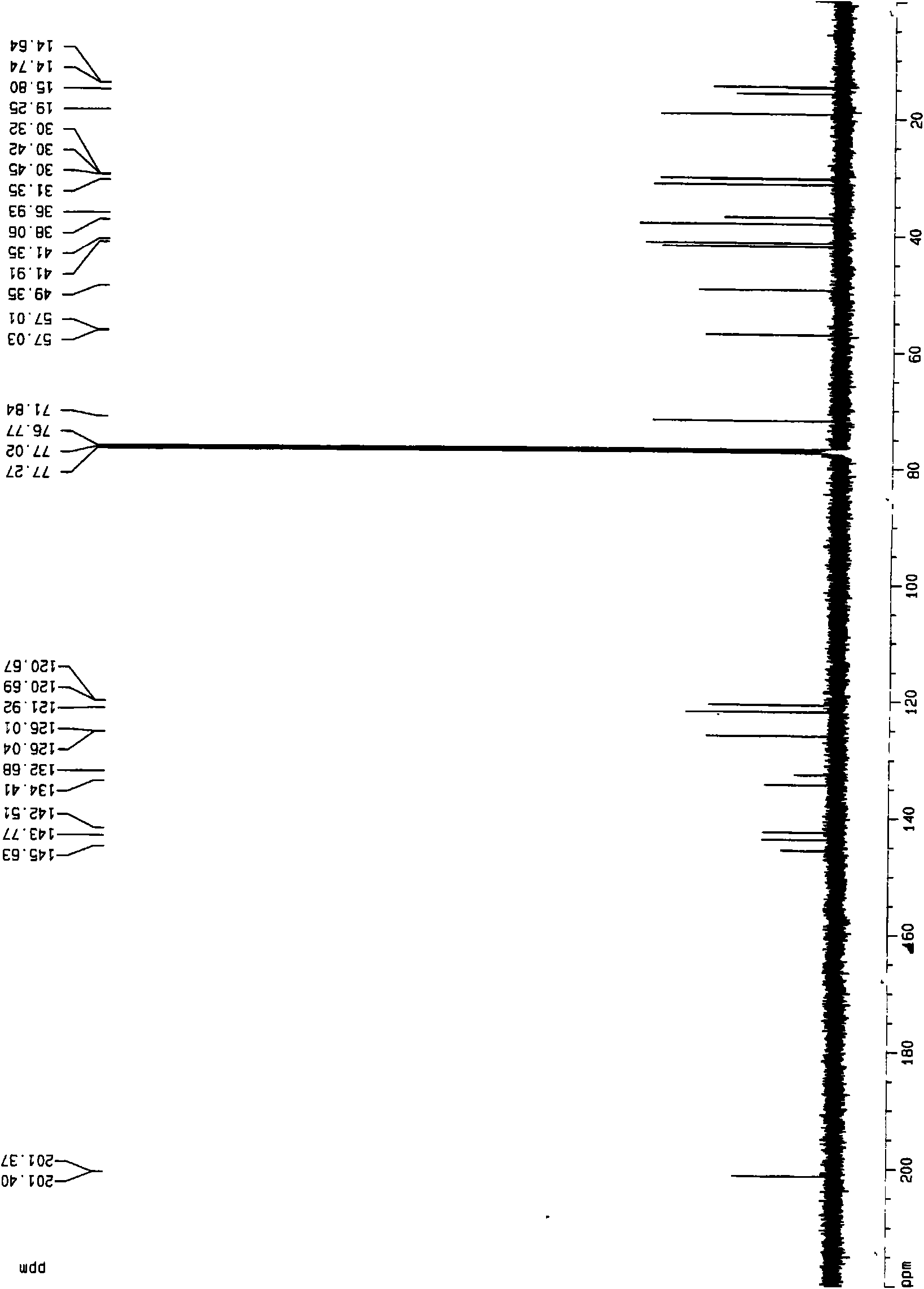
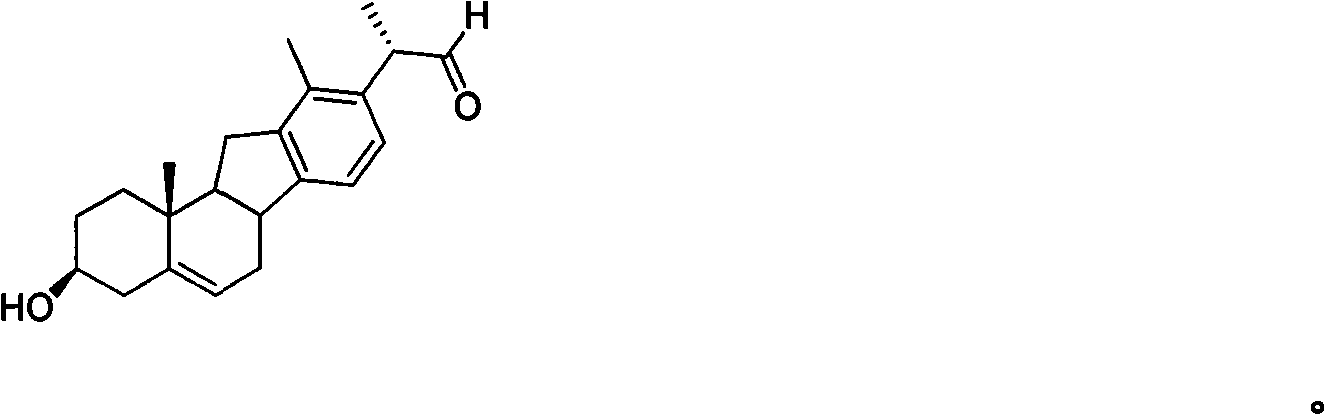
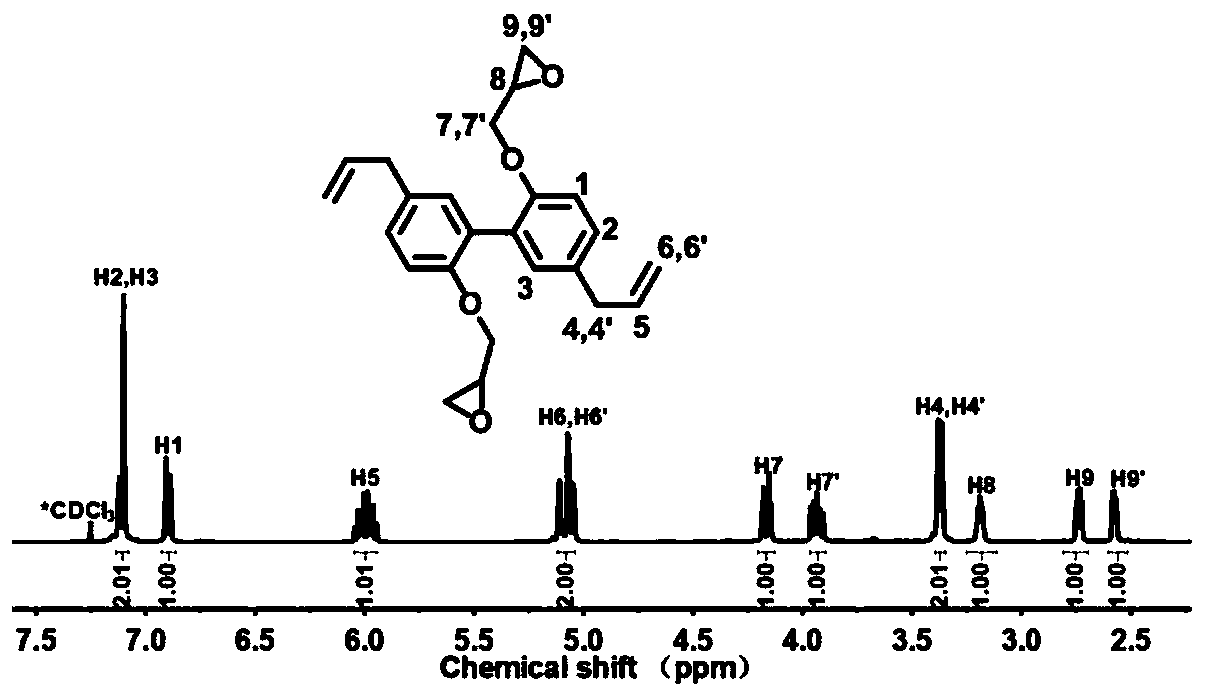
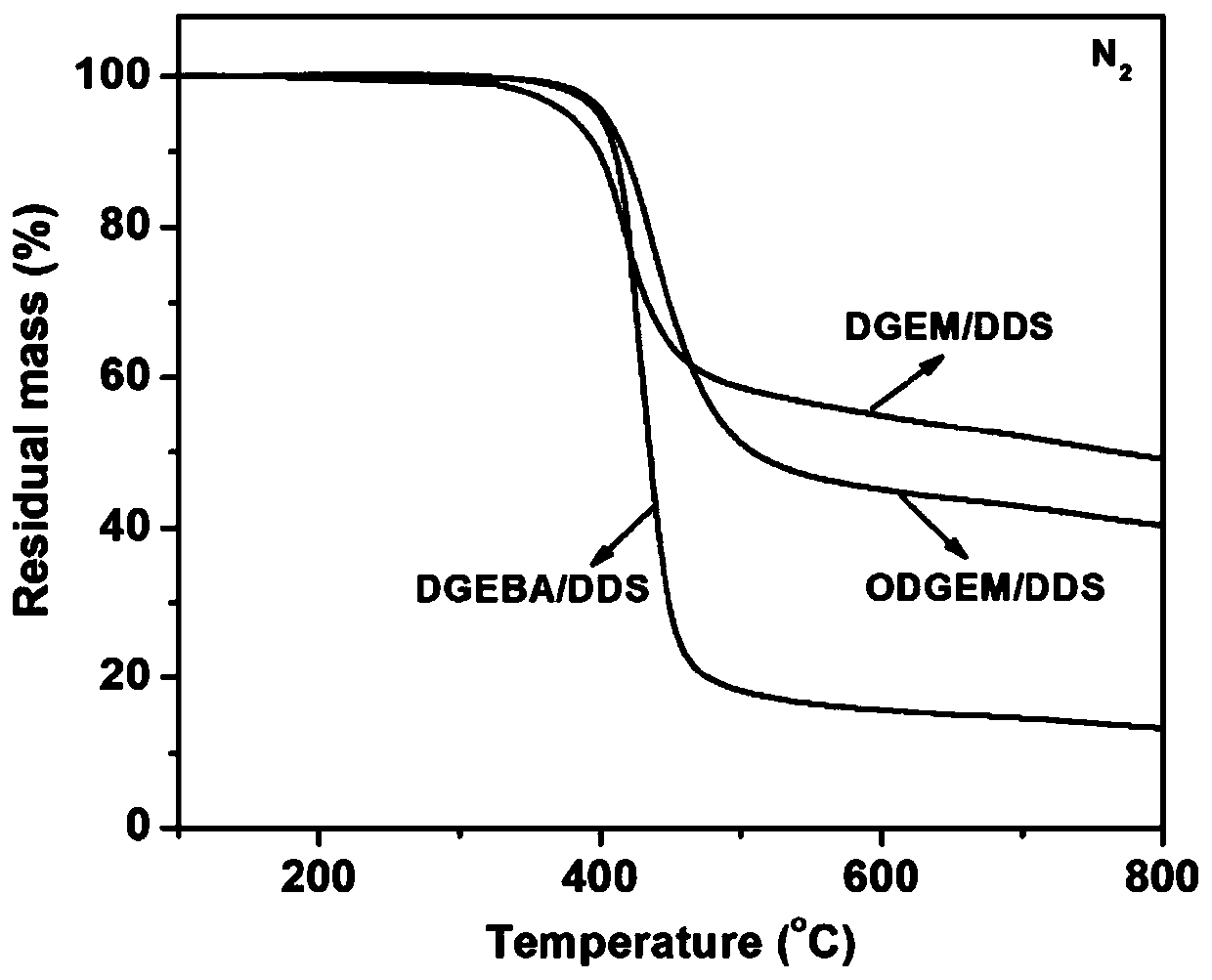
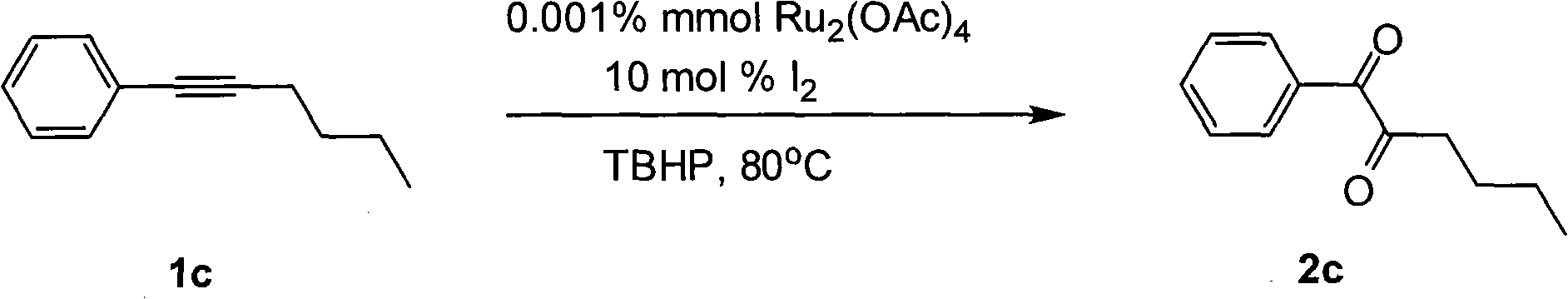Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
156 results about "Meta-Chloroperoxybenzoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
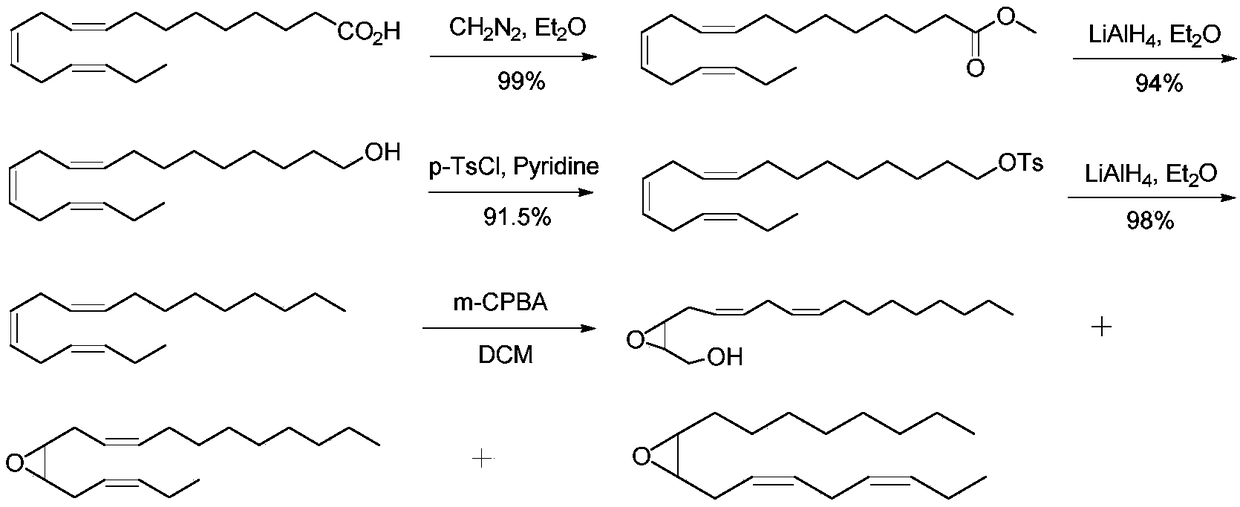
Meta-Chloroperoxybenzoic acid (mCPBA or mCPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material.
