35-55 parts of indigo, 10-15 parts of black nightshade herb, 10-15 parts of asparagus and 8-10 parts of paniculate swallowwort root. The medicament disclosed by the invention is finely extracted from pure Chinese herbal medicines and has obvious analgesic, calmative and anti-cancer effects; the effect of suppressing respiratory tract and digestive tract tumors can achieve 45%; and the medicament is low in price and is a safe and effective treatment way.
Owner:范冬锁
Monoclonal antibody for resisting cell surface ectopic expression, and preparation method and application thereof
ActiveCN103130896ASignificant technological progressAntibody ingredientsFused cellsBinding siteIn vitro test
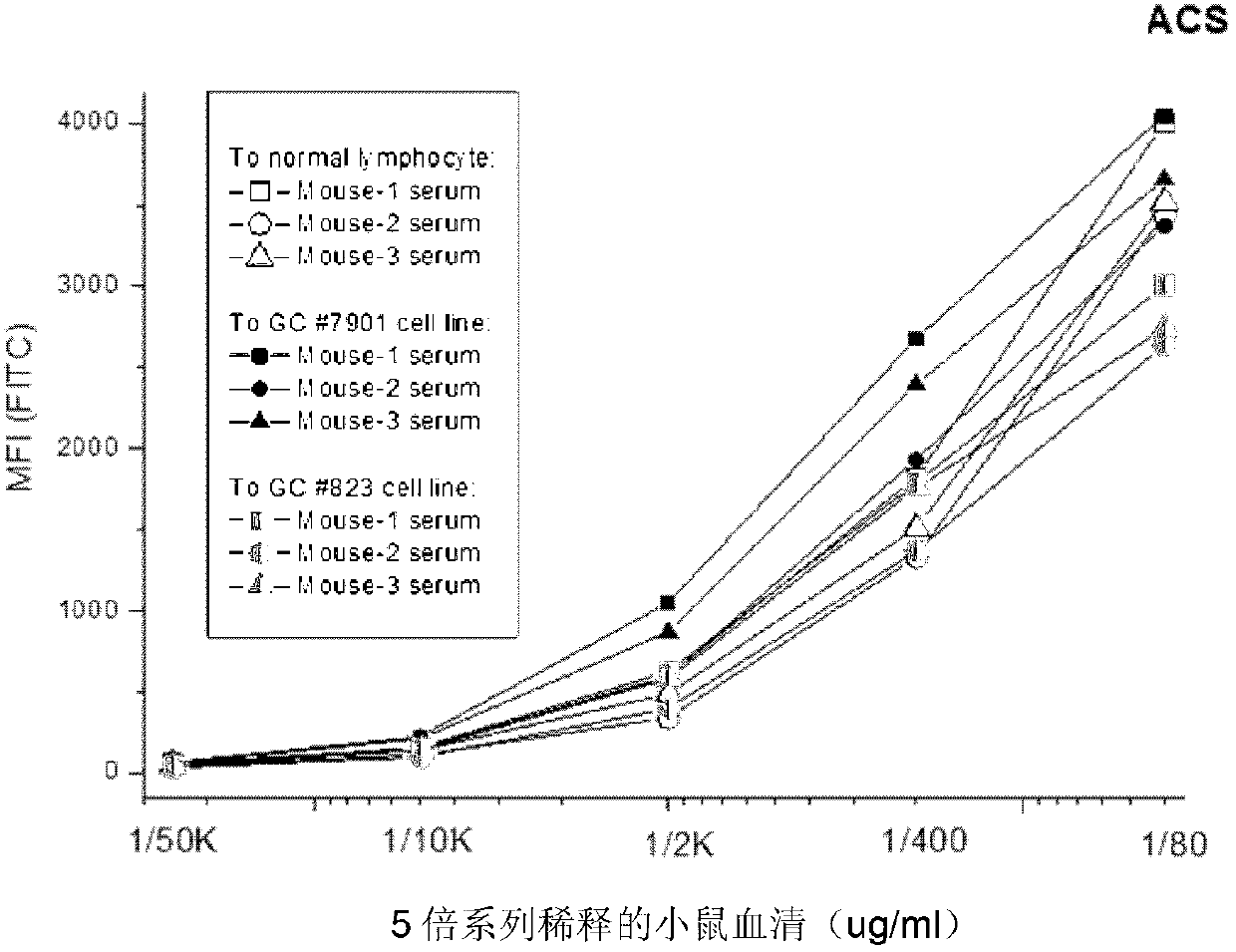
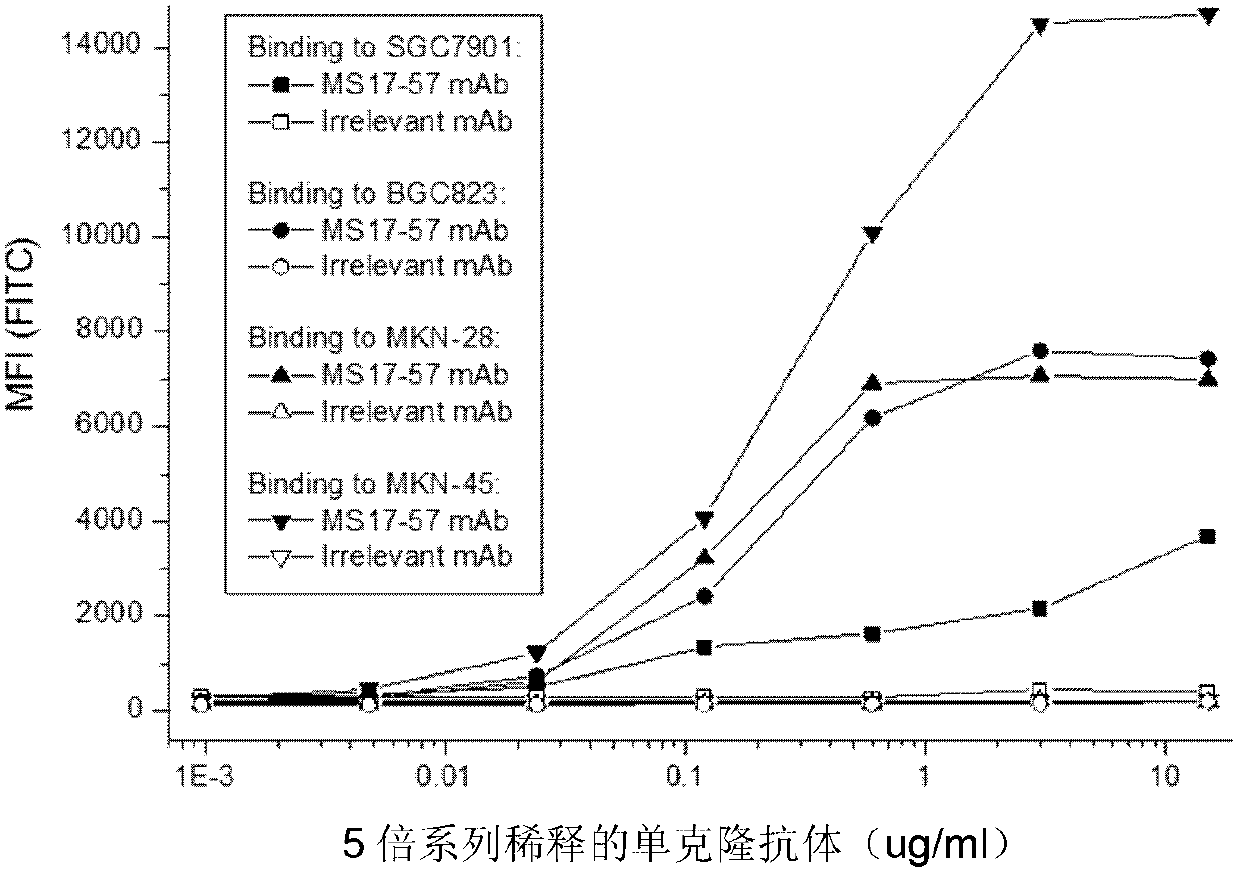
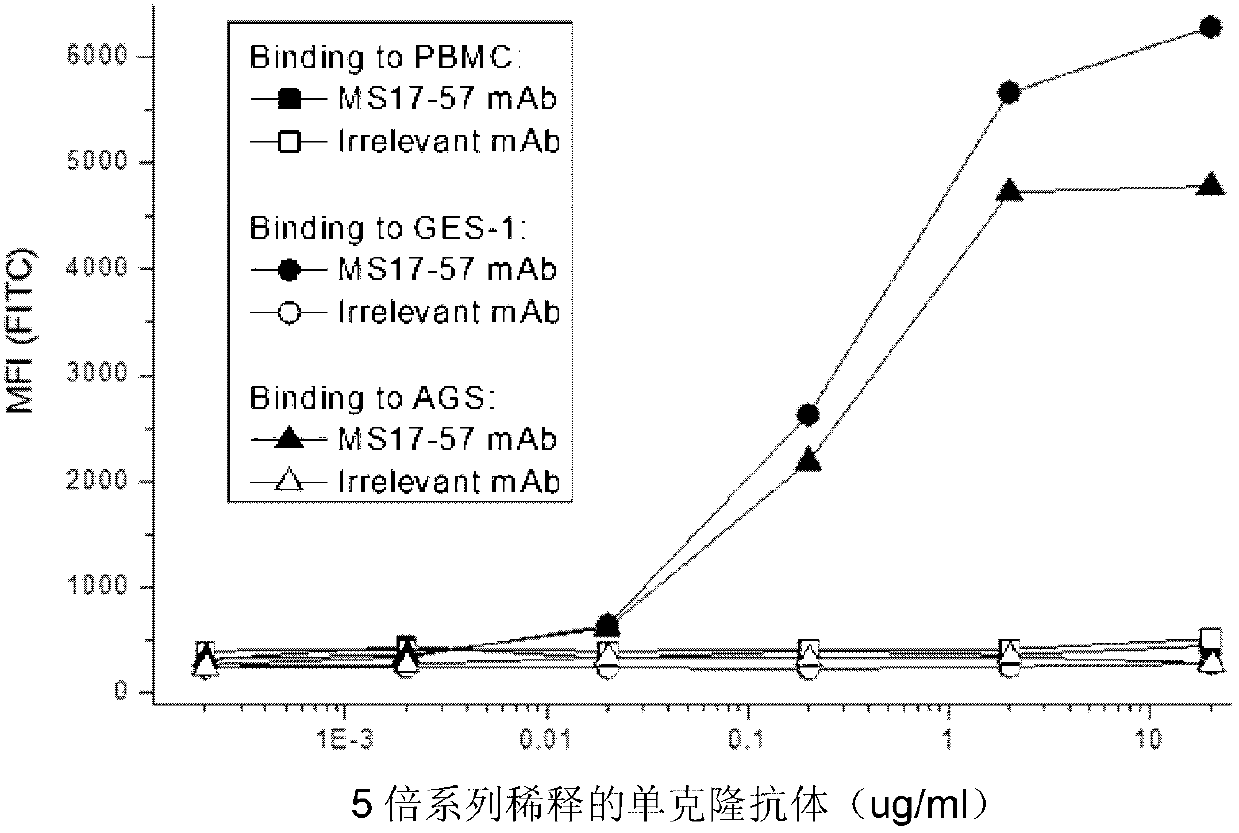
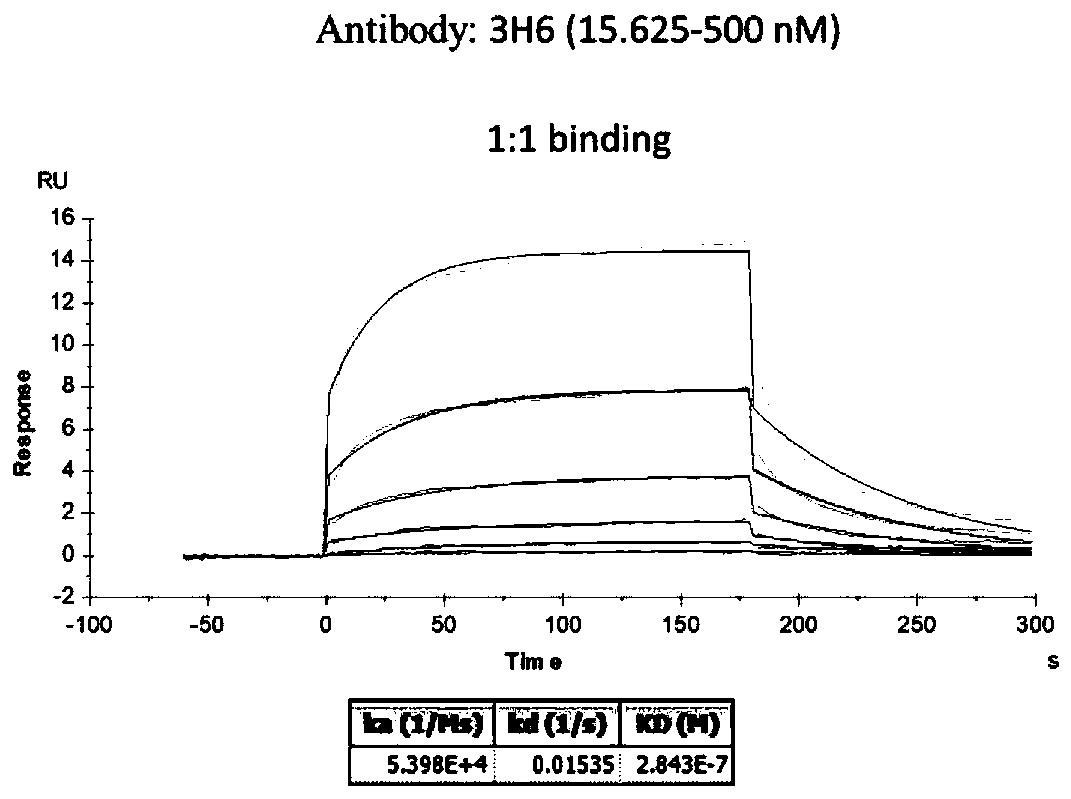
The invention belongs to the field of biological pharmacy and provides a monoclonal antibody for resisting cell surface ectopic expression. The amino acid sequence of a light chain variable region at the Fab fragment antigen-binding site of an antibody molecule is shown as SEQ ID NO:2, and the amino acid sequence of a heavy chain variable region is shown as SEQ ID NO:4. The invention also provides application of the monoclonal antibody in preparation of medicaments for treating and preventing digestive tract tumors or digestive tract tumor metastasis. The invention also provides a detection kit and medicinal composition containing the monoclonal antibody. The invention also provides a preparation method of the monoclonal antibody. A series of in vivo and in vitro tests prove that the purified and degermed monoclonal antibody has high affinity for PALP and / or IALP antigen for resisting extracellular ectopic expression of tumor cells and has a biological activating function of inhibiting growth and migration of tumor cells.
Owner:MABSTAR
Digestive tract tumor PDX model and construction method of standardized model library
PendingCN113142135AFacilitate adjuvant therapyProcess specificationCompounds screening/testingAnimal husbandryDigestive canalTumor therapy
The invention provides a digestive tract tumor PDX model and a construction method of a standardized model library. The method comprises the following steps: conducting sampling, treating a collected peripheral blood sample of a digestive tract tumor, obtaining tumor cells, conducting treating and culturing, stably passing the tumor cells to n generations, constructing a digestive tract tumor PDX model and evaluating the model. A Chinese population human-derived digestive tract tumor transplantation animal model and a corresponding primary tumor cell line are established in a digestive tract tumor PDX standardized model library, an important in-vivo model is provided for biological research of tumors, searching of diagnostic markers and drug screening, randomness and blindness of drug use are avoided, disturbance by factors such as original tissue samples is avoided, the digestive tract tumor PDX model can be stably subcultured to three generations or more, and the construction of the digestive tract tumor PDX model standard sample library provides bioinformatics for exploring new targets and searching and analyzing clinical characteristics of digestive tract tumors, and assists in research of a fatal mechanism of digestive tract tumors and treatment of patient tumors.
