Primer and probe set for diagnosis, detection or screening of digestive tract cancer
A digestive tract cancer and probe group technology, applied in the field of biomedicine, can solve the problems of low patient acceptance, low early detection rate of digestive tract cancer, and difficulty in popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
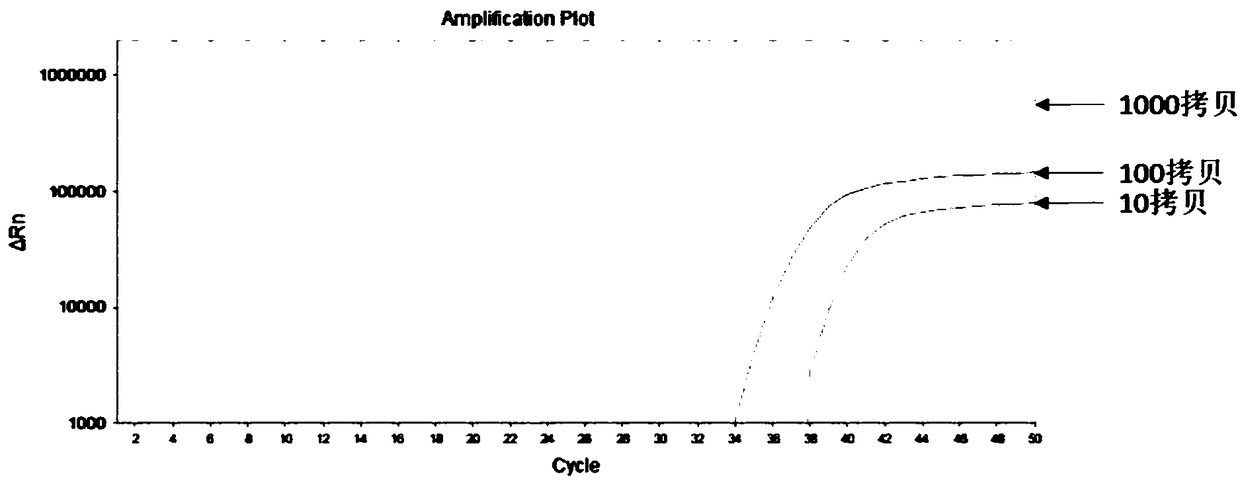
[0026] The genome sequence of a cell line known to be fully methylated with the SFRP2 gene was used as a DNA template. The DNA template was treated with bisulfite before the PCR reaction, and the ammonium bisulfite solution with a bisulfite concentration of 8M was used. The reaction conditions were: Heat at 70°C for 60 minutes, then use the Axygen gel purification kit to purify to obtain the converted DNA template, and then perform PCR.
[0027] Using SEQ ID NO.1, SEQ ID NO.17 and SEQ ID NO.37, carry out fluorescent quantitative PCR reaction, wherein the fluorescence labeled by SEQ ID NO.37 is ROX; the PCR system and reaction conditions are shown in Table 2 and Table 3.
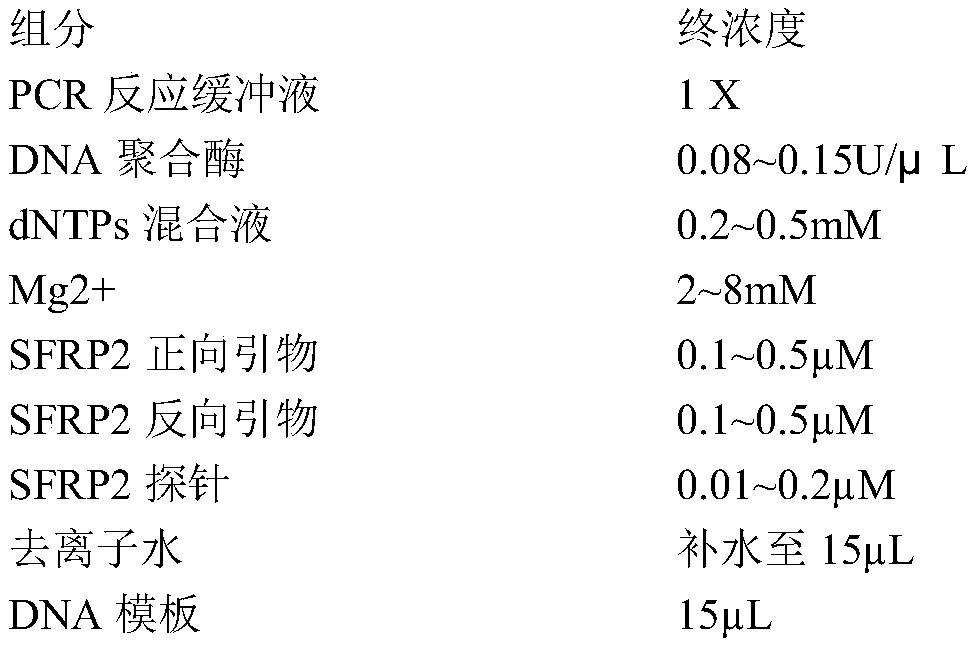
[0028] The PCR component of table 2 embodiment 1
[0029] components
Final concentration
PCR reaction buffer
1X
0.1U / μL
dNTPs mixture
0.2mM
Mg2+
5mM
SEQ ID NO:1
0.2μM
SEQ ID NO: 17
0.2μM
SEQ ID NO: 37
0.1μM...