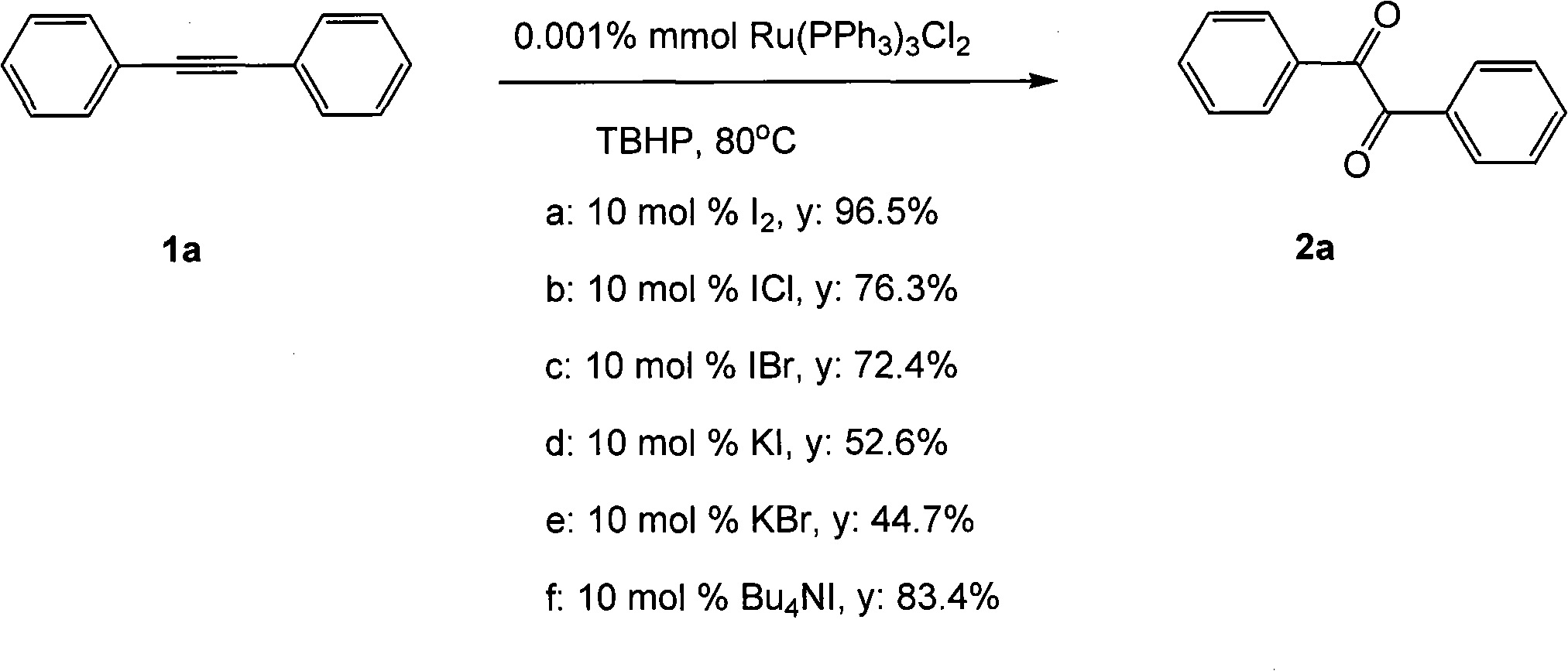
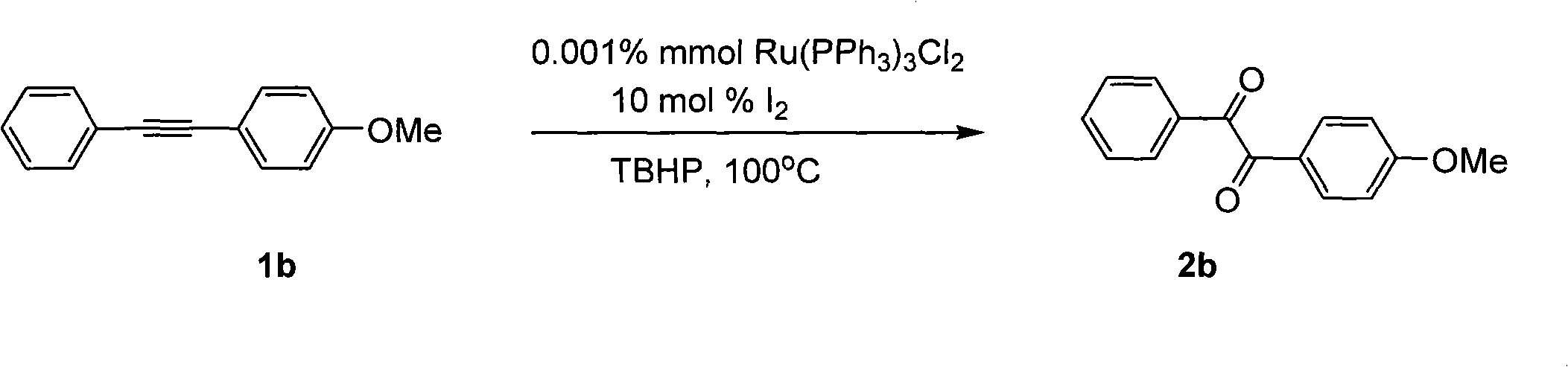
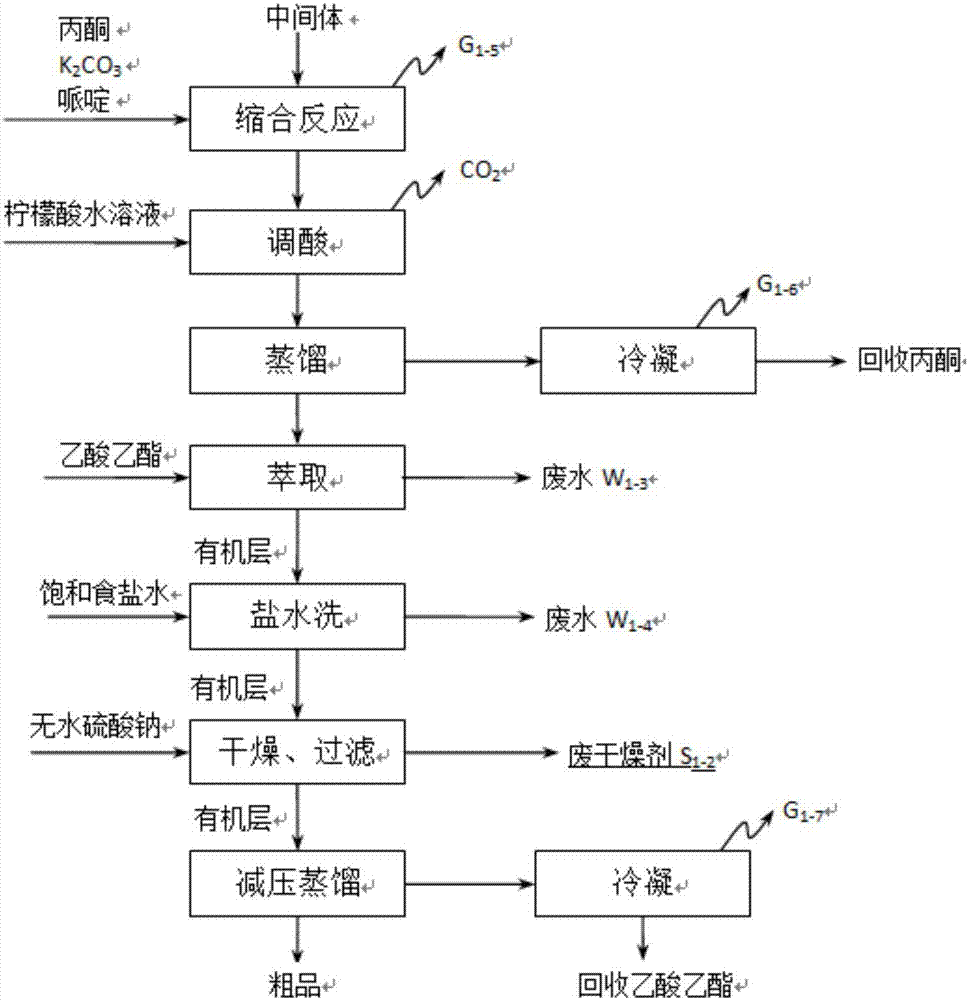
Method for preparing 1, 2-diketone by catalyzing and oxidizing alkynes
InactiveCN101624322AMild reaction conditionsMeet the requirementsCarboxylic acid nitrile preparationOrganic compound preparationCatalytic oxidationKetone
The invention belongs to the field of catalysis and oxidization, and particularly discloses a method for preparing 1, 2-diketone by catalyzing and oxidizing alkynes. The method comprises the following steps: taking alkynes R1-C-C-R2 (acetylenic link exists between C and C) as a reaction substrate, taking one of TBHP, m-chloroperoxybenzoic acid and p-benzoquinone as a oxidant, taking one of dichloro (p-cymene) ruthenium (II) dimer, tri (triphenylphosphine) ruthenous chloride, ruthenium acetate, ruthenium dichlorophenyl (II) dimer, ruthenium trichloride, BINAP ruthenous chloride, dodecacarbonyltriruthenium and tricarbonyldichlororuthenium (II) dimer as a catalyst, taking one of iodine, iodine chloride, iodine bromide, potassium iodide, tetrabutyl ammonium iodide and potassium bromide as a cocatalyst, and taking 1, 4-dioxane as a solvent to react under 40 DEG C to 100 DEG C for 4 to 24 h to prepare the 1, 2-diketone. The method is economic, environmental-friendly and mild.
Owner:SUZHOU UNIV
Veratramine degradation product veratrum fluorene aldehyde and the derivatives thereof, as well as the preparation and application thereof
InactiveCN101565446AEnhanced inhibitory effectOrganic active ingredientsSteroidsCancer cellSteroidal alkaloid
The invention provides a veratramine degradation product veratrum fluorene aldehyde 1 and derivatives thereof. The invention uses steroidal alkaloid veratramine (A) as raw material and prepares veratrum fluorene aldehyde by oxidative degradation of m-chloroperoxybenzoic acid to obtain 20 derivative compounds of the veratrum fluorene aldehyde 1 after further oxidation, reduction and condensation reaction. The 20 compounds of the invention have good inhibitory activity on cancer cells, wherein the compounds 1, 2, 5, 8, 13 and 17 have good inhibitory activity on cells of human pancreatic cancer cells BxPC-3 and SW1990, small-cell lung cancer cells NCI-H446, human colorectal cancer LOVO and the like, and the inhibitory activity of the compound veratrum fluorene aldehyde 1 is the most significant.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation method of bio-based epoxy resins based on natural Magnolia officinalis derivative
ActiveCN110408003AImprove responseReaction conditions are easy to controlOrganic chemistryHeat resistanceEpichlorohydrin
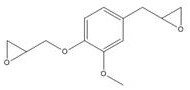
The invention belongs to the technical field of bio-based thermosetting epoxy resins, and discloses a preparation method of bio-based epoxy resins based on a natural Magnolia officinalis derivative. The preparation method comprises the following steps: the natural Magnolia officinalis derivative and epichlorohydrin undergo a one-step reaction to obtain a corresponding natural Magnolia officinalisderivative difunctional epoxy resin; and the difunctional epoxy resin is oxidized by m-chloroperoxybenzoic acid to obtain the corresponding natural Magnolia officinalis derivative tetrafunctional epoxy resin. The entire synthesis process is very simple and efficient, so large-scale production is easy. The bio-based difunctional and tetrafunctional epoxy resins based on the natural Magnolia officinalis derivative have the advantages of excellent heat resistance, excellent mechanical properties and excellent flame retardancy, and have great potential when used to replace petroleum-based bisphenol A epoxy resin.