Owner:SHANDONG PROVINCIAL HOSPITAL AFFILIATED TO SHANDONG FIRST MEDICAL UNIVERSITY
Soft lentinan capsule and its prepn
InactiveCN1887292AHigh drug loadingImprove bioavailabilityOrganic active ingredientsDigestive systemChronic hepatitisGlycerol
The present invention discloses one kind of soft lentinan capsule and its preparation process, and the soft lentinan capsule is prepared through suspending lentinan in diluent to form stable suspension and filling the suspension into soft capsule shell. Of each 1000 capsules in 334.45-824.07 g, there are lentinan 10-50 g, diluent 100-400 g, gelatin 50-180 g and glycerin 14.1-64.5 g. The inclusion contains lentinan, diluent and suspending agent; and the capsule shell contains gelatin, glycerin, water, preservative and antioxidant. The soft lentinan capsule has the functions of benefiting Qi, invigorating spleen, tonifying deficiency and strengthening body's resistance, and may be used in treating chronic hepatitis B and as the assisting medicine for radiotherapy and chemotherapy treating tumor in digestive tract. The present invention has the advantages of stable quality, convenient taking and high bioavailability.
Owner:ZHUHAI EBANG PHARMA
Anticancer embryo antigen antibody as well as preparation method and application thereof
ActiveCN110713539AIncrease lethalityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsComplementarity determining regionAntiendomysial antibodies
The invention provides an anti-CEA (carcinoembrynio antigen) antibody which comprises a heavy chain variable region and a light chain variable region, wherein the complementary determining region of the heavy chain variable region comprises CDR (complementary determining region)-H1 of which the amino acid sequences are shown in SEQ ID NO.1 in the description, CDR-H2 of which the amino acid sequences are shown in SEQ ID NO.2 in the description and CDR-H3 of which the amino acid sequences are shown in SEQ ID NO.3 in the description; and the complementary determining region of the light chain variable region comprises CDR-L1 of which the amino acid sequences are shown in SEQ ID NO.4 in the description, CDR-L2 of which the amino acid sequences are shown in SEQ ID NO.5 in the description and CDR-L3 of which the amino acid sequences are shown in SEQ ID NO.6 in the description. The inventor of the invention screens CEA scFv by using a phage display technology, and thus obtains a high-affinityanti-CEA single-chain antibody. In addition, an anti-CEA chimeric antigen receptor gene is introduced into T cells by using a genetic engineering method to prepare CEA CAR-T, so that CEA CAR-T cellsare capable of specifically recognizing and killing digestive tract tumor cells expressing CEA, and thus the anti-tumor effects of the CEA CAR-T cells can be achieved.
Owner:HUADAO SHANGHAI BIOPHARMA CO LTD
Endoscopic minimally invasive hoe scaler
InactiveCN102144932AImproved medical skillsWith strippingVaccination/ovulation diagnosticsSurgical instruments for heatingBiopsy forcepsPrior treatment
The invention discloses an endoscopic minimally invasive hoe scaler, comprising a catheter, hemostatic forceps, an operating grip and an operation control device. The endoscopic minimally invasive hoe scaler is characterized in that a head of the catheter is connected with the hemostatic forceps while a tail of the catheter is matched connection with the operating grip; the hemostatic forceps are composed of a subwarhead-shaped hollow binding clip of which two halves are closed and opened correspondingly; the hoe scaler is a plasma hoe scaler formed by a steel wire structure, and extends into a linked tube of the hemostatic forceps; the operating grip is provided with a hemostatic forceps push ring and a hoe scaler control grip capable of moving back and forth; and the operation control device comprises a hemostatic forceps operation control device and a hoe scaler operation control device. The endoscopic minimally invasive hoe scaler solves the defects in the prior treatment on digestive tumor, and comprises both the hoe scaler and the hemostatic forceps, so that cockamamie operations of repeatedly changing a plurality of prior instruments in the world at present are avoided; and after being powered on, the endoscopic minimally invasive hoe scaler has the functions of the biopsy forceps and the hemostatic forceps, and the function of incision, so that the detection, the incision and the haemostasis in time can be convenient, and the complications of bleeding can be reduced.
Owner:潘文胜
Tumor blood vessel and M1 type macrophage targeting peptide and application thereof
ActiveCN108178783AProlong biological half-lifeImprove pharmacological activityAntipyreticAnalgesicsMolecular targetingMacrophage targeting
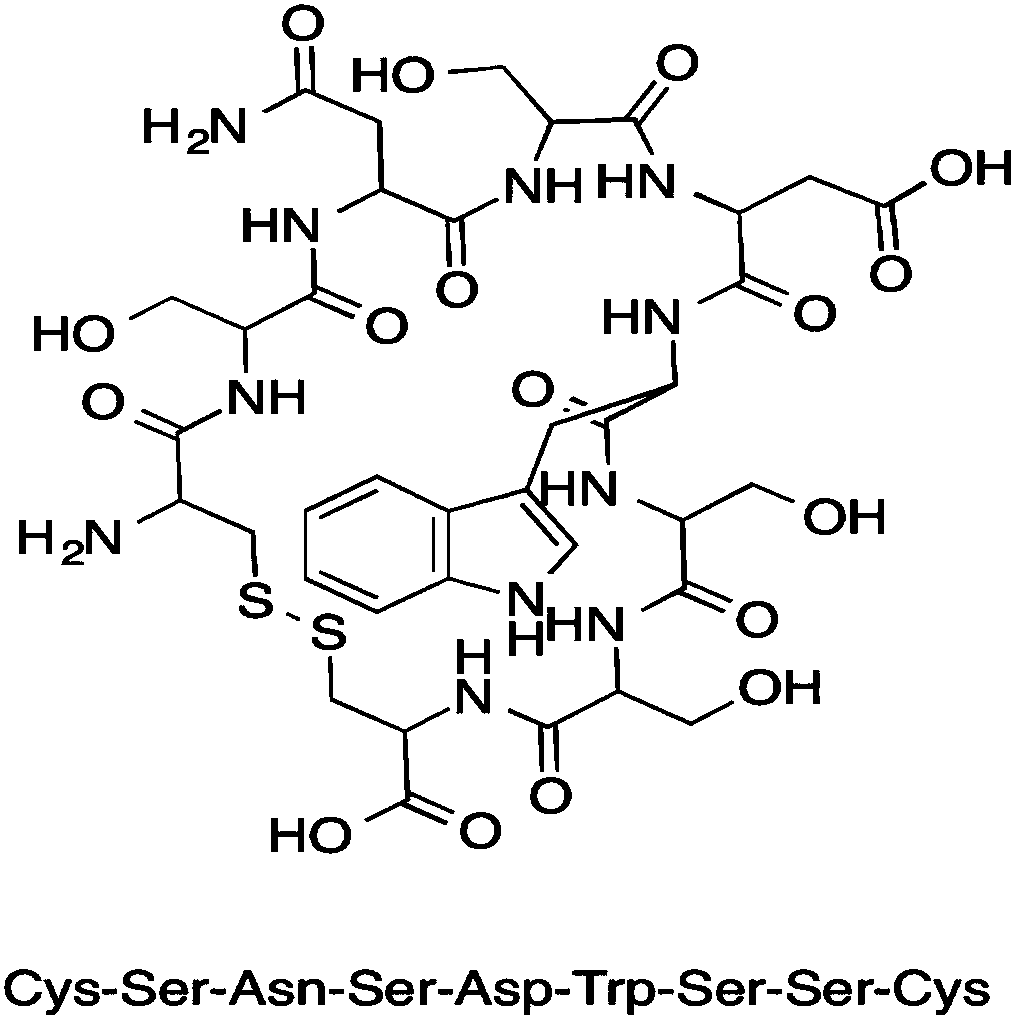
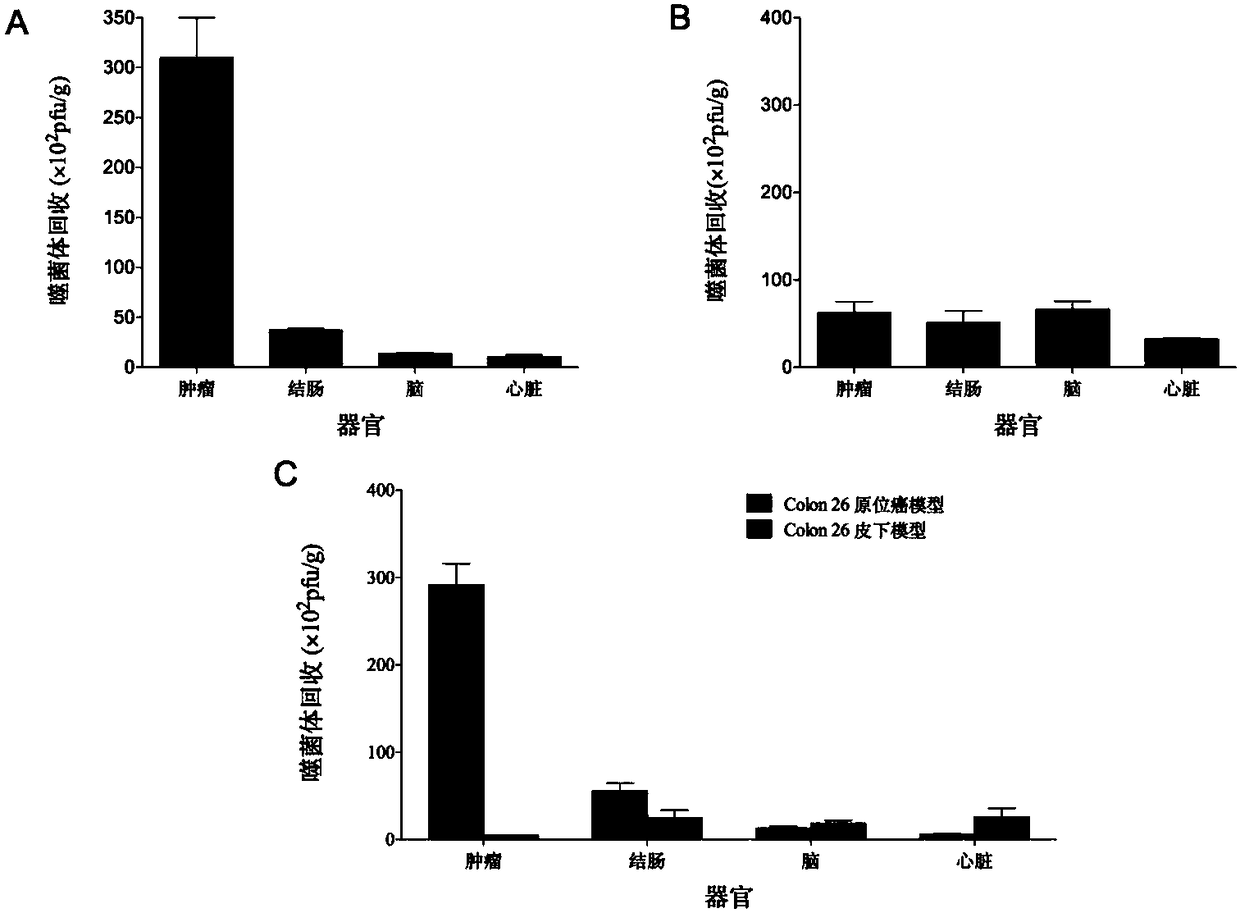
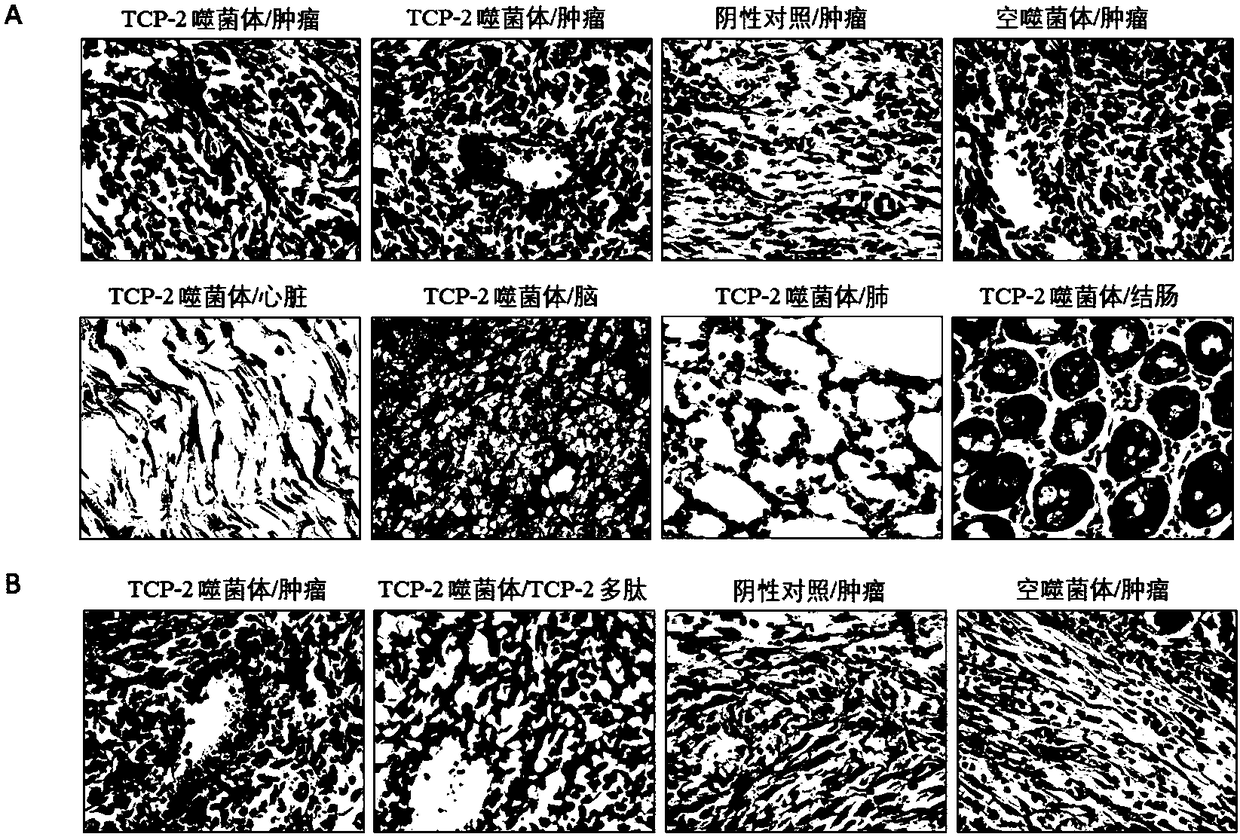
The invention belongs to the technical field of molecular biology and in particular relates to a tumor blood vessel and M1 type macrophage targeting peptide and application thereof. Aiming at the problem that a small molecular targeting peptide for preparing a medicine for diagnosing or treating tumors is lacked, the invention provides the tumor blood vessel and M1 type macrophage targeting peptide TCP-2 and a carrier thereof. The small molecular targeting peptide has an amino acid sequence shown as SEQ ID NO: 1, can be used for specifically targeting tumor blood vessels and M1 type macrophages and can be used for preparing the targeting medicine for diagnosing or treating the tumors; especially, the mall molecular targeting peptide is used for treating digestive tract tumors and providesa new choice for targeting therapy of the tumors.