Embodiment 2
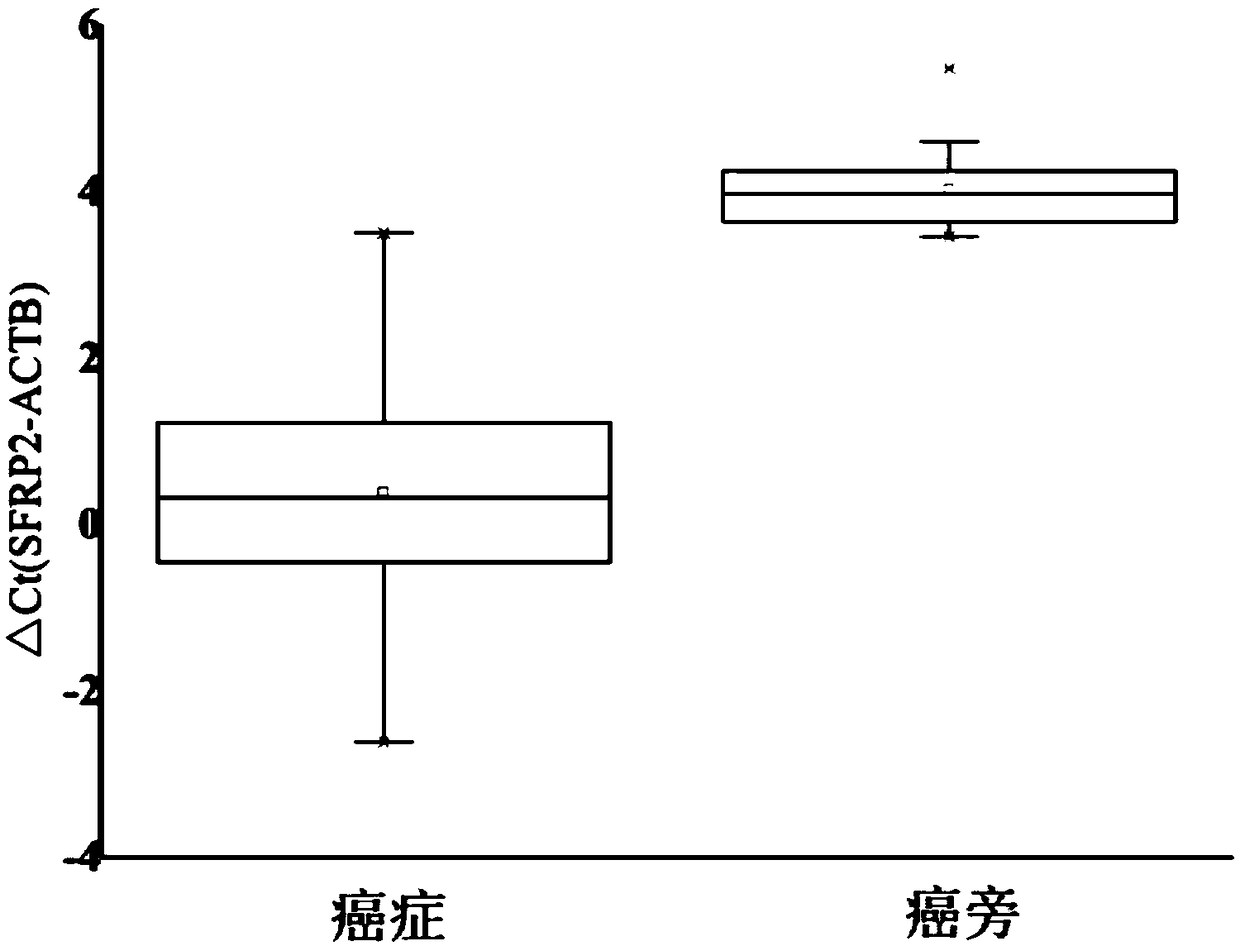
[0035] Colorectal cancer tissue and matched paracancerous tissue were used as detection objects, DNA was extracted with Qiagen’s DNeasy Blood&Tissue Kit, and then treated with 10M bisulfite, the bisulfite solution used was 6M ammonium bisulfite, A mixed solution of 3M sodium bisulfite and a mixed solution of 1M anhydrous sodium sulfate were heated at 80° C. for 40 minutes under the reaction conditions, and PCR was performed after purification using the Axygen gel purification kit.
[0036] Using SEQ ID NO.2, SEQ ID NO.22 and SEQ ID NO.31, wherein the fluorescence labeled by SEQ ID NO.31 is JOE, a fluorescent quantitative PCR reaction was used to detect 16 cases of colorectal cancer tissue and its paracancerous tissue samples. The PCR system and reaction conditions are shown in Table 4 and Table 5.
[0037] The PCR component of table 4 embodiment 2
[0038] components
Final concentration
PCR reaction buffer
1X
0.08U / μL
dN...
Embodiment 3
[0043] The test samples were 10 cases of esophageal cancer serum cfDNA, 31 cases of gastric cancer serum cfDNA and 36 cases of serum cfDNA of patients without obvious gastrointestinal diseases, and the serum volume was 1 mL. After the cfDNA extraction kit of Suzhou Weishan Biotechnology Co., Ltd. was used for DNA extraction, 9M bisulfite was used for treatment. The bisulfite solution used was 9M ammonium bisulfite solution. minutes, followed by PCR after purification using the Axygen Gel Purification Kit.
[0044] Using SEQ ID NO.5, SEQ ID NO.23 and SEQ ID NO.34, wherein the fluorescence labeled by SEQ ID NO.34 is FAM, for fluorescent quantitative PCR reaction detection, the PCR reaction system and reaction conditions are shown in Table 6 and Table 7 As shown, the test results are shown in Table 8.
[0045] The PCR component of table 6 embodiment 3
[0046] components
Final concentration
PCR reaction buffer
1X
0.15U / μL
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com