Owner:DALIAN UNIV OF TECH
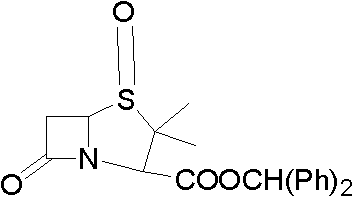
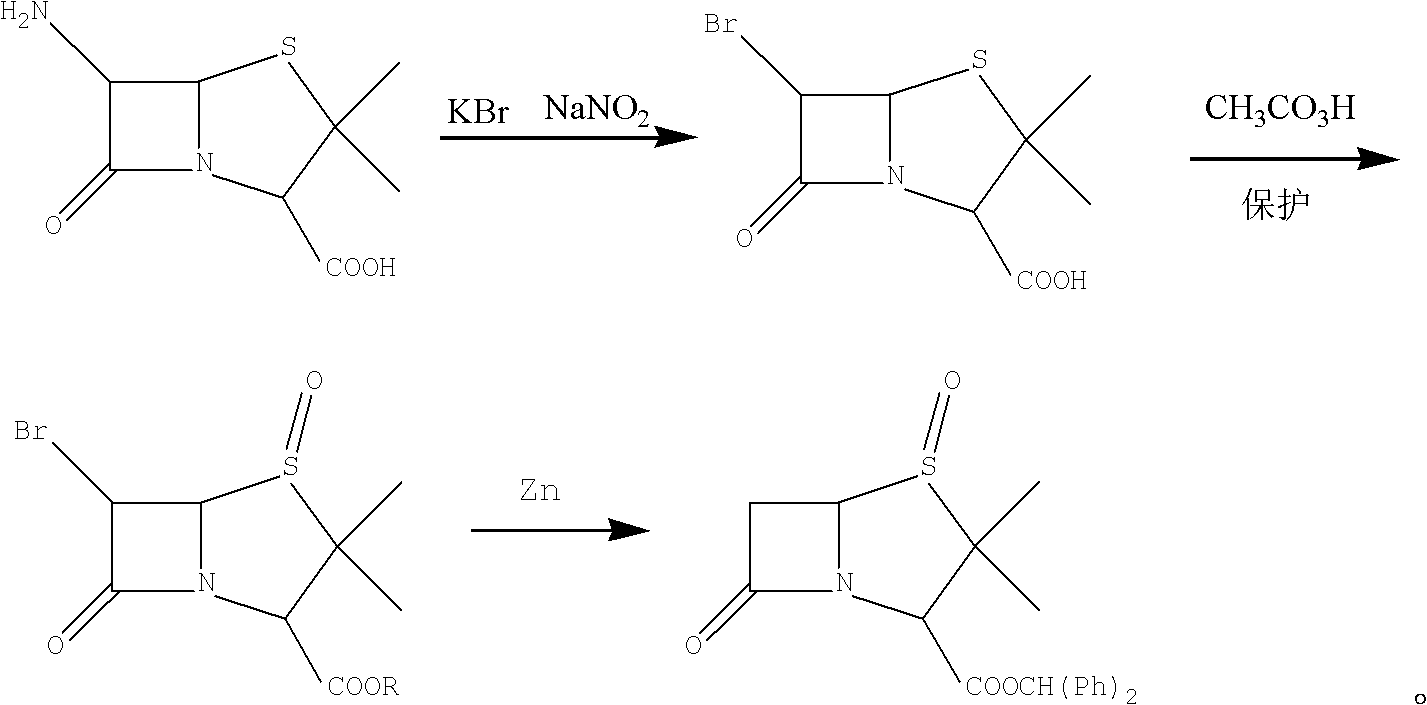
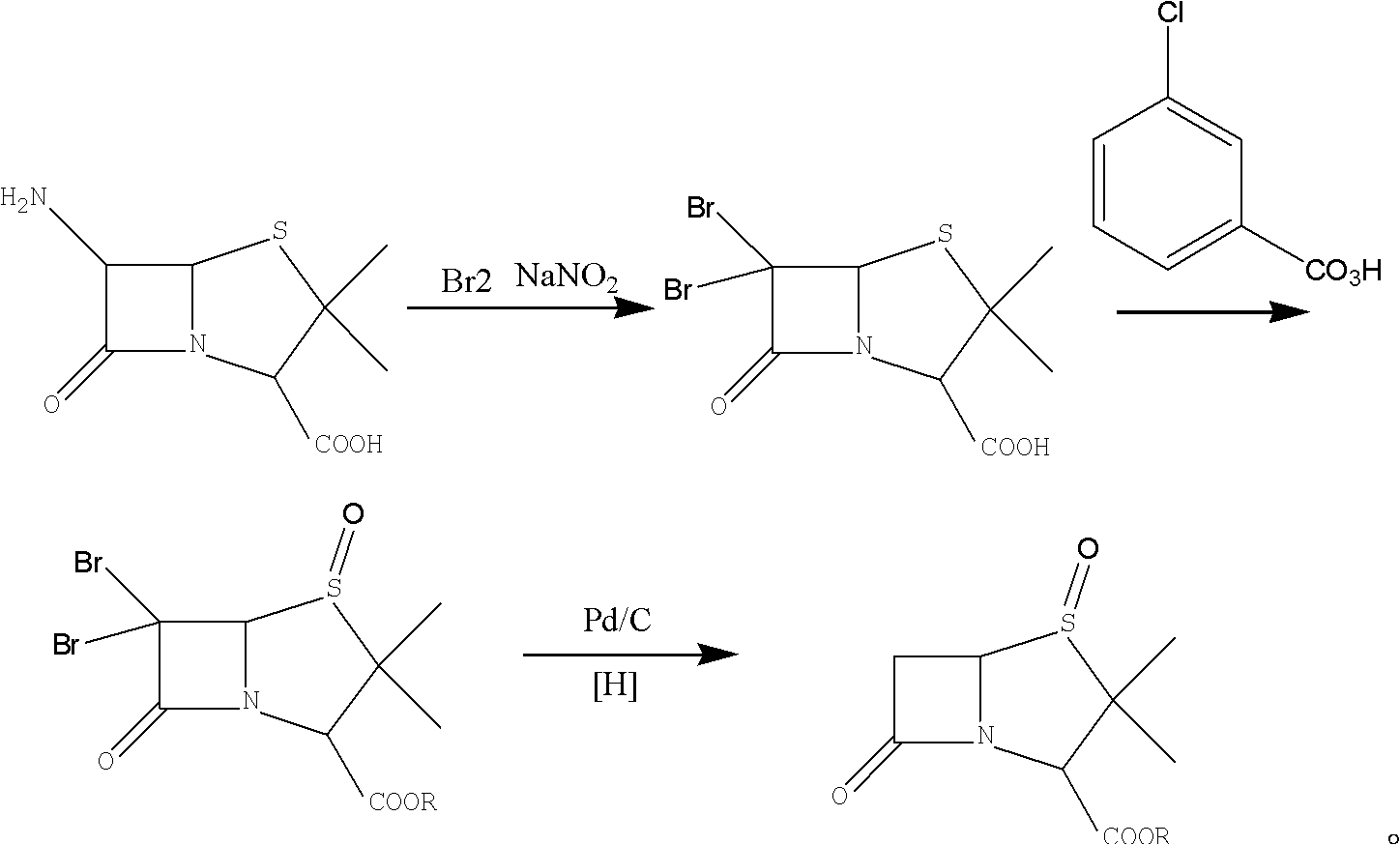
Method for preparing penicillanic acid sulfoxide diphenyl methyl ester
ActiveCN101935324AQuality improvementIncrease profitOrganic chemistryHigh pressureMeta-Chloroperoxybenzoic acid
The invention provides a method for preparing penicillanic acid sulfoxide diphenyl methyl ester. Penicillanic acid sulfoxide is prepared by the following four-step reaction. Hydrobromic acid is used as a brominating agent for substituting potassium bromide and bromine, and hydrogen peroxide is used as an oxidant for substituting peracetic acid and chloroperoxybenzoic acid, so the preparation method is safe and environment-friendly; in addition, the hydrogen peroxide is reacted with a compound (iii) under the catalysis action of acetyl molybdenum pyruvate so that the reaction mole yield can be obviously improved and reaches 90 percent; zinc powder which substitutes palladium and carbon is used as a reducing agent, so high-pressure equipment is not needed in the reaction; and the preparation method simplifies the process operation step, is easy for industrialized production, and reduces the production cost.
Owner:江西祥太生命科学有限公司
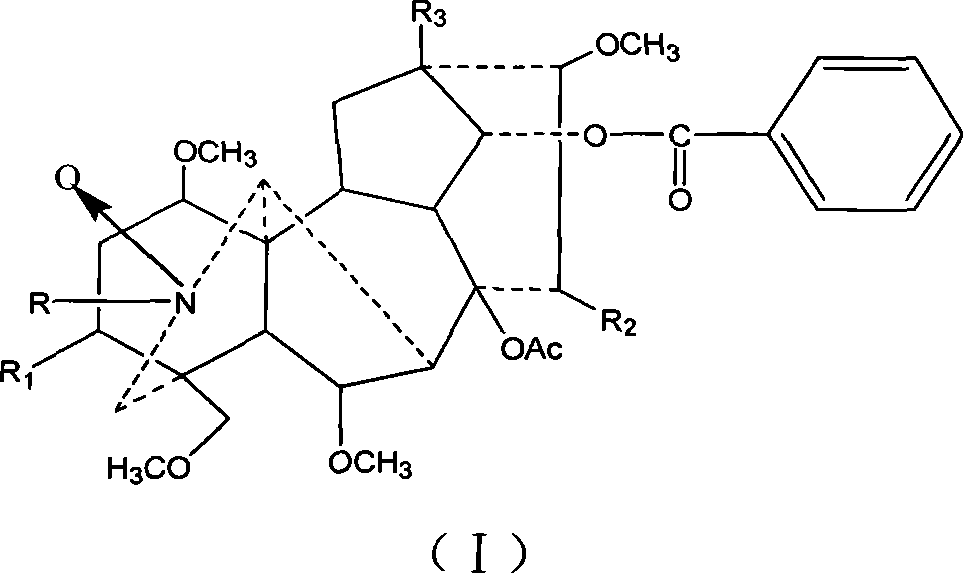
Preparation method of 16 Alpha-methyl steroidal compound
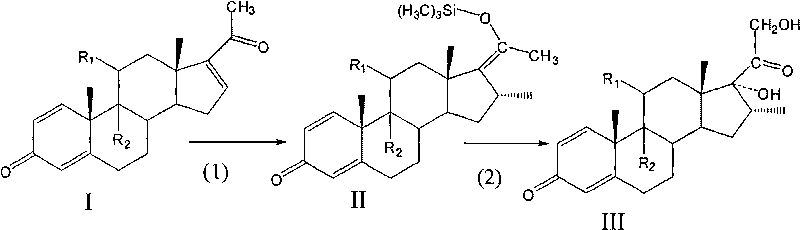
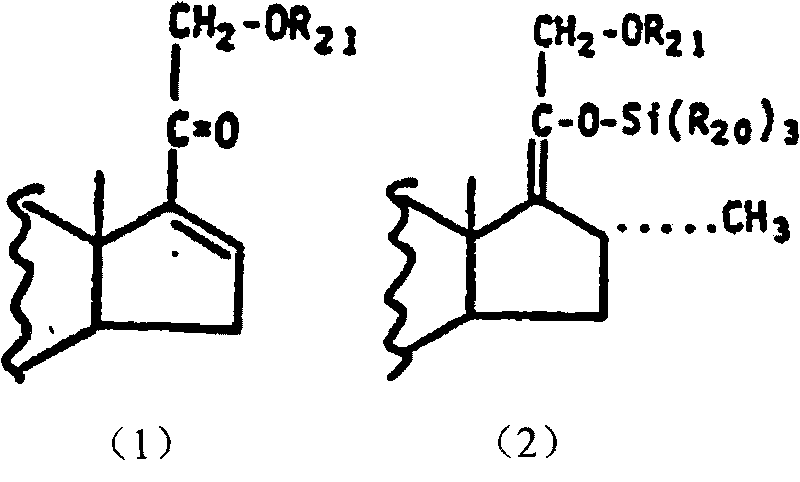
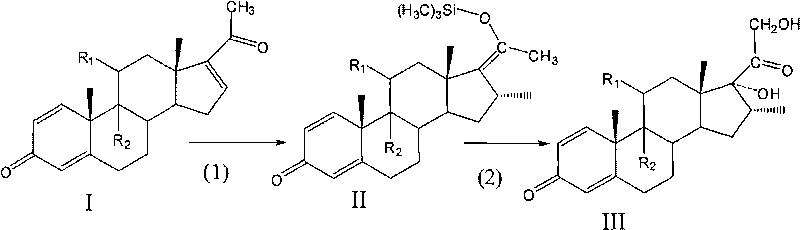
A preparation method of a 16 Alpha-methyl steroidal compound comprises: taking a formula (I) compound as a substrate, reacting with 0.2 to 0.1 time catalyst, 1 to 1.5 times trimethyl chlorosilane, 1 to 1.5 times Grignard reagent and 2 to 4 times triamine phosphate in organic solvent to obtain a formula (I) compound, and taking the formula (I) compound to react with 1 to 5 times alkali and 1 to 3 times meta-chloroperoxybenzoic acid in organic solvent to obtain a formula (III) compound.
Owner:TIANJIN JINYAO GRP
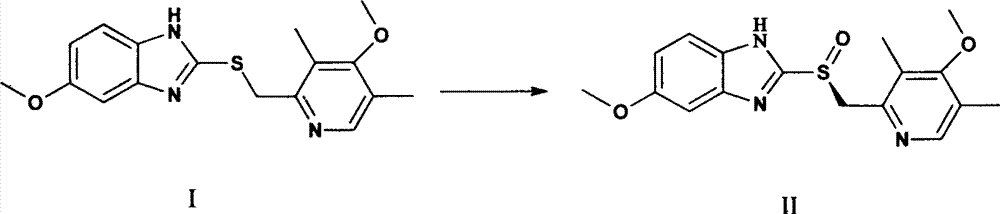
Method for synthesizing lansoprazole and salt thereof
InactiveCN101475562AHigh yieldShort reaction timeOrganic chemistryDigestive systemSodium iodideSolvent
The invention relates to a method for synthesizing lansoprazole and salts of the lansoprazole, in particular to a method for synthesizing lansoprazole sodium. The method adds sodium iodide serving as a catalyst into a reaction system, uses acetone as a reaction solvent, reduces reaction time, greatly improves product yield to 95 percent. And due to the use of m-choroperoxybenzoic acid as an oxidizer during the production process of lansoprazole, the method reduces cost, improves oxidization conditions, reduces side reactions, achieves higher product yield and purity and obvious invention technical effects.