Owner:SOUTHWEST MEDICAL UNIVERISTY
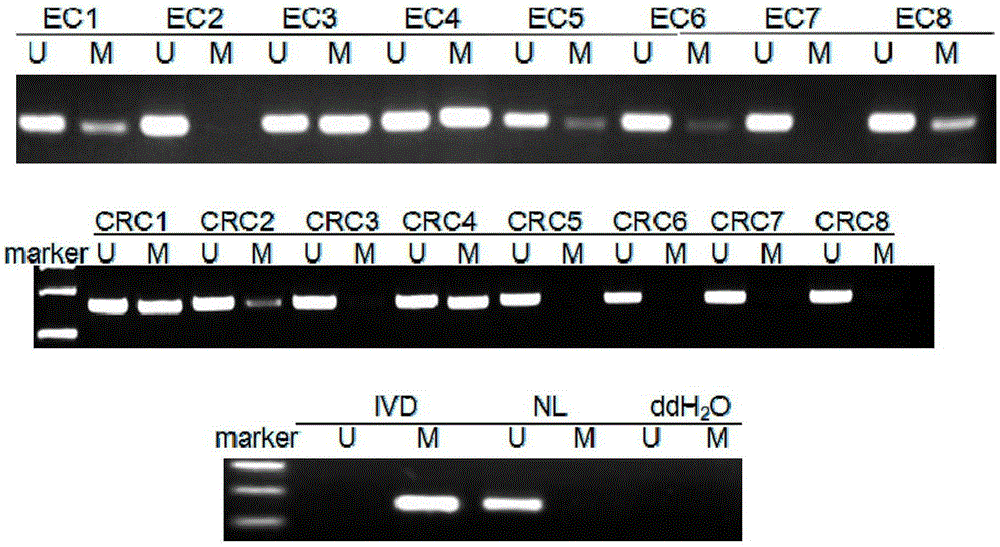
TMEM176A gene promoter region DNA methylation detection
ActiveCN105400865ASimple and fast operationImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationCancer cell
According to the present invention, TMEM176A gene is firstly adopted as a target gene, and the promoter region in esophageal cancer cells and colon cancer cells presents the high methylation state through a MSP technology; primers and a kit for detecting cell TMEM176A gene promoter region methylation state are provided, wherein the primers are a pair of methylation primers, or a pair of methylation primers and a pair of non-methylation primers; with the primers and the kit, the esophageal cancer detection specificity is good, and the sensitivity is high and achieves 0.5%, wherein the esophageal cancer detection specificity is 61.2%, the colorectal cancer detection specificity is 53.13%, and five cancer cells in 1000 cells can be detected; and the application of the kit to detect the TMEM176A gene promoter region DNA methylation state can be adopted as the powerful tool for digestive tumor diagnosis, treatment effect observation, prognosis determination, minimal residual disease detection and the like, and the advantages of easy operation, good stability, far-reaching clinical significance and promotion are provided.
Owner:GENERAL HOSPITAL OF PLA
Gastric cancer extremely-early cell marker, gastric cancer precancerous lesion early cell marker and application of markers in diagnostic kits
The inventor finds out a cell population (named as gastric cancer very early cells) which starts to appear in the low-grade heteroplasmal hyperplasia stage of the stomach, has specific molecular characteristics and has a high canceration risk, and the cell population can be used as a marker for very early diagnosis of gastric cancer. Molecular markers of gastric cancer extremely-early cells are applied to preparation of a kit for realizing early diagnosis of gastric cancer or other digestive system tumors based on gastric tissues or blood samples. The gastric cancer extremely-early cell markercan also distinguish the postoperative recurrence risk of gastric cancer and the tumor risk of digestive system organs such as intestines, pancreas and esophagus. Besides, the inventor also finds outa cell population which appears in the early stage of gastric precancerous lesions and has specific molecular characteristics, and the cell population can be used as a marker for early diagnosis of gastric precancerous lesions and is also integrated into the kit. The markers and kits can be used for early clinical diagnosis of gastric cancer and other digestive tract tumors, can also be used as an intervention target for prevention and treatment of the digestive tract tumors, and have a good application prospect.
Owner:TSINGHUA UNIV
Application of dronedarone hydrochloride in preparation of anti-digestive tract tumor drug
InactiveCN112137999AEnhanced inhibitory effectOrganic active ingredientsDigestive systemOncologyPharmacology
The invention discloses application of dronedarone hydrochloride in preparation of an anti-digestive tract tumor drug. The chemical name of dronedarone hydrochloride is N [2-butyl-3[4-[3- (dibutylamino) propoxy]phenyl]-5-benzofuran] methanesulfonamide. The application comprises the following steps: firstly, through the action of dronedarone hydrochloride on an esophageal cancer cell line, specifically evaluating the effect of dronedarone hydrochloride in an esophageal cancer in-vitro experiment. Secondly, through the action of dronedarone hydrochloride on the gastric cancer cell line, specifically evaluating the in-vitro inhibition effect of dronedarone hydrochloride on the gastric cancer cell line, and comprehensively evaluating the effect of dronedarone hydrochloride in digestive tract tumors. Results show that dronedarone hydrochloride with a proper concentration has a good inhibition effect in a digestive tract tumor cell line, and a new thought and a new basis are provided for treatment and prevention of tumors.
Owner:ZHENGZHOU UNIV
Anti-cancer preparation-kangaiping, new preparing method therefor
The present invention relates to a Chinese medicine composition and its preparation process. In particular, it relates to a Chinese medicine prescription for curing the malignant tumor of digestive tract, including carcinoma of stomach, cardiac cancer, carcinoma of esophagus and rectal cancer, etc. and its preparation process. It can be made into dripping pills and soft capsule preparation.
Owner:FUKANGREN BIO PHARMA
Digestive tract tumor marker combination, detection kit and application thereof
ActiveCN112646891AEasy to coverHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesOncologyBiology
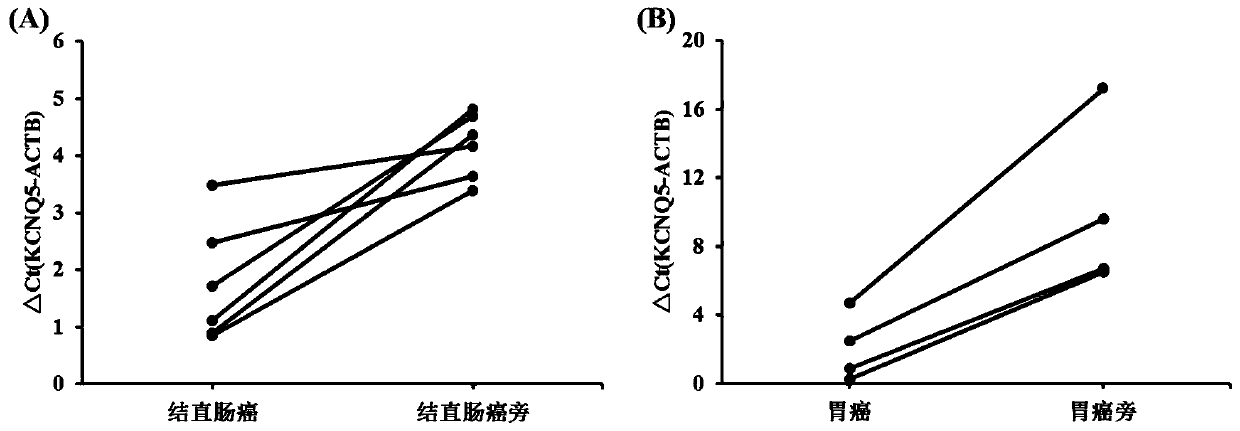
The invention belongs to the field of biomedicine, and particularly relates to a digestive tract tumor marker combination, a detection kit and application thereof. Marker genes comprise methylated KCNQ5, C9orf50, CLIP4, ZNF582, TFPI2 and ELMO1; and the sequences of the methylated KCNQ5, C9orf50, CLIP4, ZNF582, TFPI2 and ELMO1 are shown as SEQ ID NO.34-SEQ ID NO.39 respectively. According to the invention, a non-invasive method capable of simultaneously detecting esophageal cancer, gastric cancer and colorectal cancer, a marker composition and a use method thereof are developed, and a new technology for early prevention and control of digestive tract tumors is provided.
Owner:SUZHOU VERSABIO TECH INC
Antibody for resisting anti-cancer embryo antigen as well as preparation method and application thereof
ActiveCN110862456AIncrease lethalityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsComplementarity determining regionSingle-Chain Antibodies
The invention provides an anti-CEA antibody. The anti-CEA antibody comprises a heavy chain variable region and a light chain variable region, wherein a complementary determining region of the heavy chain variable region comprises CDR-H1 with an amino acid sequence shown as SEQ ID No. 1, CDR-H2 with an amino acid sequence shown as SEQ ID No. 2 and CDR-H3 with an amino acid sequence shown as SEQ IDNo. 3; a complementation determining region of the light chain variable region comprises CDR-L1 with an amino acid sequence shown as SEQ ID No. 4, CDR-L2 with an amino acid sequence shown as SEQ ID No. 5 and CDR-L3 with an amino acid sequence shown as SEQ ID No. 6. The CEA scFv is screened by an inventor of the invention by utilizing a phage display technology, so that the CEA-resistant single-chain antibody with high affinity is obtained. An anti-CEA chimeric antigen receptor gene is introduced into T cells through a genetic engineering method to prepare CEA CAR-T, so that the CEA CAR-T cellsspecifically recognize digestive tract tumor cells expressing CEA and kill the digestive tract tumor cells, and the anti-tumor effect of the CEA CAR-T cells is achieved.
Owner:HUADAO SHANGHAI BIOPHARMA CO LTD
Antibody resisting carcinoembryonic antigen and preparation method and application of antibody
ActiveCN110655581AIncrease lethalityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsComplementarity determining regionSingle-Chain Antibodies
The invention provides a CEA resisting antibody. The CEA resisting antibody comprises a heavy chain variable region and a light chain variable region, wherein the complementary determining region of the heavy chain variable region comprises CDR-H1 of which the amino acid sequence is shown as SEQID No.1, CDR-H2 of which the amino acid sequence is shown as SEQID No.2 and CDR-H3 of which the amino acid sequence is shown as SEQID No.3, and the complementary determining region of the light chain variable region comprises CDR-L1 of which the amino acid sequence is shown as SEQID No.4, CDR-L2 of which the amino acid sequence is shown as SEQID No.5 and CDR-L3 of which the amino acid sequence is shown as SEQID No.6. The inventor uses a phage display technique to screen CEA scFv, so as to obtain a high-affinity CEA resisting single-chain antibody. The chimeric antigen receptor gene for resisting CEA is transmitted into T cells by a genetic engineering method to prepare CEA CAR-T, so that the digestive tract tumor cells of CEA can be specially recognized and expressed by CEA CAR-T cells, and are killed, and the effect of resisting tumors can be realized.
Owner:HUADAO SHANGHAI BIOPHARMA CO LTD
Method for detecting imatinib metabolite in plasma of GIST patient based on non-targeted metabonomics
ActiveCN113406226AHigh sensitivityClear peak shapeComponent separationVenous bloodPrincipal component analysis
The invention discloses a method for detecting imatinib metabolite in plasma of GIST patients based on non-targeted metabonomics, which comprises the following steps: collecting peripheral venous blood of a plurality of GIST patients who do not take IM or take IM regularly for a long time, processing the peripheral venous blood, performing non-targeted detection on a sample to be detected by adopting a UPLC-QTOF / MS (Ultra Performance Liquid Chromatography-Quadrature Time of Flight / Mass Spectrometry) combined technology to obtain original data of the metabolite in the plasma, performing unsupervised principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLS-DA), Student's t-test and difference multiple analysis (FoldChange) on original data, searching differential metabolites, and searching a database to qualitatively determine the GIST metabolic marker. Technical support is provided for effective treatment of GIST patients suitable for IM treatment, and a foundation can be laid for effectiveness, pertinence, noninvasive screening and marker characterization of drug treatment after confirmation of digestive tract tumors.
Owner:SICHUAN CANCER HOSPITAL
Primer and probe set for diagnosis, detection or screening of digestive tract cancer
ActiveCN108796078AEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationForward primerBiological organism
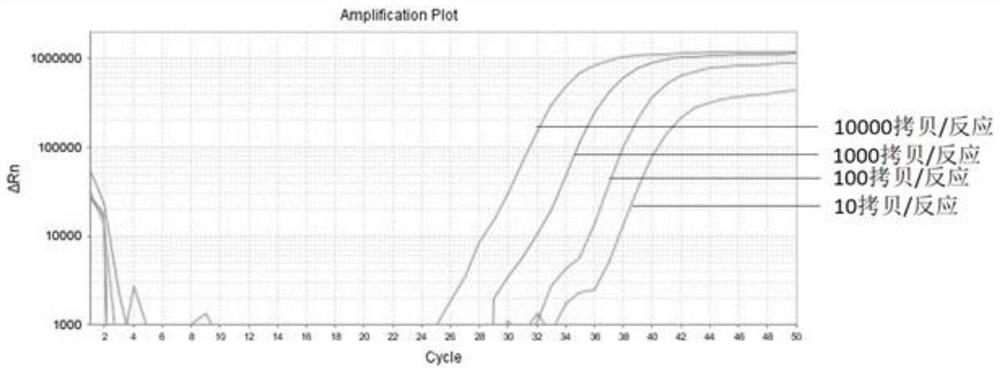
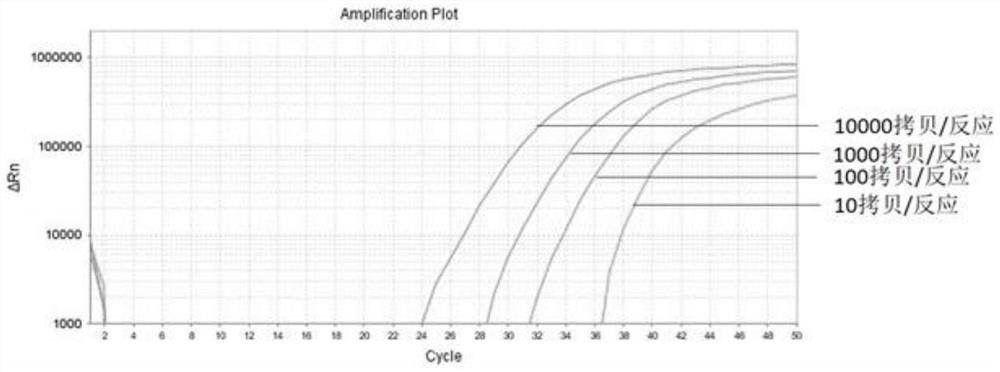
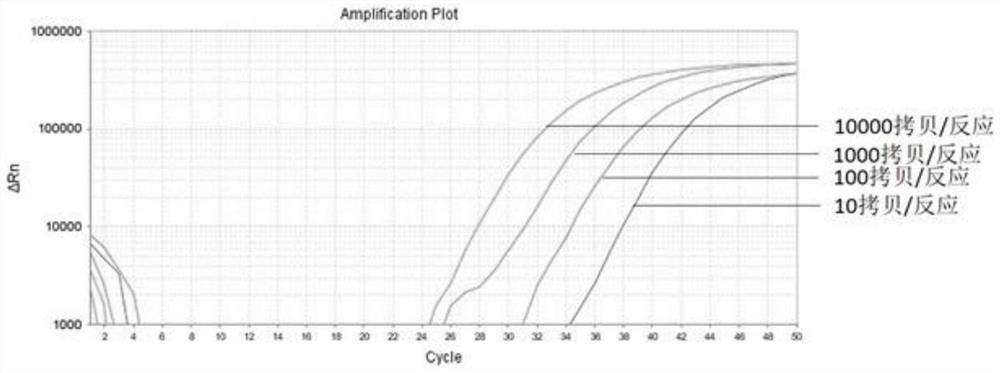
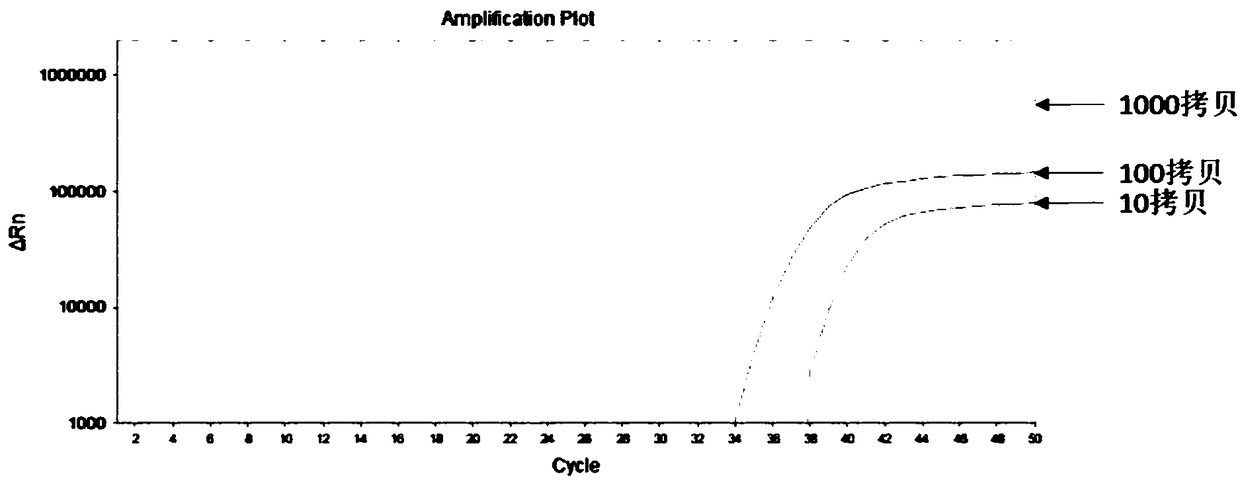
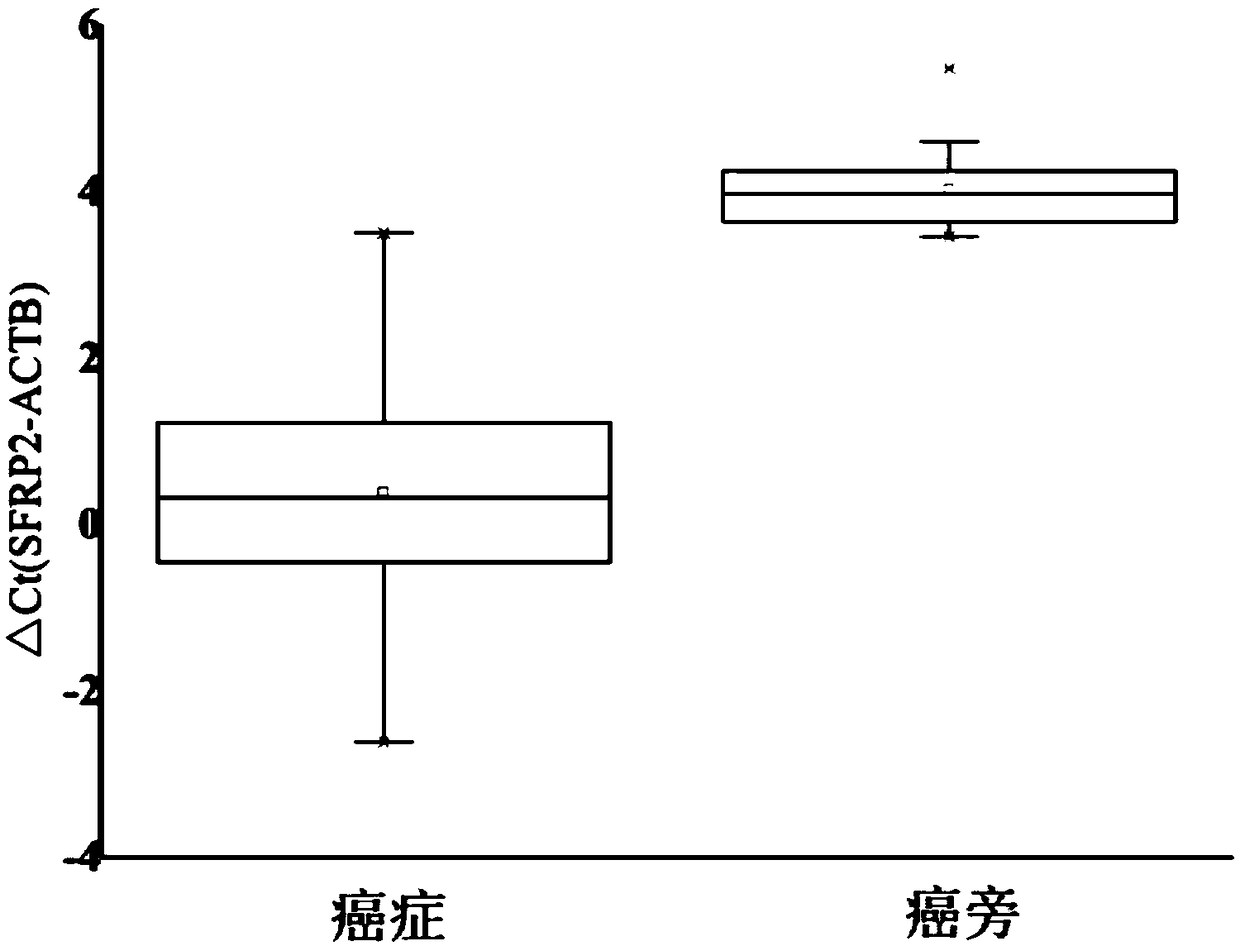
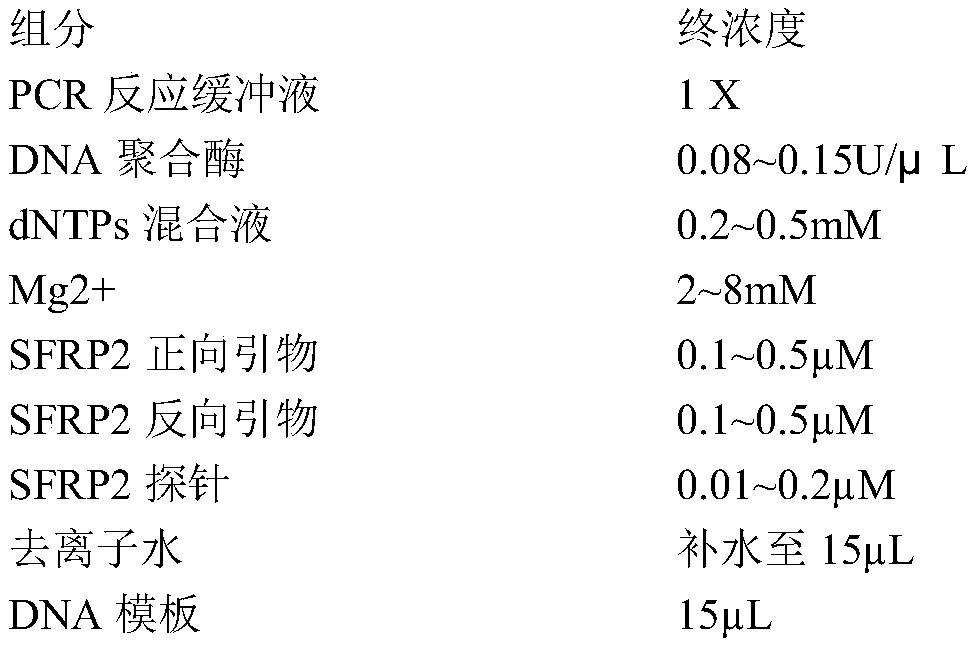
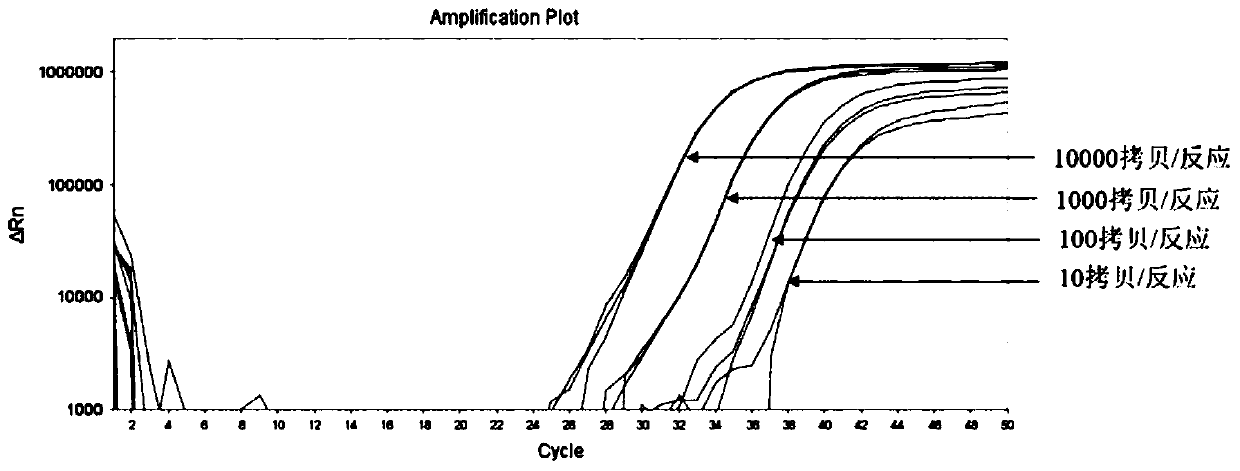
The invention belongs to the field of biomedicine and relates to a primer and probe set for diagnosis, detection or screening of digestive tract cancer. Primers include a forward primer and a reverseprimer, and probes are SERP2 probes, wherein the forward primer of SERP2 includes any one sequence of SEQ ID: 1-16, and the reverse primer of SERP2 includes any one sequence of SEQ ID: 17-30. Comparedwith the prior art, the primer and probe set has the beneficial effects of by extracting DNA in a biological sample, whether the biological sample contains methylated SFRP2 gene is detected by a fluorescent quantitative PCR technique, thereby determining whether or not a risk of digestive tract cancer exists; the SFRP2 methylation sequence can be detected very well, and the detection sensitivitycan reach 10 copies / reaction; the methylated SFRP2 gene can be used for clearly distinguishing cancer samples and normal samples of colorectal cancer, gastric cancer, esophageal cancer and other digestive tract cancer.
Owner:SUZHOU VERSABIO TECH INC
Application of mozavaptan in preparation of anti-digestive tract tumor drugs
ActiveCN112121051AInhibition of clonogenicityInhibit apoptosisAntineoplastic agentsHeterocyclic compound active ingredientsTumor therapyApoptosis
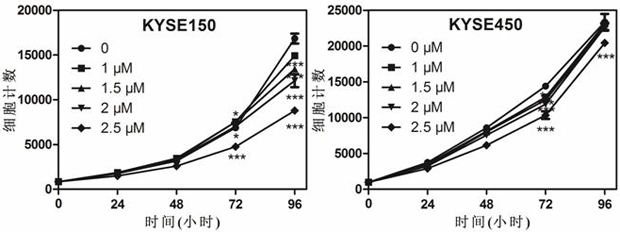
The invention discloses an application of mozavaptan in preparation of anti-digestive tract tumor drugs, the chemical name of the mozavaptan is 5-(dimethylamino)-1-[4-(2-methylbenzamido)benzoyl]-2,3,4,5-tetrahydro-1H-benzazephe, and experiments prove that the mozavaptan can inhibit esophageal squamous cell carcinoma cells KYSE150 and KYSE450, proliferation of gastric cancer cells HGC27 and AGS, clone formation of esophageal squamous carcinoma cells and induce the cell apoptosis. Therefore, it is shown that mozavaptan has a remarkable inhibition effect on esophageal squamous cell carcinoma cells. Therefore, the mozavaptan can be used as an existing clinical medicine to play a role in preventing and treating tumors. The invention provides a new application of mozavaptan in tumor treatment and prevention.