Owner:HAINAN LINGKANG PHARMA CO LTD
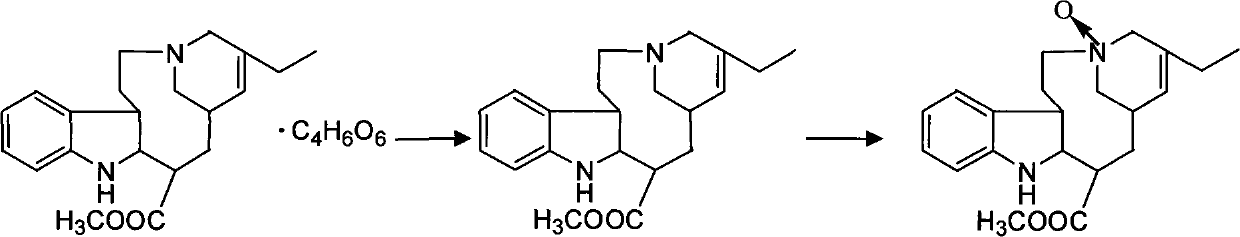
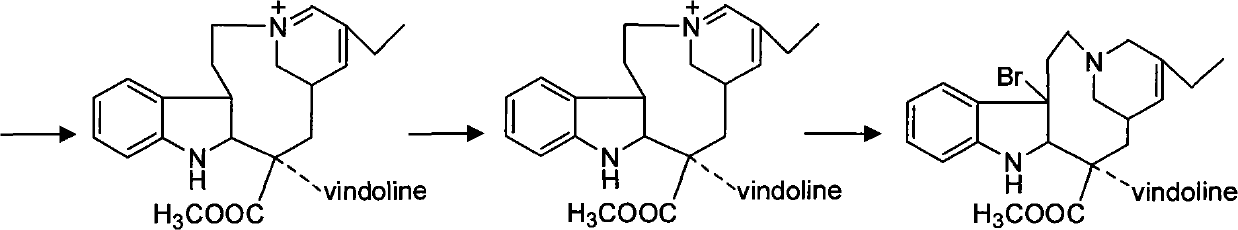
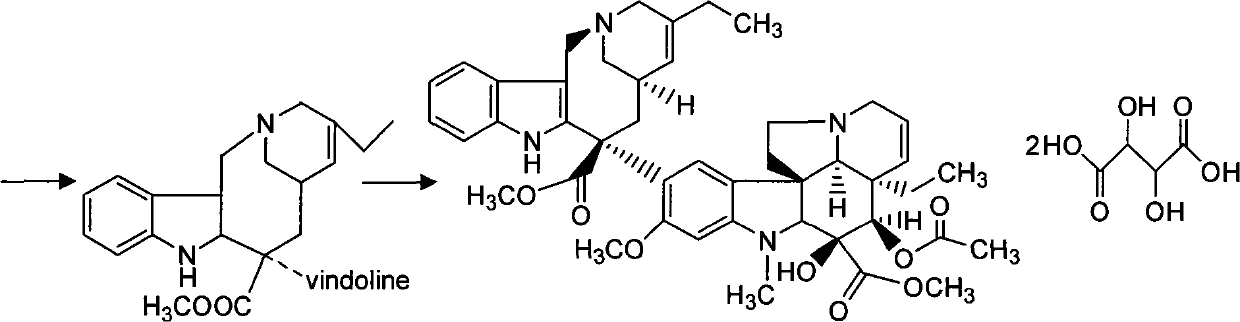
Preparation method for vinorelbine tartrate
ActiveCN102199165AReduce manufacturing costLow impurity contentCarboxylic acid salt preparationTrifluoroacetic anhydrideSodium borohydride
The invention discloses a preparation method for vinorelbine tartrate; the preparation method comprises the following steps of: taking tartrate vinblastine as a starting material; using ammonia water for dissociating the starting material, reacting the dissociated starting material with the metachloroperbenzoic acid so as to obtain vinblastine-N oxide, then reacting the vinblastine-N oxide with vindoline and trifluoroacetic anhydride, carrying out the reduction through sodium borohydride so as to obtain dehydrated vinorelbine, treating the dehydrated vinorelbine so as to obtain vinorelbine, and finally salifying the vinorelbine with tartaric acid so as to obtain the vinorelbine tartrate. The preparation method has the advantages of low cost, few side reaction, easily controlled operation process, higher yield and product purity and wide industrialized application prospect.
Owner:HUBEI HONCH PHARMA
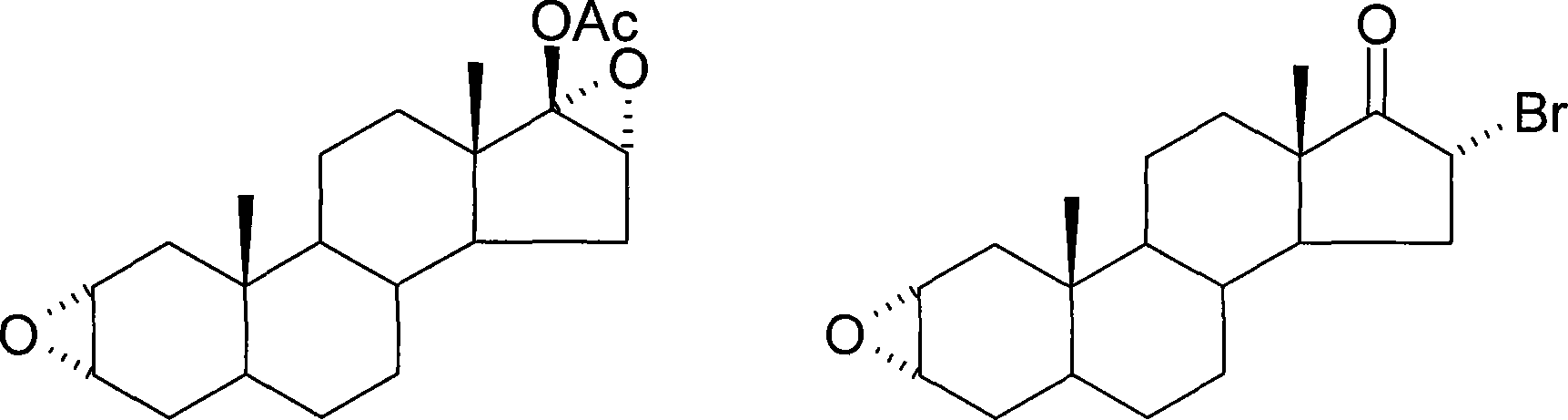
Method of synthesizing 2alpha,3alpha-epoxy-16alpha-bromo-5alpha-androsterone-17-one
The invention discloses a making method of 2 alpha, 3 alpha-epoxy-16 alpha-bromine-5 alpha-androstane-17-ketone as intermediate of steroidal muscular relaxation drug, which comprises the following steps: dissolving epiandrosterone in the benzene; adding catalyst; stirring; heating; refluxing 8-12h; preparing 5 alpha-androst-2-olefin-17-ketone; reacting the 5 alpha-androst-2-olefin-17-ketone and copper bromide in the carbinol to produce 16 alpha-bromine-5 alpha-androst-2-olefin-17-ketone; reacting the 16 alpha-bromine-5 alpha-androst-2-olefin-17-ketone and m-perbenzoic acid in the composite solvent of water and dichloromethane; producing the product through eliminating, bromizing and epoxidising.
Owner:WUHAN UNIV
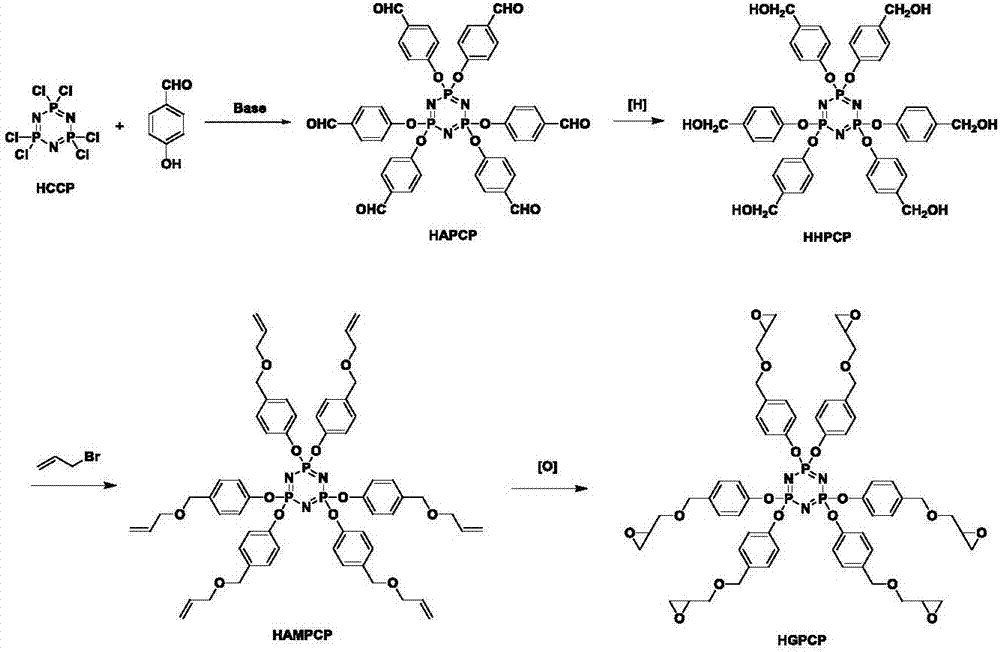
Preparation method of six-functionality epoxy resin based on cyclotriphosphazene
ActiveCN107216354AStable flame retardant propertiesImprove mechanical propertiesGroup 5/15 element organic compoundsEpoxyOxygen
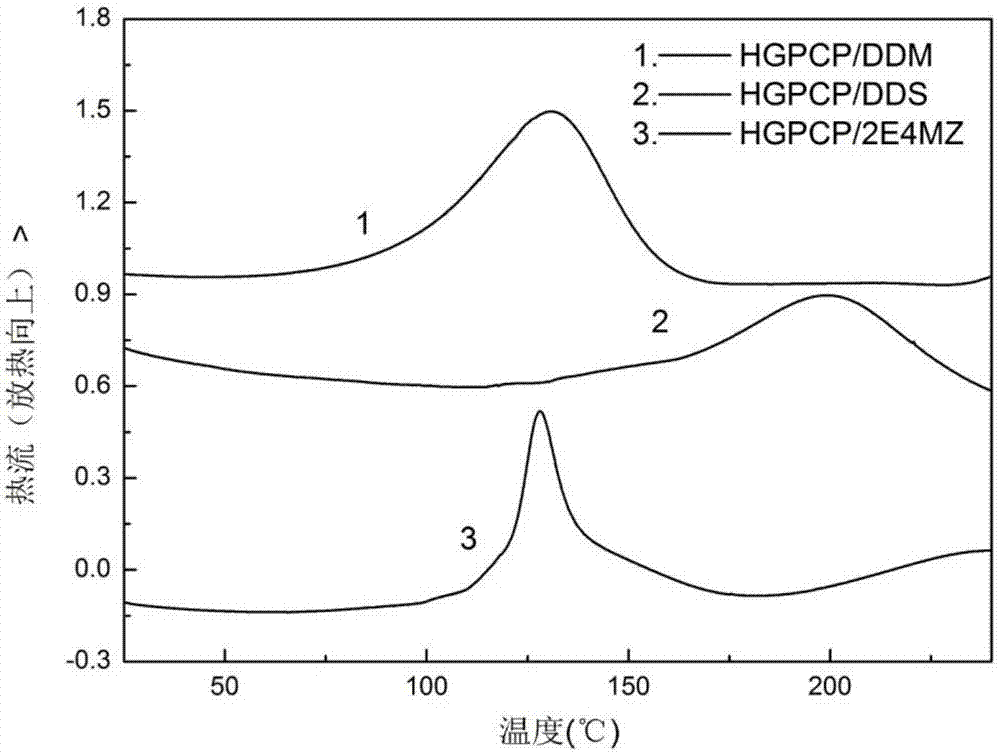
The invention discloses a preparation method of six-functionality epoxy resin based on cyclotriphosphazene, which aims at solving the technical problem of poor comprehensive property in the existing prepared epoxy resin flame-retardant system. The preparation method adopts the technical scheme with the following steps of firstly, performing nucleophilic substitution on p-hydroxybenzaldehyde and phosphonitrilic chloride trimer under the condition of alkaline existence, so as to obtain a hexaaldehyde compound HAPCP; using sodium borohydride or lithium borohydride to reduce the HAPCP, so as to obtain a hexahydroxy intermediate HHPCP; dissolving the HHPCP, bromopropylene and alkaline into an organic solvent, so as to obtain HAMPCP; oxidizing a hexaalkenyl-containing compound HAMPCM by metachloroperbenzoic acid in an organic solvent, so as to obtain a hexaepoxy target product HGPCP. The preparation method has the advantages that because the molecular structure of HGPCP contains six epoxy functional groups, the mechanical property and heat-resistant property are improved; by containing the cyclotriphosphazene structure with the flame-retardant property, the flame-retardant property is very excellent, the limited oxygen index is greater than 30%, and the UL-94 reaches V-0 level.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Novel synthesis method of 2-methyl-1,4-naphthoquinone
InactiveCN103833541AReduce consumptionEasy to separateQuinone preparation by oxidationSynthesis methodsDistillation
The invention relates to a novel synthesis method of 2-methyl-1,4-naphthoquinone. The novel synthesis method comprises the steps: respectively dissolving a defined amount of 2-methylnaphthalene and metachloroperbenzoic acid in moderate glacial acetic acid to prepare solutions (1) and (2), dropwise adding the solution (2) in the solution (1) under the stirring, after reacting for a period of time at a certain temperature, extracting a reaction solution by using chloroform, washing an extraction solution with a saturated NaHCO3 solution and water, after anhydrous Na2SO4 is dried, performing reduced pressure distillation to prepare a crude product of the 2-methyl-1,4-naphthoquinone, and recrystalizing by using alcohol as a solvent to obtain a pure product of the 2-methyl-1,4-naphthoquinone. According to the novel synthesis method, the conversion rate of the 2-methylnaphthalene can reach 80 percent, and the yield of the 2-methyl-1,4-naphthoquinone can reach 31 percent. The method disclosed by the invention has the characteristics of short reaction time, mild conditions, and the like, and products are easily separated; and no any catalyst is used in the reaction, thus the energy consumption and the environment pollution can be reduced.