Owner:ZHENGZHOU UNIV
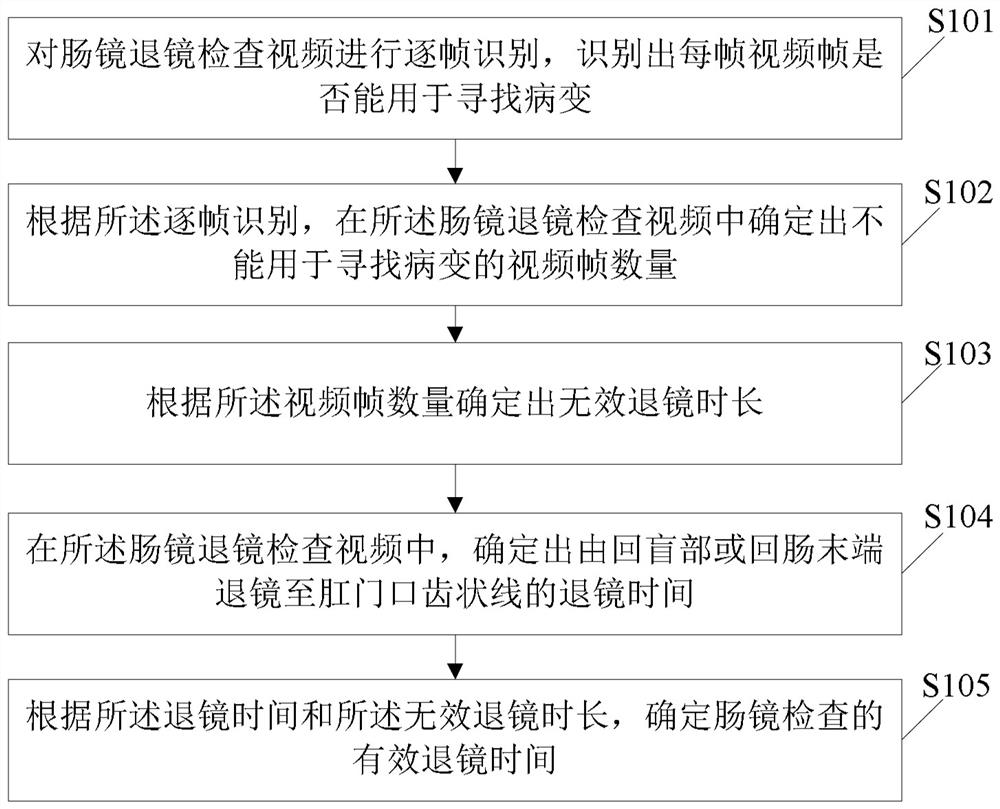
Effective mirror retreating time evaluation method and device for enteroscopy and storage medium
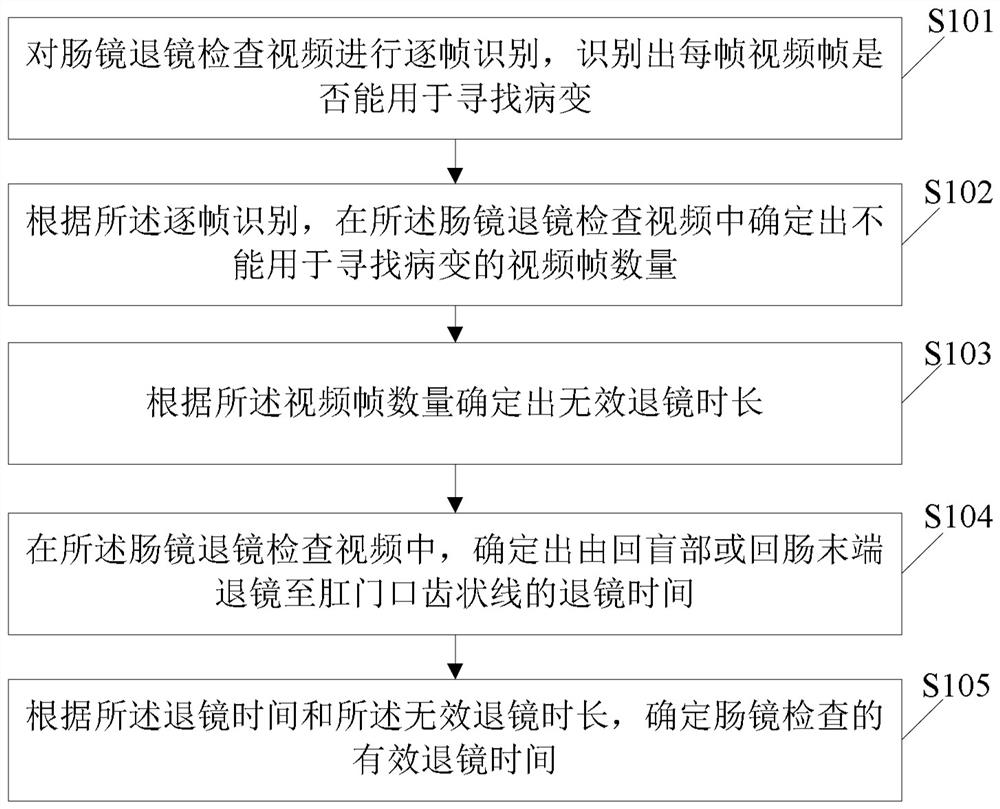
InactiveCN113962998AEffective assessment of qualityImprove the detection rateImage enhancementImage analysisDiseaseEngineering
The invention relates to an effective mirror retreating time evaluation method and device for enteroscopy and a storage medium. The effective retreating time evaluation method for enteroscopy comprises the following steps: performing frame-by-frame identification on an enteroscopy retreating video, and identifying whether each video frame can be used for finding a lesion or not; according to the frame-by-frame identification, determining the number of video frames which cannot be used for searching lesions in the enteroscopy retroscopy video; according to the number of the video frames, determining an invalid mirror retreating time length; in the enteroscope retreating examination video, determining the retreating time from the ileocecal part or the ileum tail end to the anal tooth-shaped line; and according to the mirror retreating time and the invalid mirror retreating duration, determining the effective mirror retreating time of enteroscopy. According to the application, the quality of colonoscopy can be evaluated more effectively, and the detection rate of digestive tract diseases and digestive tract tumors can be improved in an auxiliary manner.
Owner:TIANJIN YUJIN ARTIFICIAL INTELLIGENCE MEDICAL TECH CO LTD
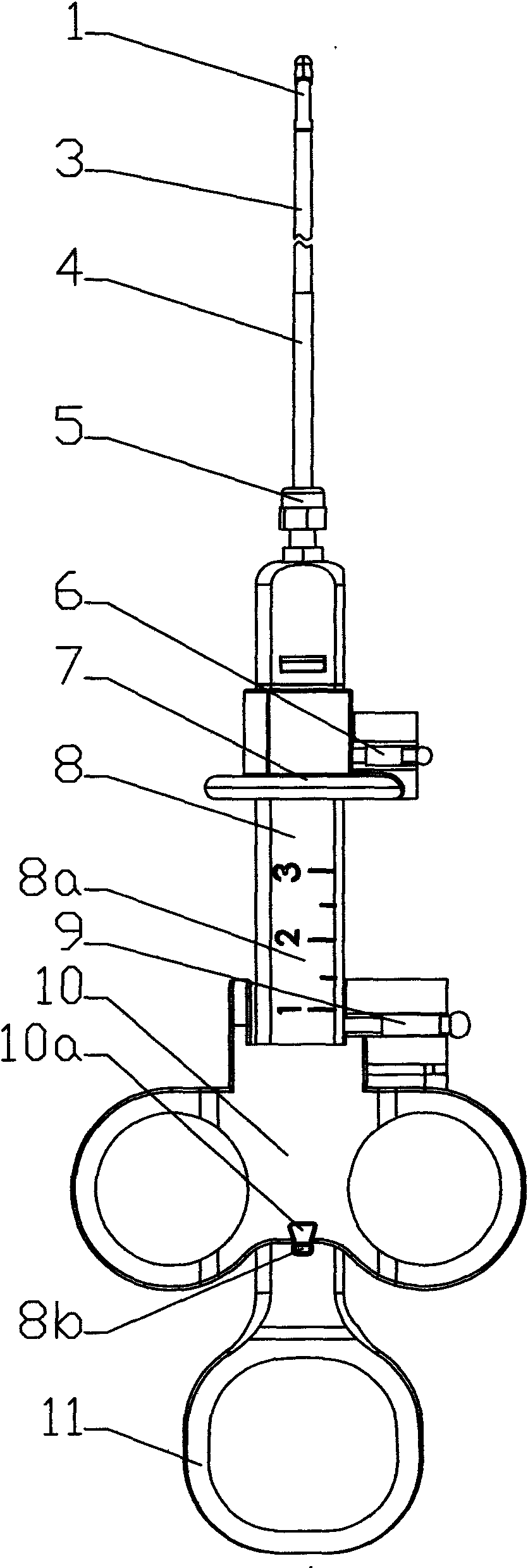
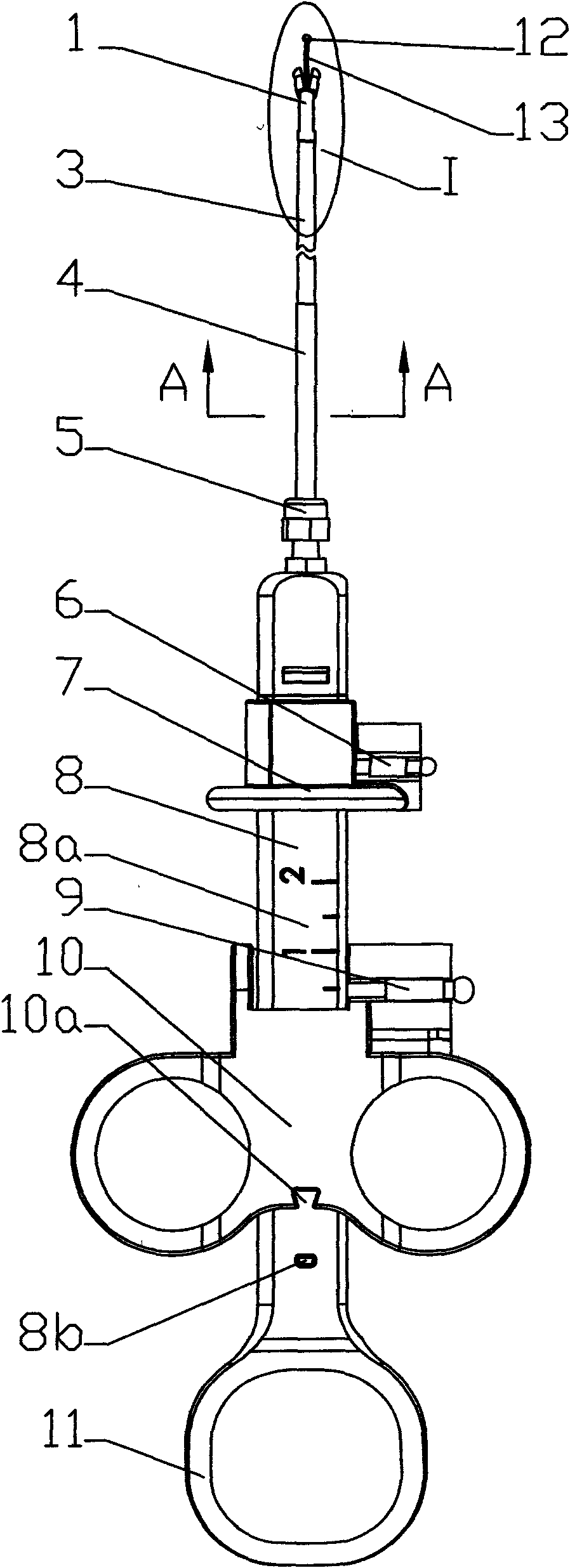
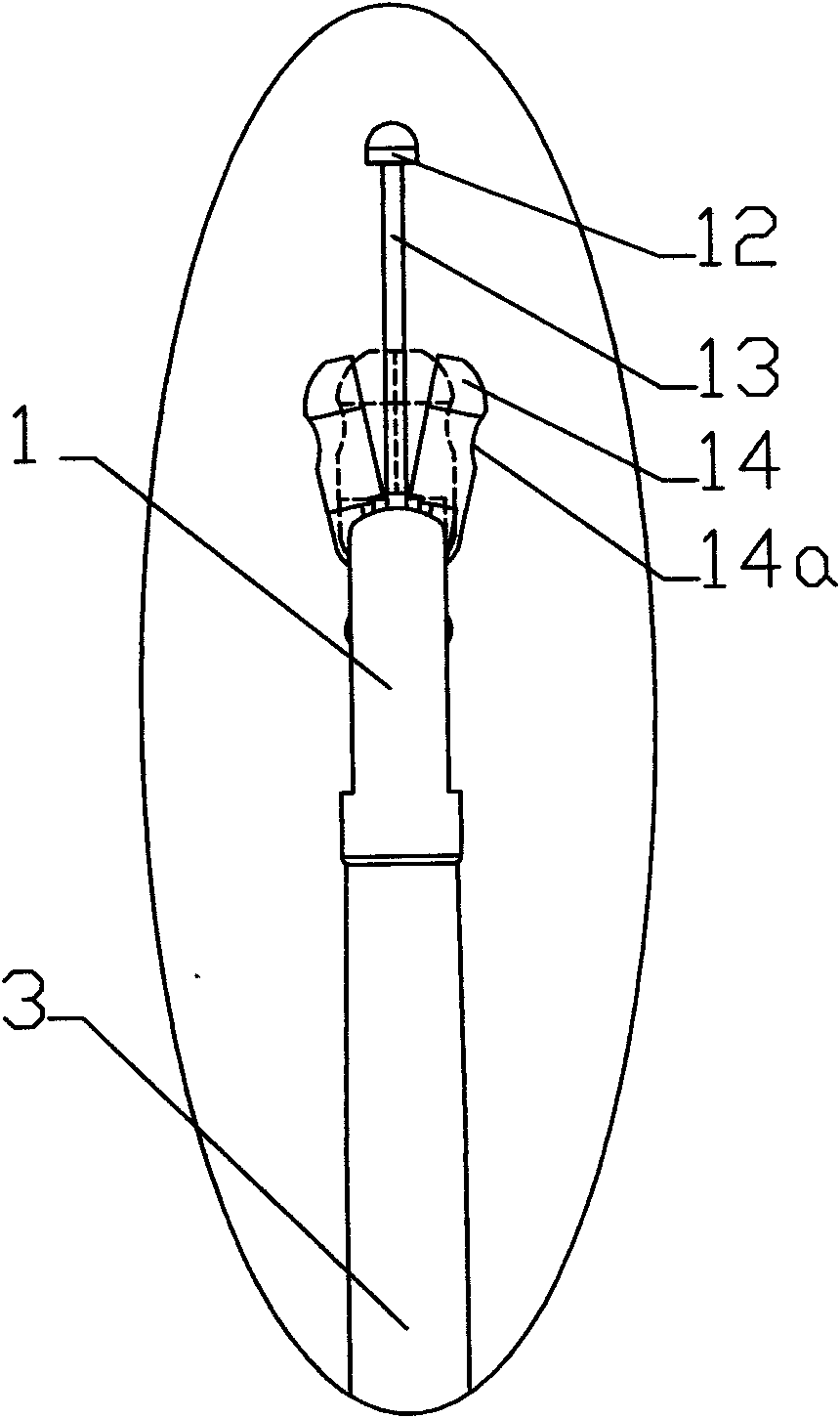
Sampler special for biopsy of digestive tract tumor
InactiveCN109998602AThe sampling process is clear and accurateWon't hurtSurgeryVaccination/ovulation diagnosticsAbnormal tissue growthMedicine
The invention relates to the technical field of medical treatment, in particular to a sampler special for biopsy of the digestive tract tumor. The sampler comprises a catheter, a sampling box, a sampling mechanism and a pair of clamping cutters. The sampling box is arranged on the head of the catheter through two rotation shafts, a sampling port is formed in the cylinder face of the head of the catheter, and the head of the sampling box fits the sampling port of the catheter; the two rotation shafts penetrate through the sampling box and are inserted in the two ends of the sampling port in thelength direction, a pair of clamping cutters are symmetrically arranged on the head of the sampling box, the tail of each clamping cutter can rotatably sleeve the rotation shaft at the correspondingposition, and the sampling mechanism is arranged in the sampling box and the catheter. The sampler is simple in structure and convenient to operate, all the structures are located in the catheter or fit the outer wall of the catheter, the digestive tract cannot be injured, and during sampling, the clamping cutters clamp and cut the sampling tissue, so that the sampling tissue does not easily falldown and is not easily lost.
Owner:HENAN PROVINCE HOSPITAL OF TCM THE SECOND AFFILIATED HOSPITAL OF HENAN UNIV OF TCM
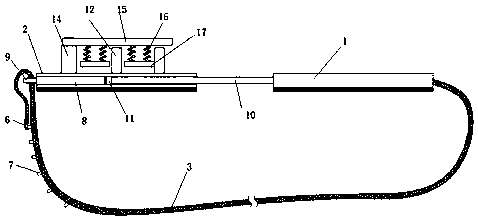
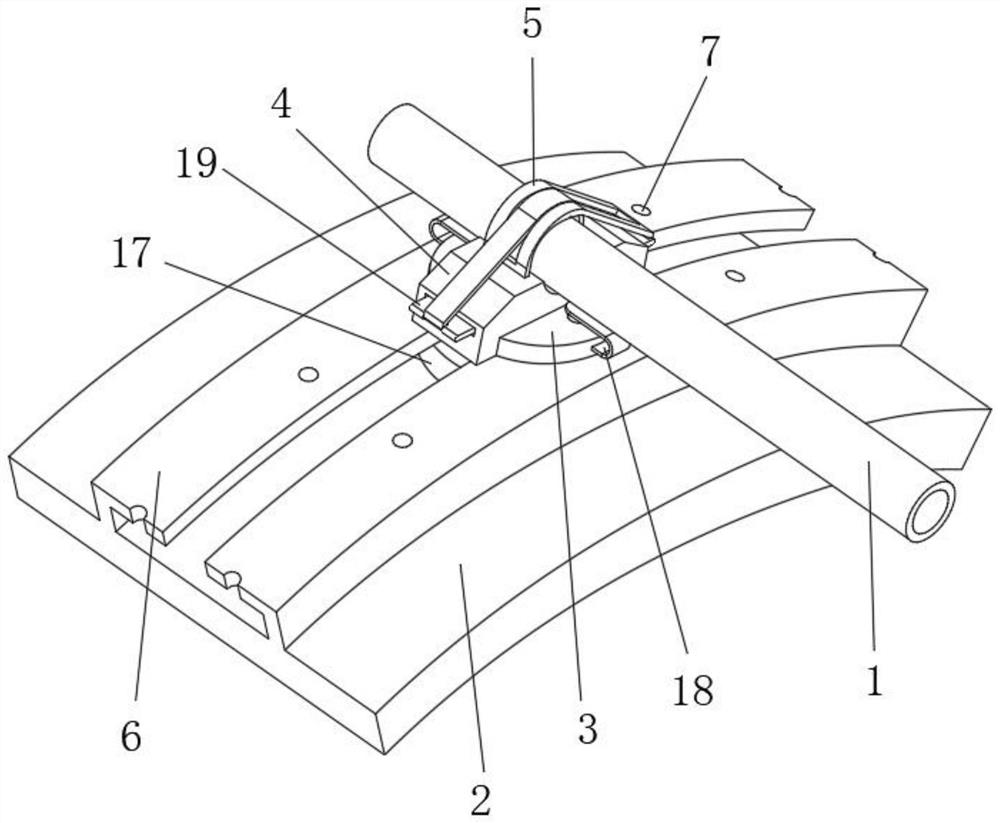
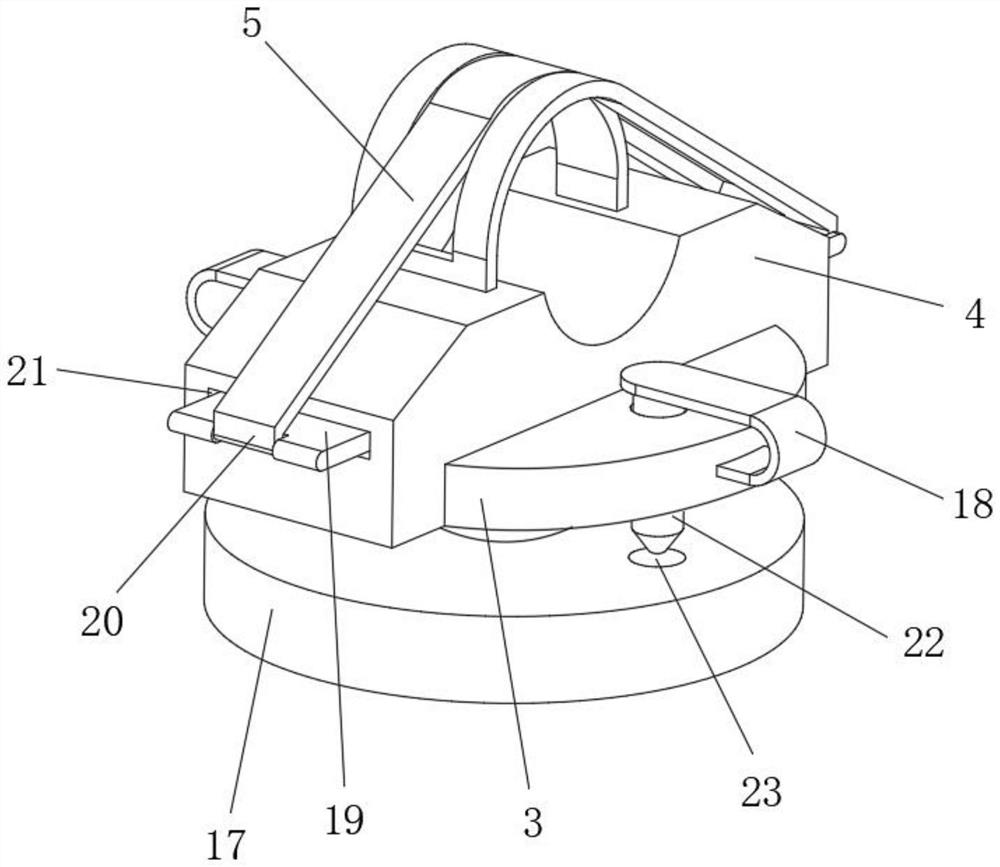
Abdominal cavity drainage fixing device for digestive tract tumor internal medicine
InactiveCN110841175ARelieve painAvoid shakingCatheterIntravenous devicesAbdominal cavityDrainage tubes
The invention relates to the field of medical instruments, and particularly discloses an abdominal cavity drainage fixing device for digestive tract tumor internal medicine, which comprises a first fixing plate, a second fixing plate and a tightening belt, wherein a first semicircular groove opening is formed in the middle on one side of the first fixing plate, one end of the tightening belt is connected to the other side of the first fixing plate, hooks are fixedly connected to the other end of the tightening belt, hanging rings are formed at one end, close to the hooks, of the tightening belt at intervals along the length direction of the tightening belt, a second semicircular groove opening is formed in the middle on one side of the second fixing plate, and a rectangular limiting framewith a hollow square shape is fixedly arranged on the other side of the second fixing plate. The first fixing plate and the second fixing plate are butted with each other, a drainage tube passes through a circular opening formed by the first semicircular groove opening and the second semicircular groove opening and is wound around two vertical limiting columns in an S shape, and a limiting and pressing device presses and fixes the drainage tube to prevent the drainage tube from shaking, and thus the drainage efficiency is ensured, and the pain of patients is reduced, so that the device is suitable for popularization and use.
Owner:SHANDONG RES INST OF TUMOUR PREVENTION TREATMENT
Preparation method of rabdosia amethystoides fermentation preparation and application thereof in anti-tumor treatment
InactiveCN113151037ARelease fullyEfficient releaseBacteriaPharmaceutical delivery mechanismGut floraFermentation
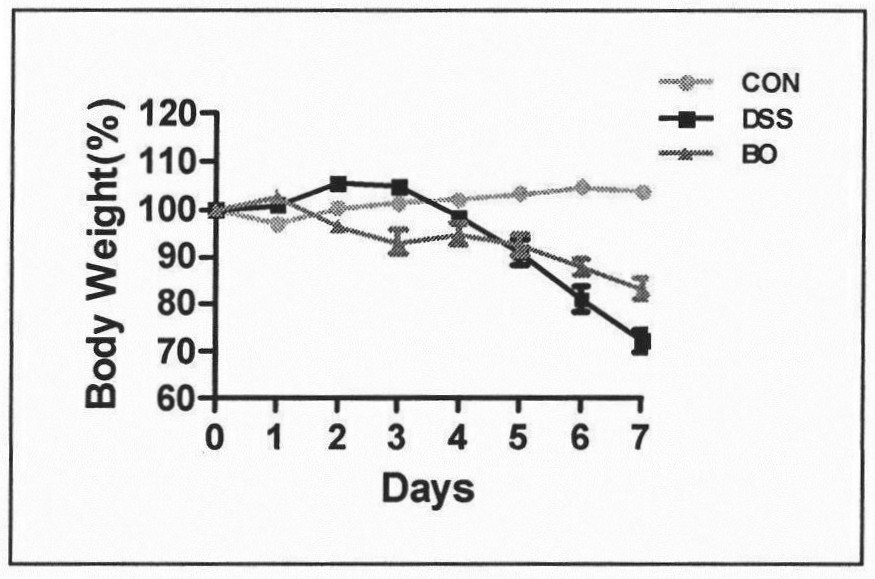
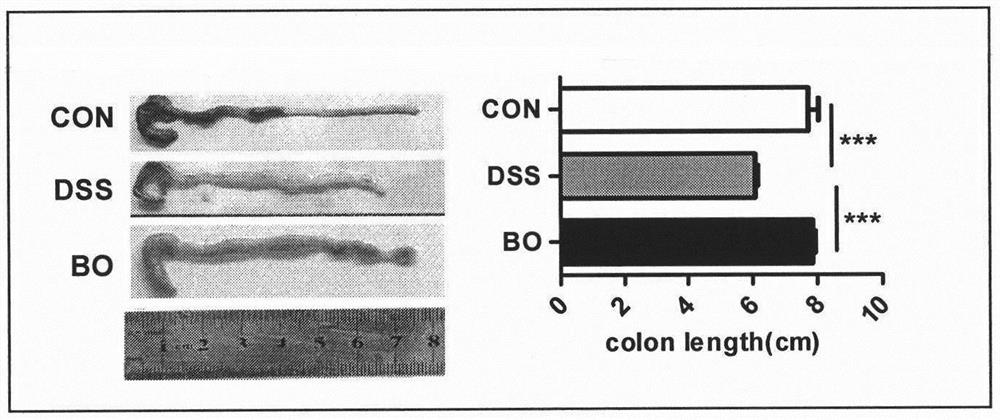
The invention belongs to the technical field of microbial fermentation of traditional Chinese medicines, and discloses a preparation method of a rabdosia amethystoides preparation fermented by edible fungi and an active effect of the rabdosia amethystoides preparation in resisting digestive tract tumors. The method is characterized in that according to the physicochemical properties of the rabdosia amethystoides, bacillus subtilis (preserved in the China General Microbiological Culture Collection Center with the CGMCC number of 1.15792) is determined as a zymophyte main body by screening food strains, the rabdosia amethystoides is fermented according to a dosage ratio and an optimal culture condition, and the efficient preparation method of the rabdosia amethystoides fermentation preparation is provided. Experiments prove that the bacillus subtilis can effectively degrade crude fiber components of a rabdosia amethystoides plant, active substances of the rabdosia amethystoides plant is fully released, and prepare a rabdosia amethystoides bacteria agent oral liquid. In a mouse AOM / DSS induced colorectal cancer model, the result shows that the oral liquid can inhibit tumor generation and development of a model mouse, enhance the anti-tumor immune effect of the body, prolong the life cycle of the model mouse and improve intestinal flora disorder of the model mouse, and has important application value in digestive tract tumor immunotherapy.
Owner:NANJING UNIV
Primer probe group, kit, detection method and application for alimentary canal tumor marker detection
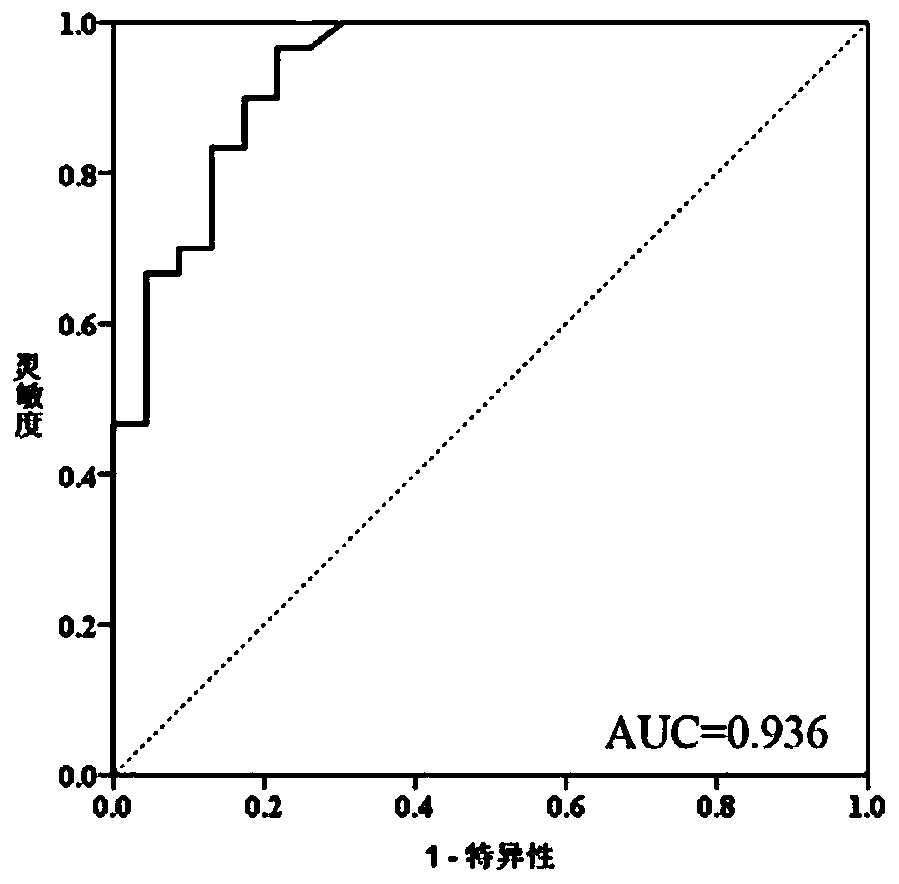
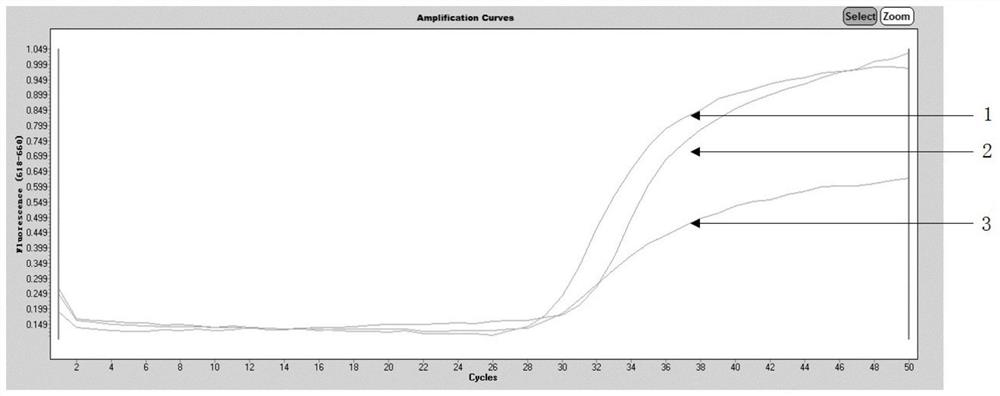
The invention discloses a primer probe group, a kit, a detection method and application for alimentary canal tumor marker detection, and belongs to the technical field of biological medicines. A disclosed marker is a methylated KCNQ5 gene and has at least one modified CpG locus, and the detection method comprises the following steps: extracting DNA in a human-derived biological sample; convertingthe extracted DNA through methylated bisulfite, and converting the unmethylated cytosine C into uracil U; carrying out methylation real-time fluorescent quantitative PCR reaction by adopting the kit;and interpreting according to the Ct value of the KCNQ5 gene, and judging whether the subject suffers from digestive tract tumor or not. The kit is used for detecting specific DNA methylation markersin biological samples of high-risk groups and patients with malignant tumors of digestive tracts, and a high-sensitivity and non-invasive detection means can be provided for early screening, early diagnosis and personalized treatment of malignant tumors of digestive tracts.