Owner:QILU UNIV OF TECH
Synthetic method of sex pheromone intermediate of Hyphantria cunea
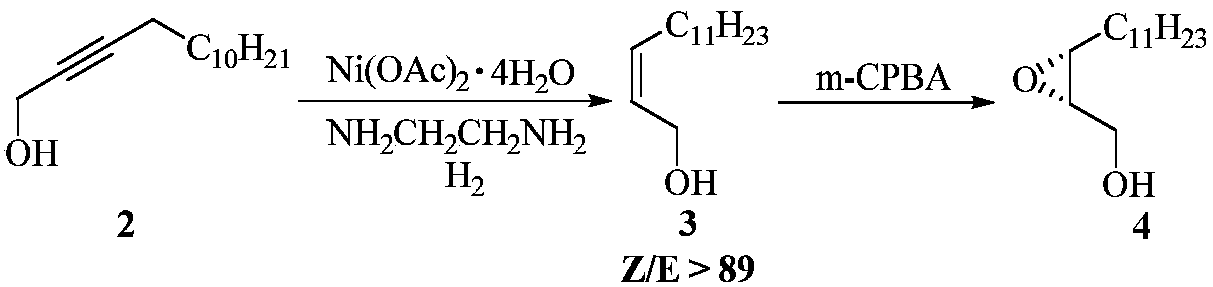
ActiveCN108299342AHigh Z/E valueHigh optical purityOrganic chemistry methodsEthylenediamineHalohydrocarbon
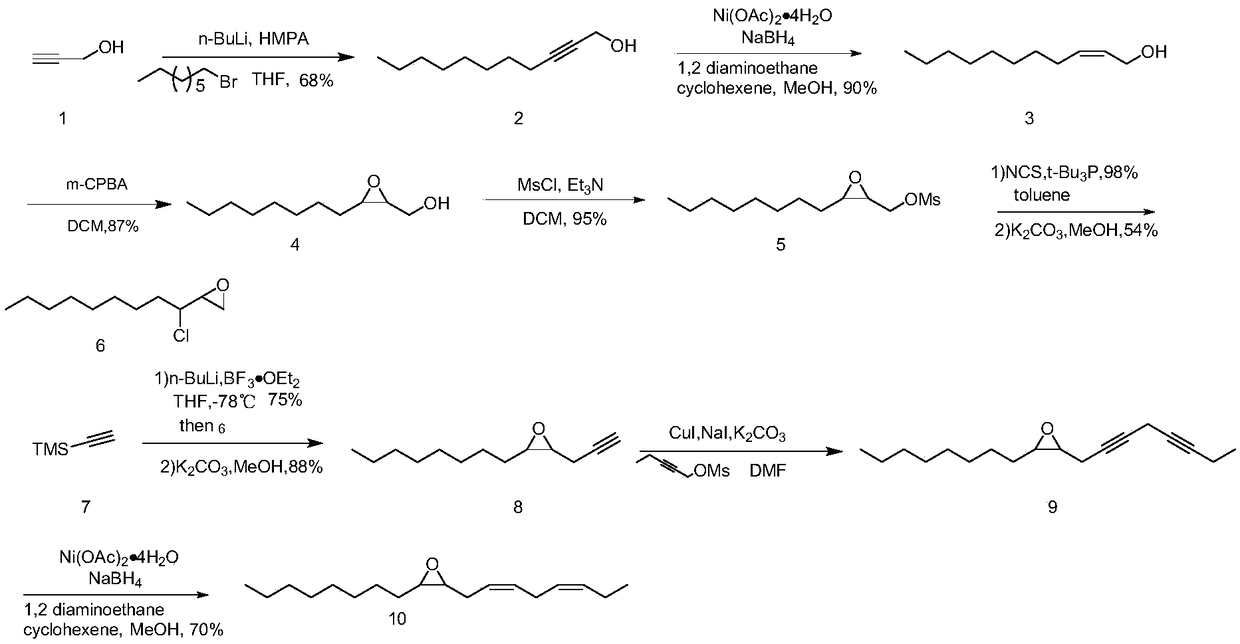
The invention discloses a synthetic method of sex pheromone intermediate (2S,3R)-2,3-epoxy-1-tetradecanol (4) of Hyphantria cunea. According to the method, firstly, nickel acetate tetrahydrate, sodiumborohydride, ethylenediamine as well as an alcohol as a solvent are added to a hydrogenation reactor for a reaction, then, an alcoholic solution with 2-tetradecynol dissolved is added continuously, areaction is performed after hydrogen replacement, and (Z)-2-tetradecene-1-ol is obtained; (Z)-2-tetradecene-1-ol is dissolved in a halogenated hydrocarbon solvent, a carbonate solution and a halogenated hydrocarbon solution of 3-chloroperoxybenzoic acid are added for a reaction, and (2S,3R)-2,3-epoxy-1-tetradecanol is obtained. The method is environmentally friendly and suitable for industrial production, cost is low, and reaction conditions are mild.
Owner:ZHEJIANG UNIV
Method for totally synthesizing natural product (+/-)-rupestine G and resolving enantiomers
ActiveCN107129462AOptically-active compound separationOrganic racemisationLithium hydroxideEnantiomer
The invention relates to a method for totally synthesizing a natural product (+ / -)-rupestine G and resolving enantiomers. The method comprises the following steps: oxidizing the raw material 2-methyl-5-bromopyridine (1) by m-chloroperoxybenzoic acid to obtain a nitrogen oxidation product compound 2, carrying out a Reissert-Henze reaction to perform cyano substitution so as to obtain a compound 3, carrying out a decarboxylation reaction on the compound 3 and potassium monoethyl malonate to obtain beta-ketoester 4, carrying out alkylation under the condition of sodium ethylate to obtain a compound 5, carrying out a coupling reaction to obtain a compound 6, carrying out an intramolecular olefin double replacement reaction on the compound 6 to obtain a key intermediate compound 7, carrying out reduction with sodium borohydride to obtain a compound 8, carrying out a reaction under the condition of pyridine / MsCl to obtain a compound 9, hydrolyzing the compound 9 under the action of lithium hydroxide, carrying out methyl esterification by using iodomethane to obtain a compound 10, carrying out palladium-carbon catalytic hydrogenation to obtain a pair of diastereoisomers 11 and 12, and carrying out resolution by a semi-preparative high performance liquid chromatograph to obtain the natural product rupestine G and three stereoisomers, namely a compound 13, a compound 14 and a compound 15.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Mesaconitine acylated product nitrous oxide, preparation and use
InactiveCN101434580ABiologically activeOrganic active ingredientsBiocideDistillationPressure reduction
The invention discloses a preparation method of a nitrogen oxide (with a compound structure shown as the picture) of an aconitine acylate product and an application thereof, pertaining to the technical field of medical and pesticide synthesis. The preparation method of the nitrogen oxide of the aconitine acylate product comprises the steps that the aconitine acylate product which is served as a raw material and a metachloroperbenzoic acid (mCPBA) which is served as an oxidant are stirred for reaction for 3 hours to 20 hours at temperature of 10 DEG C to 40 DEG C; extraction is carried out after pH value is adjusted to between 8 to 10; then the nitrogen oxide of the aconitine acylate product is obtained by means of column chromatography and separation after pressure reduction and distillation. The compound has obvious desinsection activity and remarkable activities of easing pain, resisting against arrhythmia and tumors, and the like, and can be used for developing new products in respect of medicines, pesticides, etc. In the formula, R refers to alkyl with 1 to 4 carbon atoms while R1, R2 and R3 refer to hydrogen atoms, hydroxyl, aliphatic acyl or aromaticacyl radical.
Owner:SOUTHWEAT UNIV OF SCI & TECH
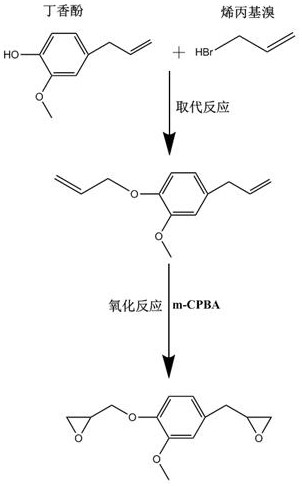
Recyclable eugenol-based epoxy resin Vitrimer material and preparation method thereof
The invention relates to a recyclable eugenol-based epoxy resin Vitrimer material and a preparation method of the recyclable eugenol-based epoxy resin Vitrimer material. Biomass-based eugenol is used as a raw material, an eugenol-based epoxy resin matrix similar to DGEBA is prepared through a two-step method including an allyl bromide substitution reaction and a m-chloroperoxybenzoic acid oxidation reaction, then the eugenol-based epoxy resin matrix is mixed with a dicarboxylic acid curing agent system containing and not containing dynamic bonds, curing is conducted in a gradient heating mode, and theeugenol-based epoxy resin Vitrimer material with a dynamic reversible exchange key anddisulfide bond is otained. The epoxy resin Vitrimer material has the advantages of high physical remodeling reprocessability, high chemical recovery efficiency and the like, and overcomes the defects that the traditional epoxy resin takes petrochemical resources as raw materials and is difficult to recycle after being used; and green development and sustainable utilization of the thermosetting polymer material are promoted.
Owner:SHAANXI UNIV OF SCI & TECH
Tetra-epoxy cage type sesquialter siloxane and preparation thereof
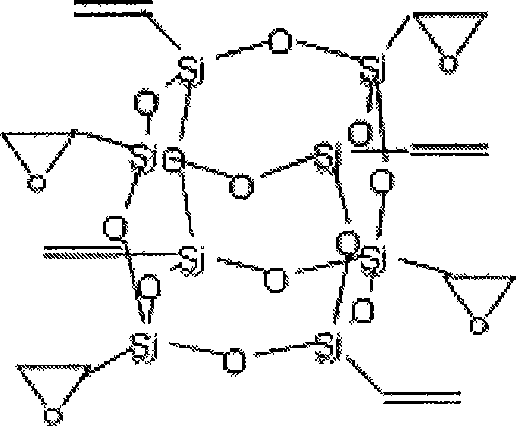
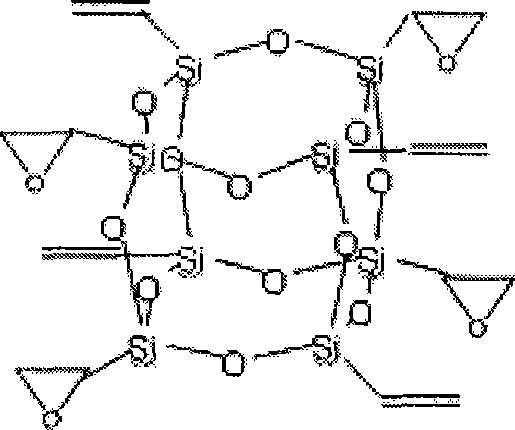
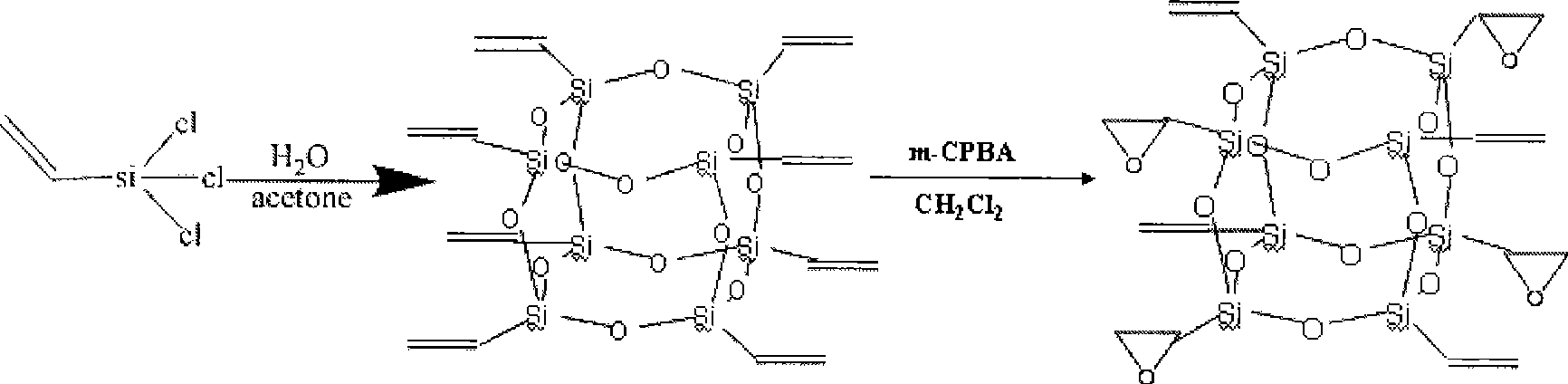
InactiveCN101508698AHigh heat resistanceImprove mechanical propertiesSilicon organic compoundsEpoxySilsesquioxane
The invention relates to polyhedral oligomeric silsesquioxane and a preparation method thereof, in particular to polyhedral oligomeric silsesquioxane with four epoxy groups and the preparation method thereof. Industrialized vinyltrichlorosilane is taken as a primary raw material to synthesize polyhedral oligomeric silsesquioxane with eight vinyl groups, and then the obtained polyhedral oligomeric silsesquioxane is oxidized by m-chloroperoxybenzoic acid or hydrogen peroxide to obtain the polyhedral oligomeric silsesquioxane with four epoxy groups. The preparation method has simple preparation process and low cost, and can be used for modified nylon, epoxy resin and the like to prepare high-performance macromolecular hybrid resin and used for refining chemical reagents.
Owner:BEIJING UNIV OF CHEM TECH
Synthesis method of (3Z,6Z)-9,10-epoxy-octadecadiene
InactiveCN109336846ARaw materials are cheap and easy to getSimple and fast operationOrganic chemistryEpoxySynthesis methods
The invention discloses a synthesis method of (3Z,6Z)-9,10-epoxy-octadecadiene which is one of tea geometrid sex pheromone components. The synthesis method comprises the following steps: coupling propynyl alcohol with bromooctane to produce undec-2-yn-1-ol, and catalytically hydrogenating the undec-2-yn-1-ol to obtain cis-undec-2-en-1-ol; performing a reaction on the cis-undec-2-en-1-ol and m-chloroperoxybenzoic acid to obtain undec-2,3-epoxy-1-ol; sulfonylating hydroxyl groups of the undec-2,3-epoxy-1-ol by p-methylsufonyl chloride to obtain p-methylsulfonyl ester; performing ring opening onan epoxidized compound under the conditions of tri-tert-butylphosphine and chlorosuccinimide to obtain 3-chloro-2-hydroxyundecyl methanesulfonate, and then performing ring closure under an alkali condition to obtain 3-chloro-1,2-epoxyundecane; then, performing ring opening under the conditions of n-butyl lithium and boron trifluorideetherate, and performing ring closure under the condition of potassium carbonate to obtain 2-octyl-3-(prop-2-yn-1-yl)oxirane; then, obtaining 2-(octane-2,5-diyn-1-yl)-3-octyloxirane under the condition of an iodo coupling reagent; and finally, performing hydrogenation catalysis to obtain a final product. The synthesis method is relatively low in cost, and is suitable for scale preparation.
Owner:CHANGZHOU UNIV +1
Method of modifying and reinforcing cement-based composite via thermally-reduced graphene-carbon nanotubes
The invention discloses a method of modifying and reinforcing cement-based composite via thermally-reduced graphene-carbon nanotubes. The method includes: adding water into a dispersant, heating, stirring to allow full dissolution, adding modified multi-walled carbon nanotubes, magnetically stirring, carrying out ultrasonic treatment, cooling to room temperature, adding a defoaming agent before stirring, pouring into a cement mortar mixer, adding cement, standard sand, water, a water reducer, silica fume-adsorbed pre-dispersed nano silica and thermally-reduced graphene dispersion, mixing well,loading in a standard cement triplex mold, smoothing, vibrating, forming, covering with wet cloth, demolding, transferring into a standard maintenance box, and carrying out standard maintenance to obtain a cement-based composite. Carbon nanotubes are treated with dichloromethane and metachloroperbenzoic acid; the treated carbon nanotubes are used to prepare cement mortar having high compressive strength, breaking strength and flexural strength; good reinforcement can be achieved just via a low content of modified carbon nanotubes.
Owner:太和县鑫泰高科新型建筑材料有限公司
Obeticholic acid preparation method
The invention discloses an obeticholic acid preparation method, which comprises that (1) hyodeoxycholic acid II reacts with an alcohol compound III under the action of a catalyst to generate an ester compound IV; (2) the ester compound IV is subjected to PDC oxidation in dichloromethane to generate a compound V; (3) the compound V and trimethyl chlorosilane are subjected to a reaction at a temperature of -70 to -20 DEG C in tetrahydrofuran by using lithium diisopropylamide as an alkali to generate a silyl enol ether compound VI; (4) the silyl enol ether compound VI is subjected to m-chloroperoxybenzoic acid oxidation and deprotection in dichloromethane to generate a compound VII: (5) the compound VII and Yield generated from ethyltriphenylphosphonium bromide under the action of a strong alkali are subjected to a Wittig alkenylation reaction at a temperature of 0-70 DEG C to convert the ketone into the vinyl so as to generate a compound VIII; (6) the double bond of the compound VIII is subjected to catalytic hydrogenation reduction in a mixing solvent to generate a compound IX; and (7) the compound IX is hydrolyzed under an alkaline condition to generate the obeticholic acid.
Owner:XIAMEN HALOSYNTECH CO LTD
Synthetic method of 25-hydroxycholesterol
The invention discloses a synthetic method of 25-hydroxycholesterol. The synthetic method comprises the following steps: (1) protecting 3-hydroxy of 24-dehydrocholesterol so as to obtain acylated 24-dehydrocholesterol; (2) carrying out epoxidation reaction on double bonds on the 24th site of the product obtained in the step (1), namely carrying out epoxidation reaction on the acylated 24-dehydrocholesterol and an epoxidation reagent in a solvent II under the catalytic action of manganese salt so as to obtain an epoxidation product; and (3) carrying out reduction ring opening on the epoxidation product obtained in the step (2), namely carrying out reduction ring opening on the epoxidation product obtained in the step (2) and a reduction reagent in a solvent III so as to obtain the 25-hydroxycholesterol. The synthetic method of the 25-hydroxycholesterol has the advantages that peroxyacetic acid and meta-chloroperoxybenzoic acid which are non-toxic and low in cost are used as the epoxidation reagents and environmentally-friendly, the reaction is carried out at a low temperature, and the manganese salt is used as a catalyst and is high in selectivity.
Owner:ZHEJIANG UNIV +1
Synthesis technology ofminoxidil
The invention discloses a synthesis technology of minoxidil. The technology comprises steps as follows: firstly, meta-chloroperoxybenzoic acid is utilized to oxidize 2,4-diamino-6-chloropyrimidine, 2,6-diamino-4-chloropyrimidine-1-oxide as an intermediate is obtained, then 2,6-diamino-4-chloropyrimidine-1-oxide as the intermediate is subjected to condensation with piperidine under the alkaline condition, and a crude product of minoxidil is obtained; finally, the crude product of minoxidil is crystallized in isopropanol and purified, and a refined finished minoxidil product is obtained. The technology adopts a simple principle and is convenient to operate, and the product is high in yield and good in purity.
Owner:安徽拜善晟制药有限公司
Method for preparing benzophenone through biomimetic catalytic oxidation
ActiveCN104402685AHigh selectivityHighly selective oxidationOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsDiphenylmethaneReaction temperature
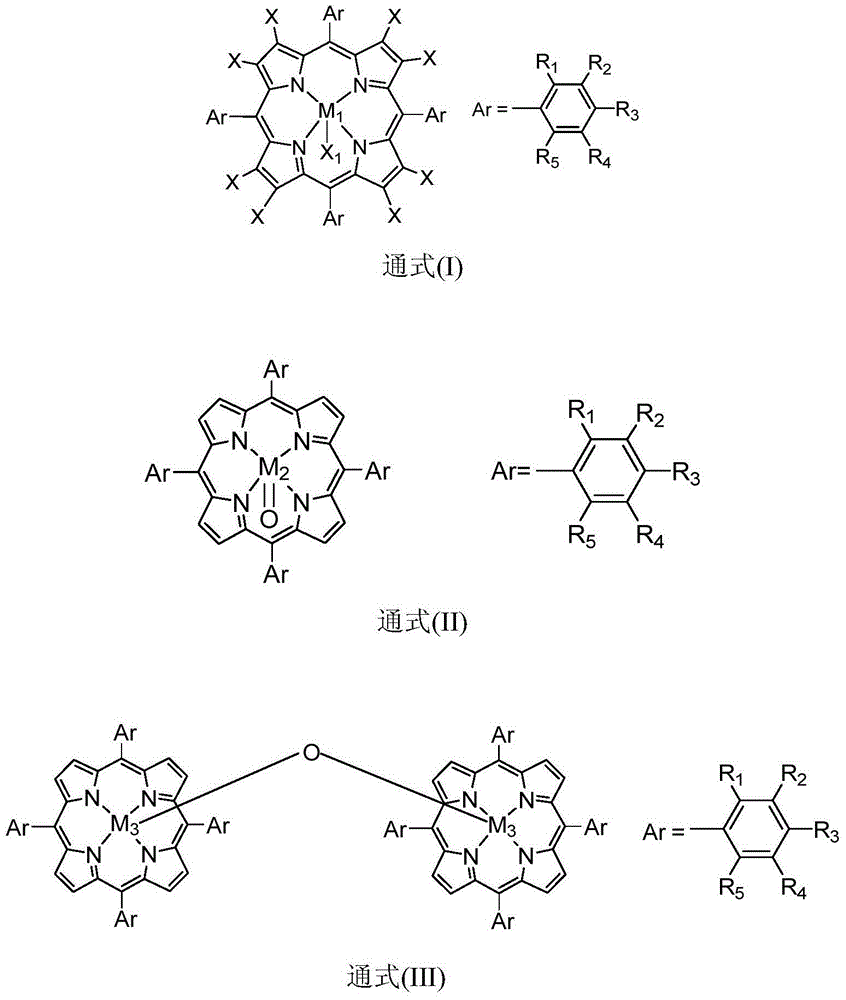
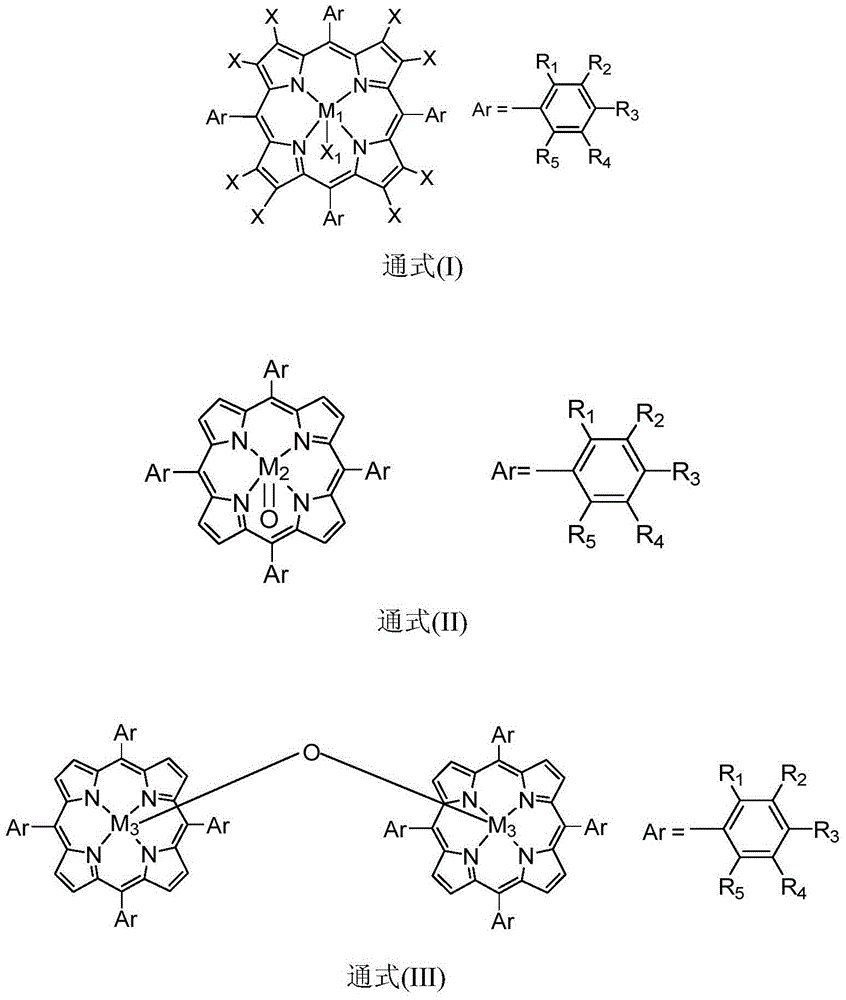
The invention discloses a method for preparing benzophenone through biomimetic catalytic oxidation, which takes diphenylmethane as a raw material, takes a metalloporphyrin compound as a catalyst, and takes tert-butyl hydroperoxide and metachloroperbenzoic acid or hydrogen peroxide as a oxidizing agent, and benzophenone can be obtained through catalytic reaction by controlling the reaction temperature at 40-100 DEG C. The method has the advantages of mild reaction condition, good catalysis effect, high products selectivity and simple process.
Owner:HUIZHOU RES INST OF SUN YAT SEN UNIV
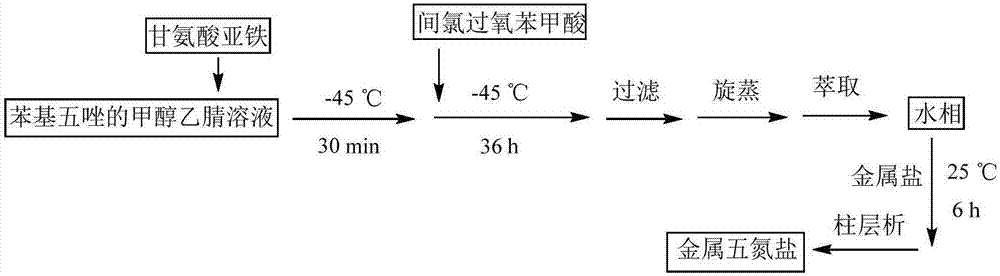
Pentazole composite salt and preparation method thereof
The invention discloses a pentazole composite salt and a preparation method thereof. By cutting off a C-N bond of substituted phenyl pentazole, a pentazolate anion is prepared. By adopting m-chloroperoxybenzoic acid and a ferrous salt as an oxidizing agent and a protective agent, respectively, a pentazolate-anion-containing composite salt [(N5)6(H3O)3(NH4)4Cl] is prepared. According to the invention, the pentazolate-anion-containing composite salt is achieved by cutting off the C-N bond of the substituted phenyl pentazole and successfully separating out the pentazolate anion. Also the synthesized pentazole composite salt can stably exist at normal temperature, and has great application values in the field of energetic materials.