Owner:SUZHOU VERSABIO TECH INC
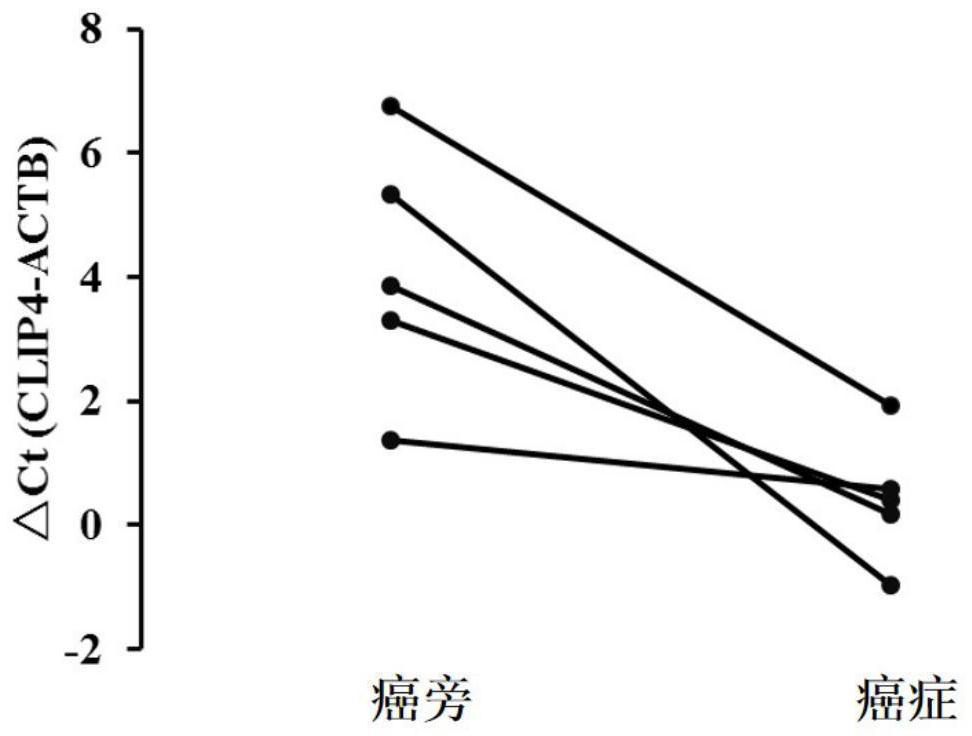
Primer probe set and reagent kit for detecting digestive tract tumor marker and application of reagent kit
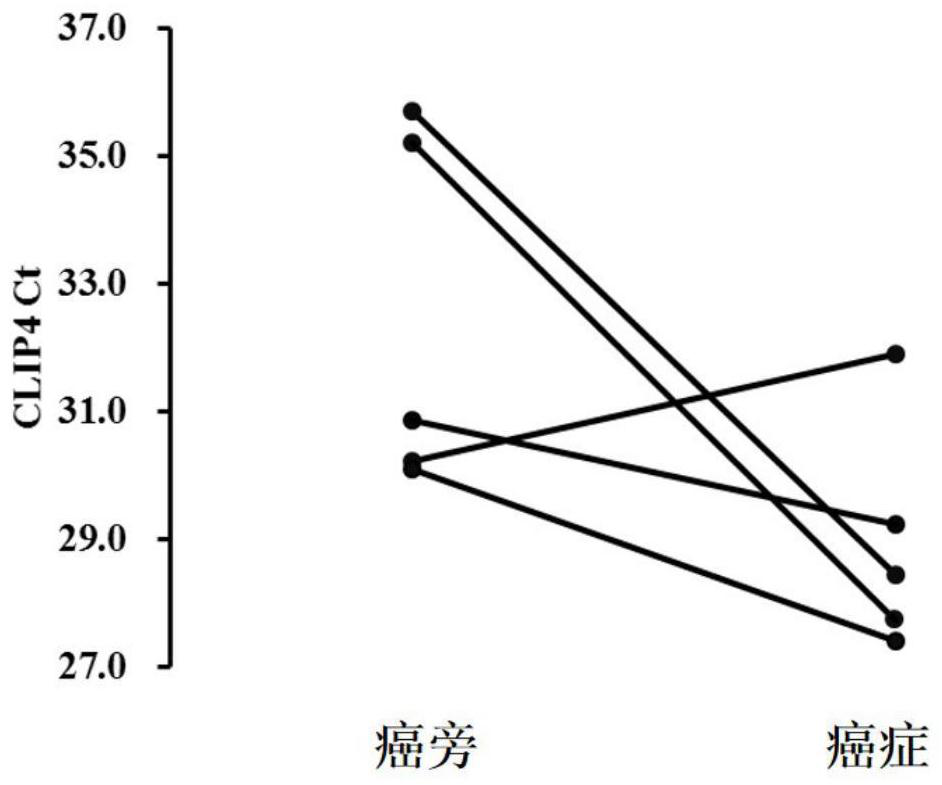
PendingCN111793688AMicrobiological testing/measurementDNA/RNA fragmentationCpG siteIndividualized treatment
The invention discloses a digestive tract tumor marker. The maker is a methylated CLIP4 gene, and has one or more modified CpG sites. The invention further discloses a primer probe set and reagent kitfor detecting the digestive tract tumor marker. The sequence of the primer probe set comprises one or more base sites being in complementary pairing with the modified CPG sites. The digestive tract tumor maker is used for detecting a CLIP4 gene of the special DNA methylation marker in biology samples of digestive tract malignant tumor high-risk groups and patients, and a high sensitivity non-invasive detection means is provided for early screening, early diagnosis and individualized treatment for digestive tract malignant tumors.
Owner:SUZHOU VERSABIO TECH INC
Gastrointestinal tumor cell collector
ActiveCN108245200BEasy to collectEasy to useSurgical needlesVaccination/ovulation diagnosticsMedical equipmentAnatomy
The invention discloses a digestive tract tumor cell collector, which relates to the technical field of medical equipment and includes an upper cell collection room, a gastric cell collection room, an intestinal cell collection room and a general controller, the upper cell collection room, the Both the gastric cell collection chamber and the intestinal cell collection chamber are in the shape of a three-dimensional crescent, and the three together form an ellipsoid. A master controller is arranged inside the ellipsoid, and a soluble capsule is wrapped outside the upper cell collection chamber. Shell, the soluble capsule shell is equipped with an electric door, and a pressure sensor is installed on the surface of the electric door. A first chamber is arranged inside the electric door, and a circular groove is arranged on the outer surface of the first chamber. The invention not only can well collect tumor cells in different parts of the digestive tract, but also is convenient to use, has good detection effect, has no side effects, and does not cause patients to suffer great pain, so it is very suitable for collecting tumor cells in the digestive tract.
Owner:THE THIRD AFFILIATED HOSPITAL OF XINXIANG MEDICAL UNIV
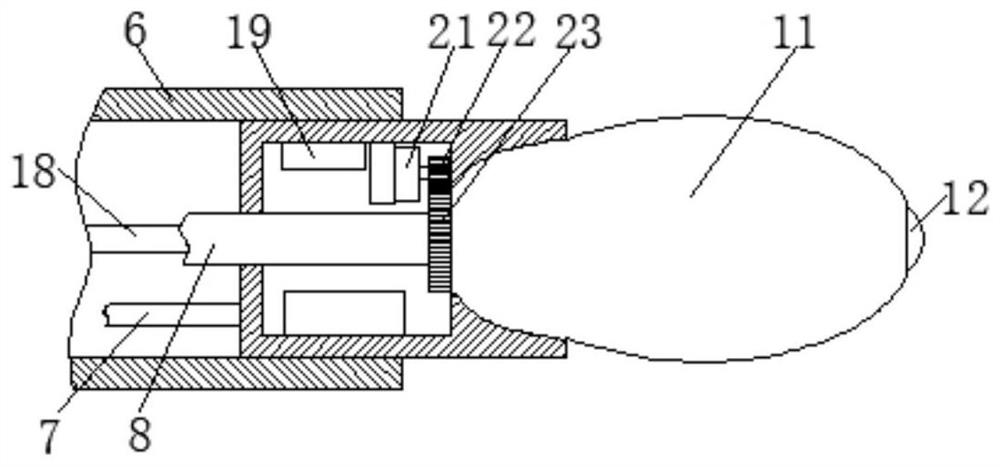
Biopsy sampler for digestive tract tumor
InactiveCN113057687AEasy to cleanEasy to sampleSurgical needlesVaccination/ovulation diagnosticsVentilation tubeAnatomy
The invention provides a biopsy sampler for a digestive tract tumor, belonging to the technical field of medical instruments. The biopsy sampler for the digestive tract tumor comprises a placing plate, wherein the upper surface of the placing plate is provided with a display device and an inflation device, the lower surface of the placing plate is fixedly connected with an outer tube, the outer tube is internally provided with a connecting steel wire and a ventilation tube, the ventilation tube is internally provided with a cable conductor, one end of the connecting steel wire is fixedly connected with a pull ring, and the other end of the connecting steel wire is fixedly connected with a connecting base. Under the action of an inner support frame, an ellipsoidal air bag is greatly deformed at an elastic bag, so a sampling blade drives the fine hair of insoluble dietary fiber to move out of a sampling groove so as to clean dirt on the surface of the tumor, and then a soluble dietary fiber adhesion layer is dissolved, so the fine hair of the insoluble dietary fiber is separated and enters a digestive tract; after the ellipsoidal air bag is deflated, the elastic bag retracts, the sampling blade cuts the tumor and brings the tumor into the elastic bag, and then the tumor can be taken out after inflation.
Owner:邵珠琳
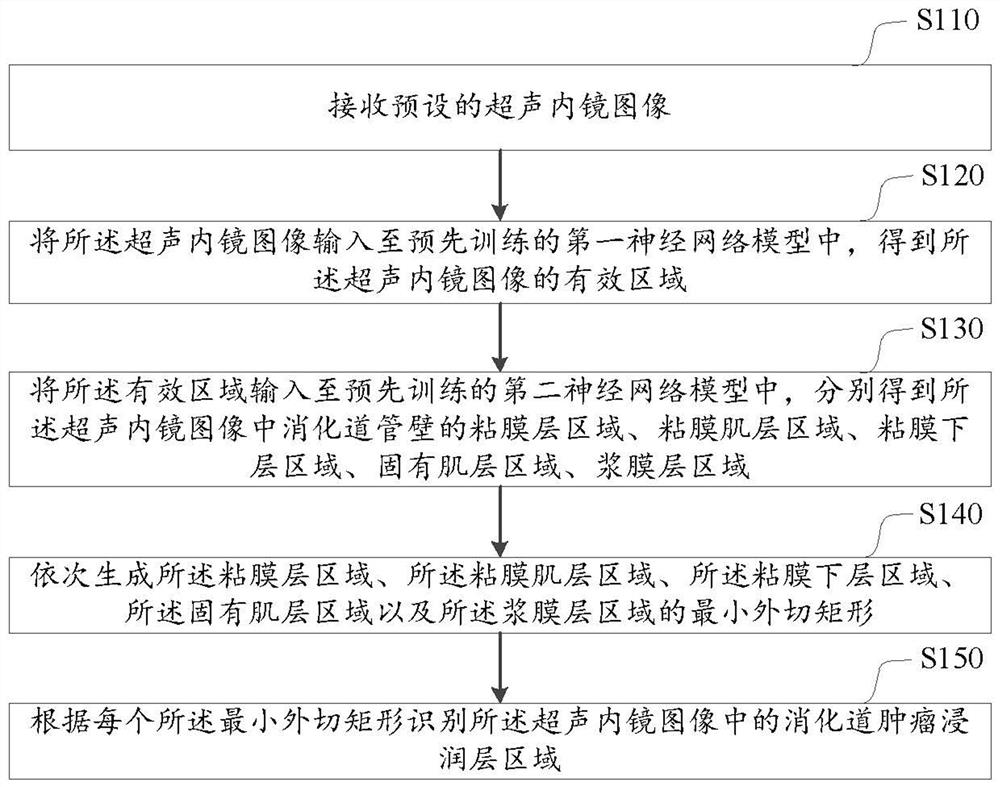
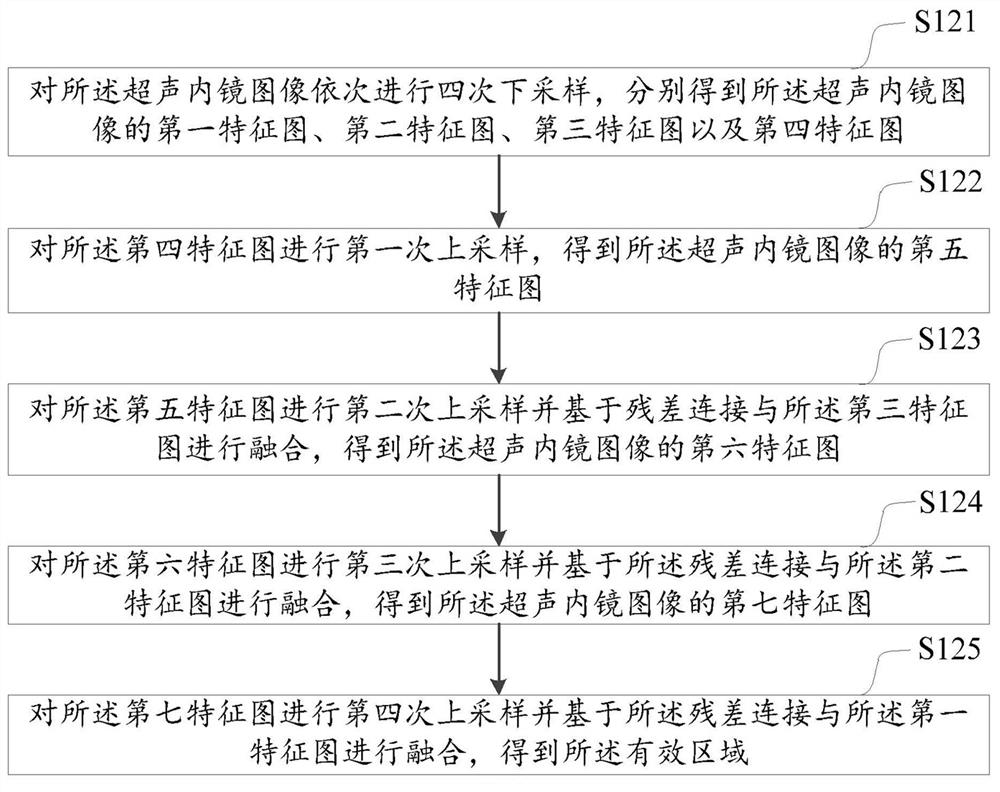
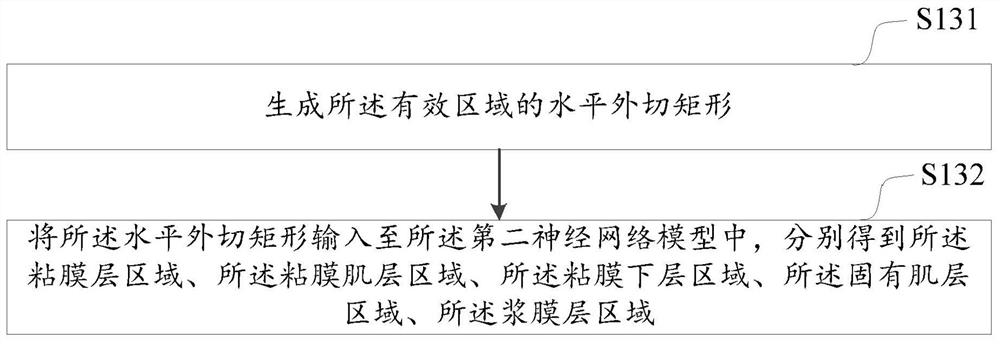
Digestive tract tumor infiltration layer identification method and device, computer equipment and medium
ActiveCN113793335AImprove accuracyAccurate identificationImage analysisCharacter and pattern recognitionMuscle layerSubmucosa
The embodiment of the invention discloses a digestive tract tumor infiltration layer identification method and device, computer equipment and a medium. The method comprises the following steps: inputting a received ultrasonic endoscope image into a pre-trained first neural network model to obtain an effective area of the ultrasonic endoscope image; inputting the effective area into a pre-trained second neural network model to respectively obtain a mucosa layer area, a mucosa muscle layer area, a submucosa layer area, an innate muscle layer area and a serosa layer area of the digestive tract wall in the ultrasonic endoscope image; sequentially generating minimum circumscribed rectangles of the mucosa layer area, the mucosa muscle layer area, the submucosa layer area, the innate muscle layer area and the serosa layer area; and identifying the alimentary canal tumor infiltration layer area in the ultrasonic endoscope image according to each minimum circumscribed rectangle. According to the method, the digestive tract tumor infiltration layer area of the ultrasonic endoscope image is accurately identified by adopting an artificial intelligence technology, and the accuracy of identifying the digestive tract tumor infiltration layer is improved.