Owner:NANJING UNIV OF SCI & TECH
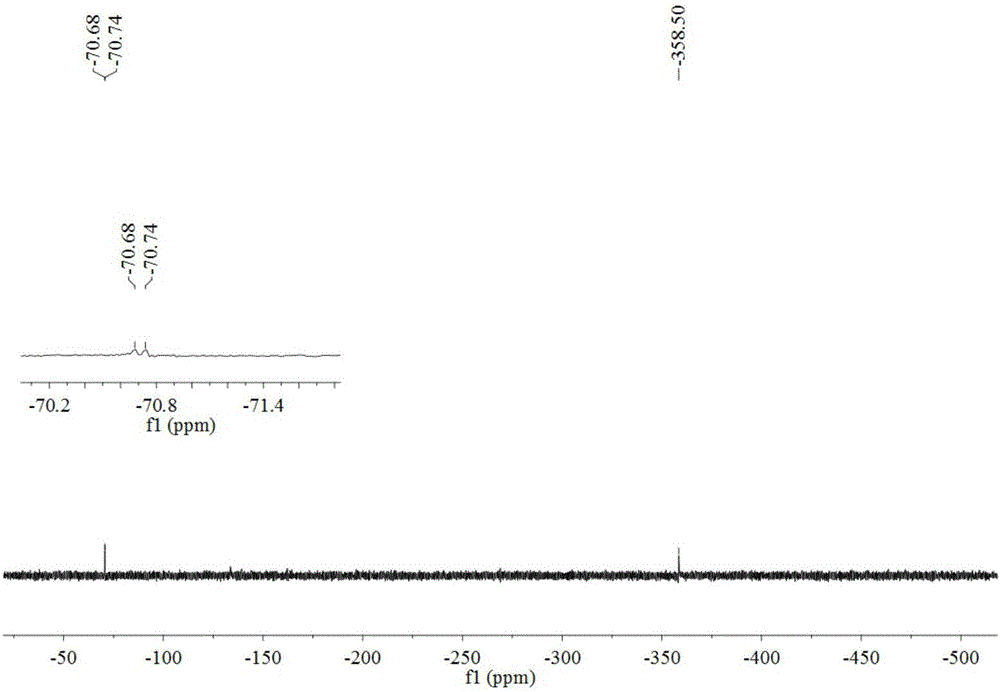
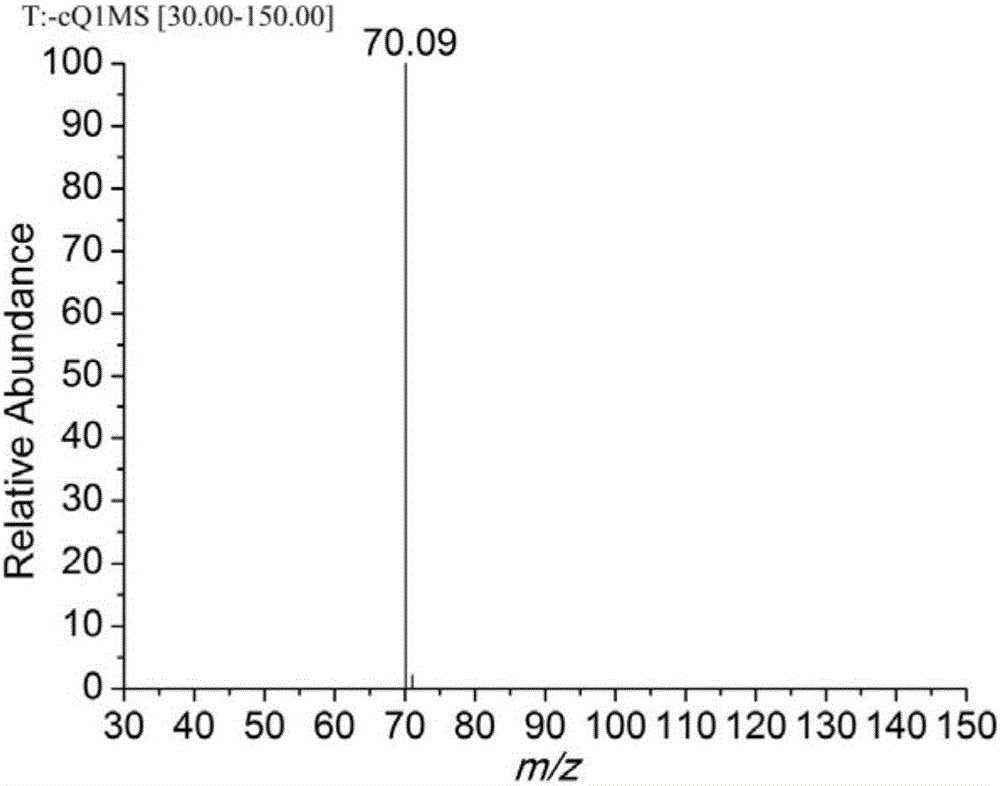
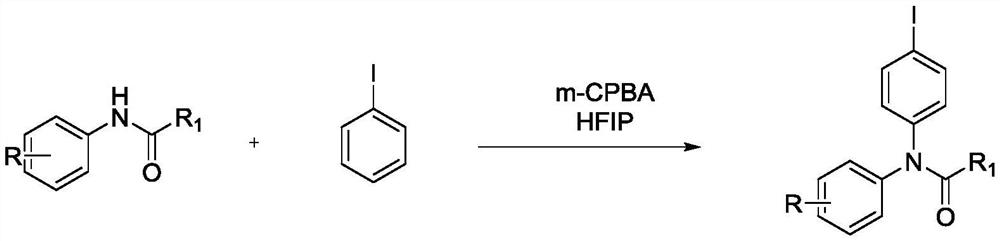
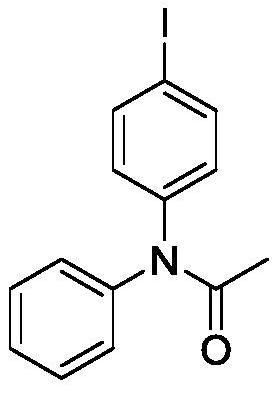
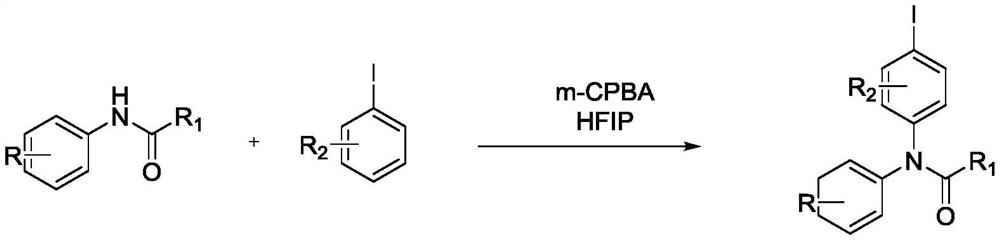
Preparation method of iodobenzene para-amination compound promoted by m-chloroperoxybenzoic acid
ActiveCN111646917ABiologically activeMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationBenzoic acidChromatographic separation
The invention relates to a preparation method of an iodobenzene para-aminated compound promoted by m-chloroperoxybenzoic acid, and the method comprises the following steps: adding substituted acetanilide, iodobenzene, m-chloroperoxybenzoic acid and hexafluoroisopropanol into a reactor, putting the reactor into an oil bath pot at 40-80 DEG C, condensing, refluxing, magnetically stirring, and heating to react for 4-10 hours; pouring the obtained reaction liquid into a separating funnel, adding distilled water, extracting for three times by using an organic solvent, carrying out reduced pressuredistillation on an organic phase to obtain a crude product, and carrying out column chromatography separation and purification to obtain the iodobenzene para-aminated compound. The method is mild in reaction condition, high in selectivity, high in yield and environmentally friendly, and the synthesized aryl amide compound has certain biological activity and can be applied to the fields of medicinesynthesis, pesticide synthesis, paint and dye synthesis and the like.
Owner:HUBEI UNIV OF TECH
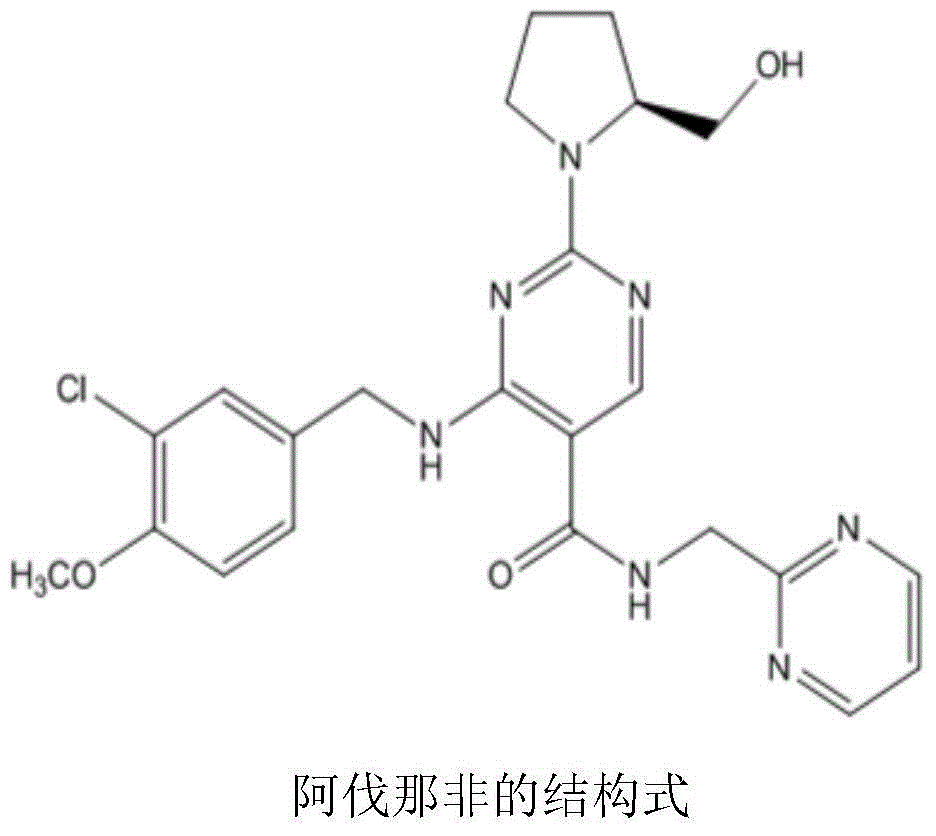
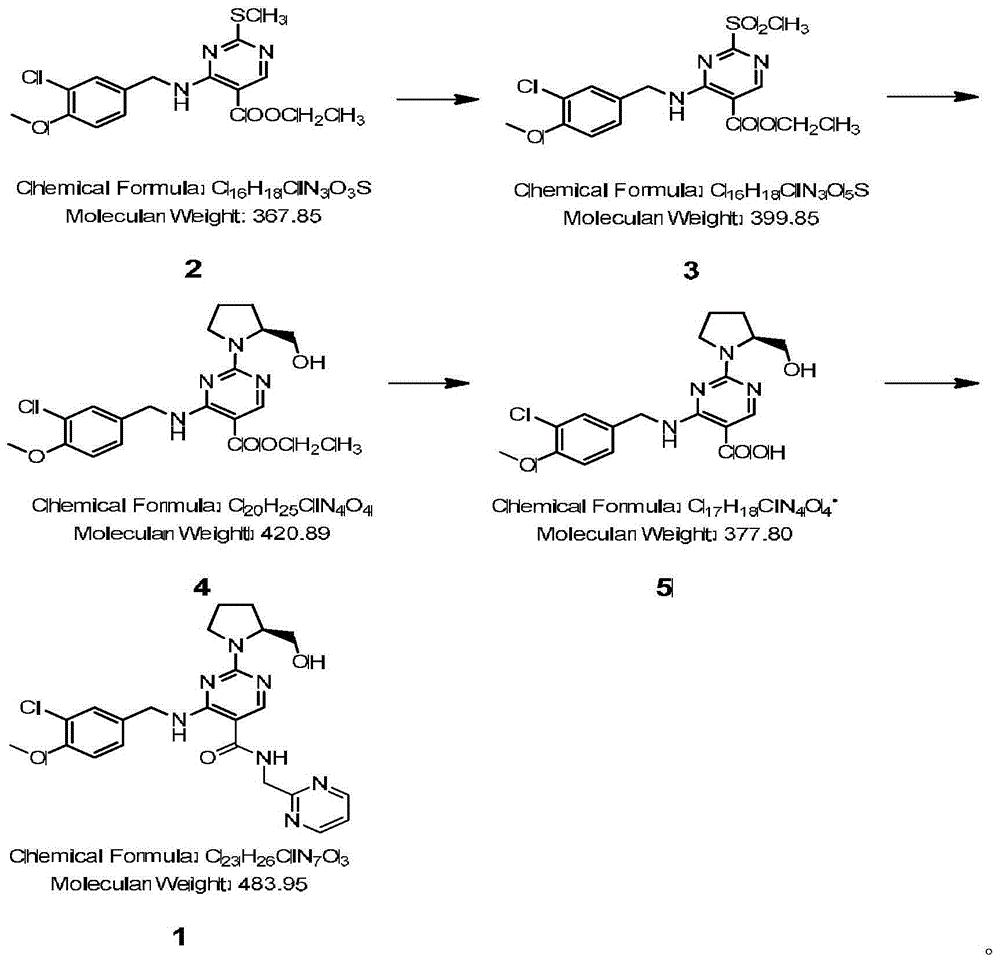
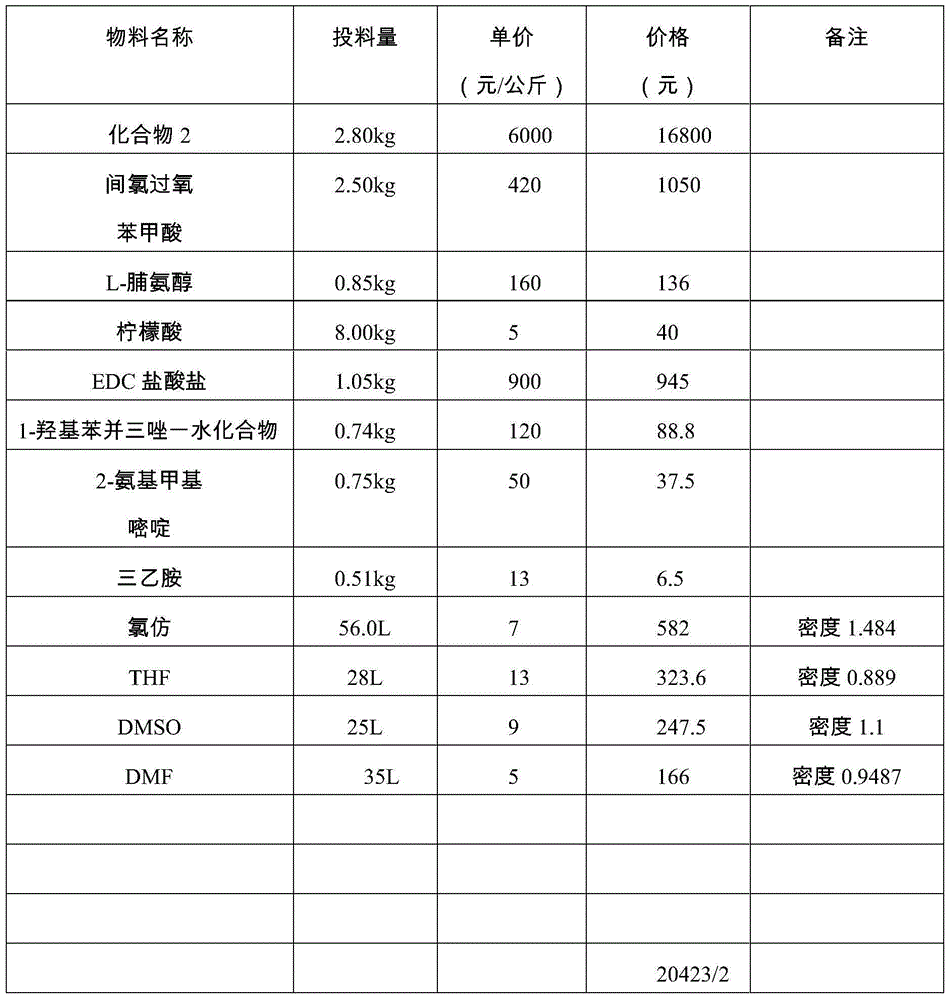
Avanafil preparation method
InactiveCN104530017ASolve purificationSolve the problem of racemization of chiral centersOrganic chemistryCarboxylic acidFormamide
The invention relates to an avanafil preparation method. The method comprises the steps that a hydrolysis reaction is carried out on 4-(3-chlorin-4-methoxy benzyl amino)-2-methylehio pyrimidine-5-carboxylic acid ethyl ester, namely a compound (2) to obtain 4-(3-chlorin-4-methylehio pyrimidine)-2-methylehio pyrimidine-5-carboxylic acid, namely a compound (6); an acylation reaction is carried out on the compound (6) and thionyl chloride, and then the product and 2-sulfadoxine-pyrimethamine or a salt of 2-sulfadoxine-pyrimethamine are condensed to obtain 4-(3-chlorin-4-methylehio pyrimidine)-2-methylehio pyrimidine-N-(2- pyrimidine methyl)-5-pyrimidine formamide, namely a compound (7); the compound (7) is oxidized by metachloroperbenzoic acid to obtain 4-(3-chlorin-4-methylehio pyrimidine)-2-methylsulfonyl pyrimidine-N-(2-pyrimidine methyl)-5-pyrimidine formamide, namely a compound (8), a nucleophilic substitution reaction is carried out on the compound (8) and L-prolinol to obtain avanafil. The method is low in cost, easy and convenient to operate, high in product purity, high in reaction yield and suitable for industrialization scale production.