Owner:WUHAN UNIV
Hericium erinaceus solid beverage
InactiveCN111493262AGuaranteed sizeGood control effectFood ingredient functionsOrganic compound food ingredientsBiotechnologyDigestive canal
The invention discloses a hericium erinaceus solid beverage. The hericium erinaceus solid beverage is prepared from the following raw materials in parts by weight: 4-5 parts of black hericium erinaceus, 2-3 parts of tremella fuciformis, 15-20 parts of stevioside, 2-3 parts of thiamine hydrochloride, 2-3 parts of riboflavin, 2-3 parts of pyridoxine hydrochloride and the balance of water. Accordingto the invention, the particle size is ensured, the functional components can be fully absorbed by a human body, a health care effect is obtained, hericium erinaceus is adopted as a main raw material,and every 100 g of dry hericium erinaceus contains 26.3 g of a protein, 4.2 g of fat and 856 mg of phosphorus, carbohydrate, calcium, iron, carotene, vitamin B1, vitamin B2 and other components are further used, protein contains various amino acids, the digestive tract tumor treatment effect is good, and thiamine hydrochloride serves as a nutritional supplement to participate in carbohydrate intermediate metabolism in vivo.
Owner:陈靓
Sterile liquid food production device for digestive tract tumor patients to eat
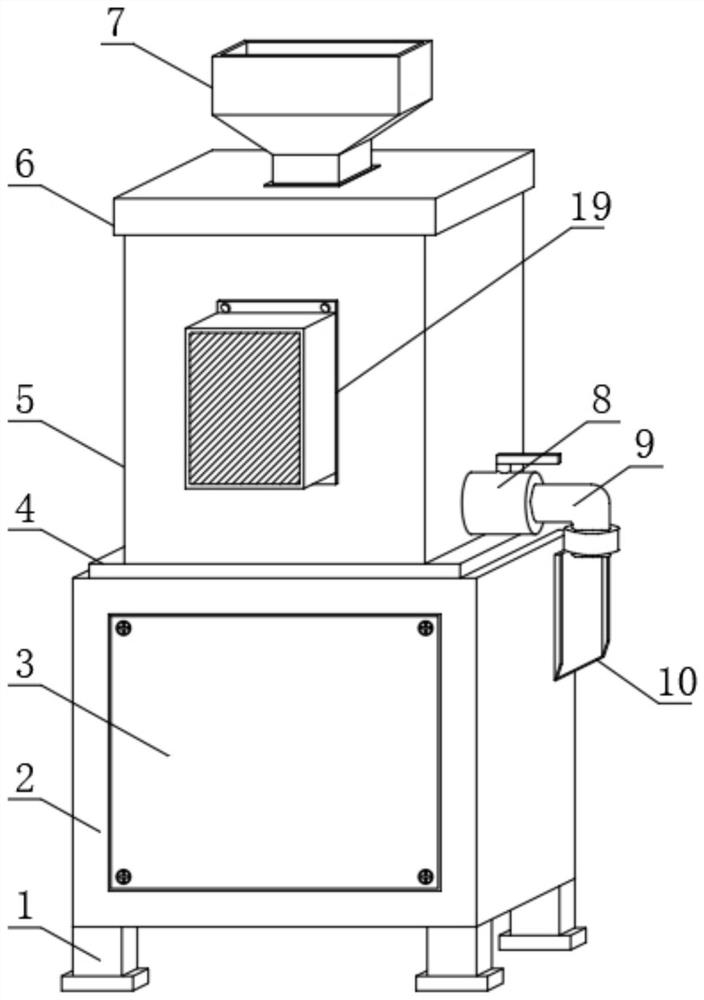
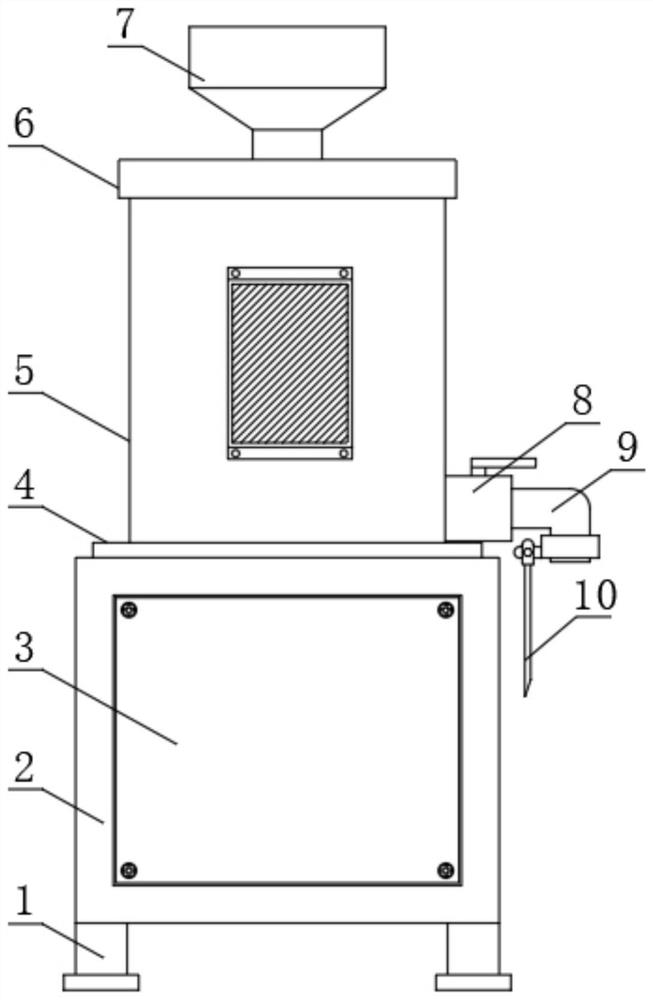
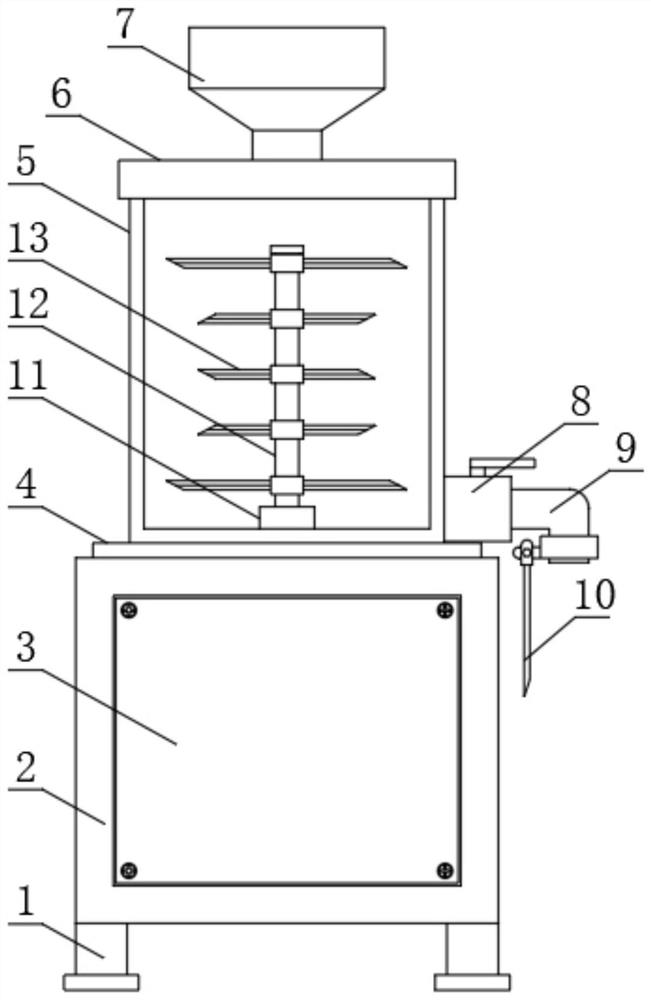
PendingCN114455527AWill not affect normal useAvoid wasteful situationsBarrels/casks fillingFood shapingAnimal scienceObstetrics
The invention discloses a sterile liquid food production device for digestive tract tumor patients to eat, which comprises a power case and a liquid food production tank, the liquid food production tank is mounted at the upper end of the power case, a fixing flange is sleeved outside the circular bottom side of the liquid food production tank, and the fixing flange is fixedly connected with the power case through screws. According to the sterile liquid food production device, the novel discharging guide device is additionally arranged outside the opening of the L-shaped discharging pipe, and after the novel discharging guide device is fixed to the outer portion of the opening of the L-shaped discharging pipe, the conveying direction of liquid food from the opening of the L-shaped discharging pipe can be changed; at the moment, the liquid food conveyed out of the opening of the L-shaped discharging pipe can be guided through the discharging guiding device, so that the liquid food can be accurately conveyed into the liquid food storage barrel without scattering, and the situation that the liquid food is wasted can be avoided.
Owner:JIAMUSI UNIVERSITY
Abdominal drainage fixing device for digestive tract medical oncology
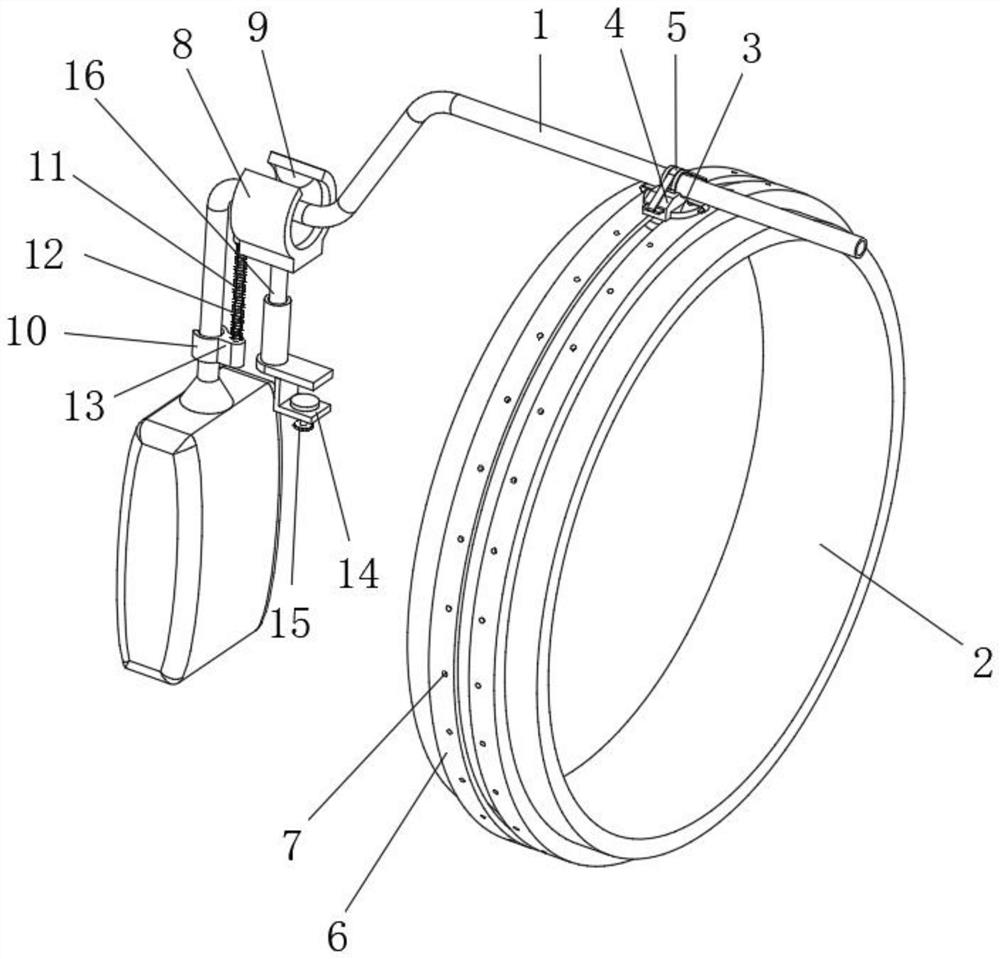
InactiveCN114522285AAvoid discomfortAvoid stayingMedical devicesCatheterEngineeringApparatus instruments
The invention belongs to the technical field of medical instruments, and particularly relates to an abdominal drainage fixing device for digestive tract medical oncology, which comprises a main body, a waistband for limiting the main body is arranged on the outer side of the main body, a support for fixing one end of the main body is arranged on the outer side of the waistband, and a support block for supporting the main body is arranged at the upper end of the support. After the main body is placed at the upper end of the supporting block, the pressing belt is pulled to enable the clamping plate to be connected into the clamping groove in a clamped mode, at the moment, pressure can be formed on the main body through elasticity of the pressing belt to fix the main body, discomfort of the wound portion of a patient during operation can be avoided, and the using effect is improved; after the sliding block is connected into the limiting seat in a sliding mode, the limiting pin is connected into the sliding hole in a clamped mode, the support and the limiting seat can be connected into a whole to be fixed, the structure is simple, the using position of the body can be conveniently adjusted according to the wound surface positions of different patients, and meanwhile impurities can be prevented from being left on the outer side of the skin of the patient.
Owner:SECOND AFFILIATED HOSPITAL OF COLLEGE OF MEDICINEOF XIAN JIAOTONG UNIV
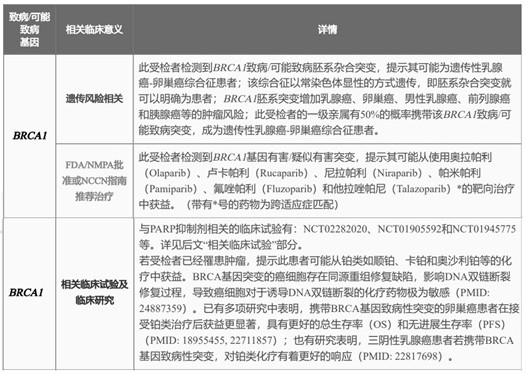
Gene panel, kit and application for detecting multiple tumors
ActiveCN114438218BWhere the guidance appliesMicrobiological testing/measurementFermentationOncologyPARP inhibitor
The invention discloses a gene Panel, a kit and an application for detecting various tumors. The gene panel includes hereditary breast and ovarian cancer syndrome BRCA-related genes, Lynch syndrome-related genes, other genes related to the multiple tumors and key genes of homologous recombination repair pathway. Detection of germline level or combined tumor tissue level can be performed for hereditary breast and ovarian cancer syndrome BRCA-related genes, Lynch syndrome-related genes, other genes related to the various tumors and key genes of homologous recombination repair pathway , and accurately assess the genetic risk of these genes related to gynecological tumors, breast cancer, prostate cancer and digestive tract tumors according to the test results, and guide tumor patients about the application of platinum-based chemotherapy drugs and PARP inhibitors. Comprehensive guidance for precise treatment of various tumors such as gynecological tumors, breast cancer, prostate cancer and digestive tract tumors can be achieved through one test.
Owner:普瑞基准科技(北京)有限公司 +2
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com