Owner:NANJING XIAOZHUANG UNIV
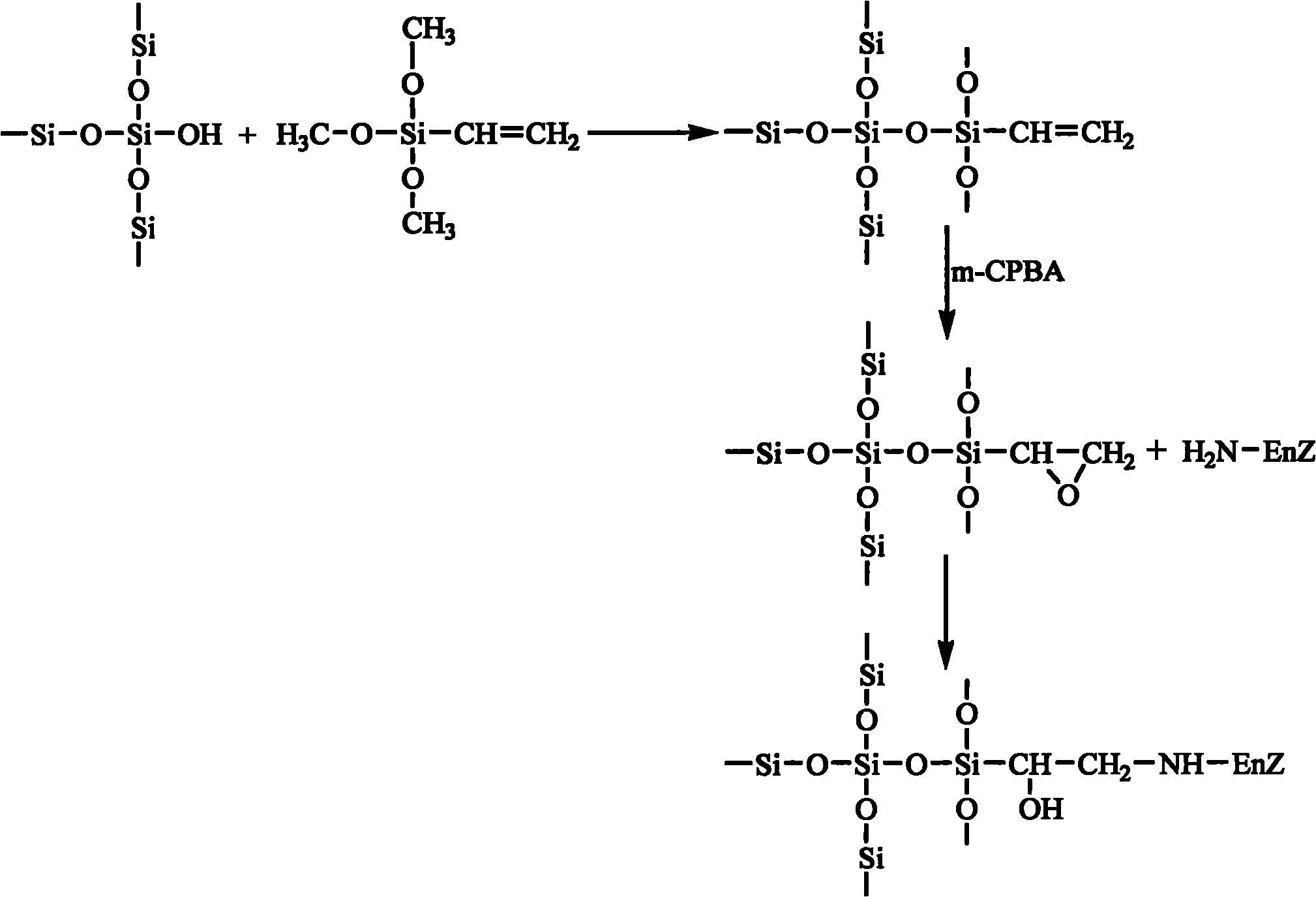
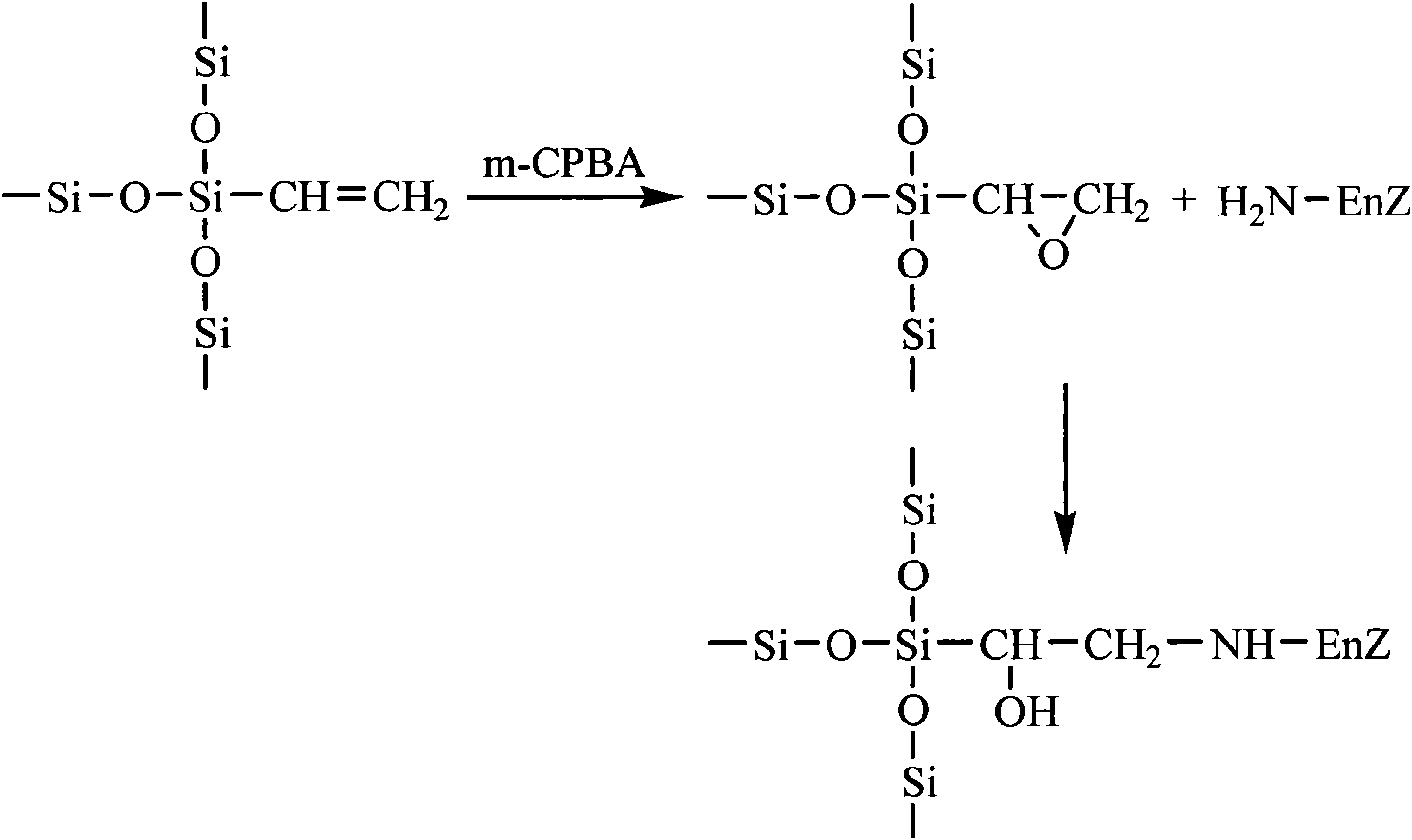
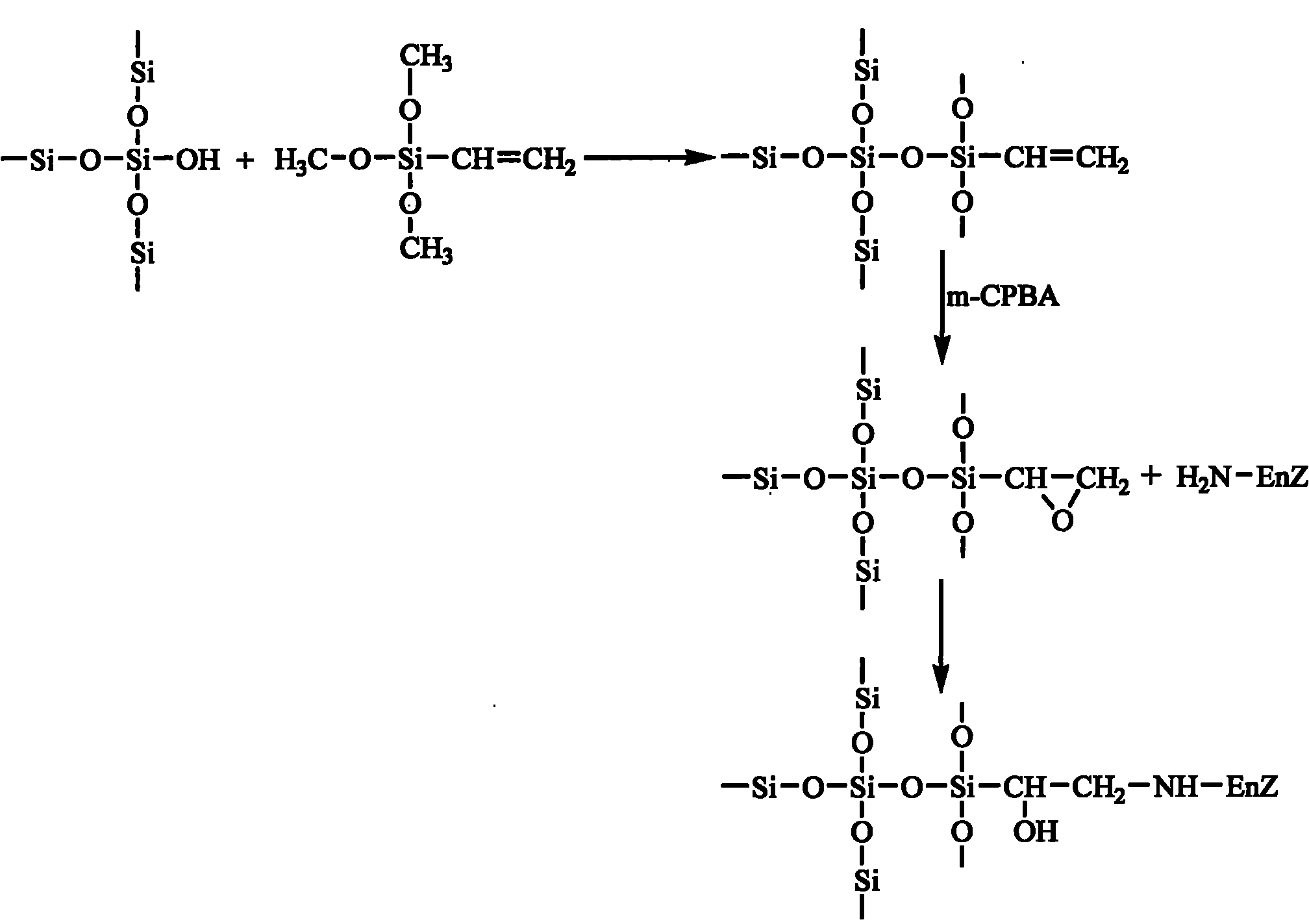
Epoxy mesoporous molecular sieve for use in bio-enzyme immobilization and preparation method thereof
The invention discloses an epoxy mesoporous molecular sieve for use in bio-enzyme immobilization and a preparation method thereof. In the invention, cyclo groups of the mesoporous molecular sieve which contains vinyl and has a cubic phase Ia3d structure and the mesoporous molecular sieve which has a surface vinyl function are oxidized by using metachloroperbenzoic acid to introduce epoxy functional groups with a shorter chain length onto the surface of the mesoporous molecular sieve while the influences of a surface functionization process on the aperture, the specific surface area and the pore volume of the mesoporous molecular sieve are reduced as much as possible, so the bio-enzyme can be directly immobilized on the surface of the mesoporous molecular sieve in a covalent bonding form without further activation, and the performance of the immobilized enzyme can be improved. The epoxy mesoporous molecular sieve can be used for the immobilization of water soluble bio-enzymes such as penicillin acylase, glucose isomerase, glucosidetransferase, parenzyme and diastase, in particular for the immobilization of the penicillin acylase; and the activity of the obtained immobilized enzyme is 8416U / g, and 90 percent of original activity of the immobilized enzyme is retained after 10 times of recycling.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of N-iodobenzene-N-phenyl amide compound
ActiveCN111606820ABiologically activeMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationBenzoic acidChromatographic separation
The invention relates to a preparation method of an N-iodobenzene-N-phenyl amide compound, which comprises the following steps: adding substituted acetanilide, substituted iodobenzene, m-chloroperoxybenzoic acid and hexafluoroisopropanol into a reactor, putting the reactor into an oil bath pot at 40-80 DEG C, carrying out reflux condensation, magnetically stirring, and heating to react for 4-10 hours; pouring the obtained reaction liquid into a separating funnel, adding distilled water, extracting for three times by using an organic solvent, carrying out reduced pressure distillation on an organic phase to obtain a crude product, and carrying out column chromatography separation and purification to obtain the N-iodobenzene-N-phenyl amide compound. The method has the advantages of mild reaction conditions, high selectivity, high yield and environmental friendliness. The synthesized N-iodobenzene-N-phenyl amide compound has certain biological activity and can be applied to the fields ofdrug synthesis, pesticide synthesis, paint and dye synthesis and the like.
Owner:HUBEI UNIV OF TECH
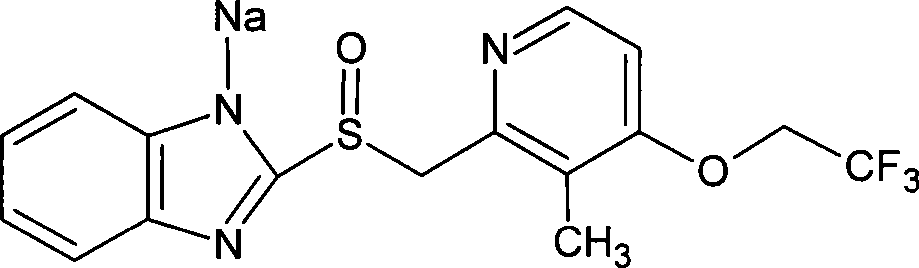
Production method of high-optical-purity esomeprazole
The invention belongs to the field of pharmacy and relates to a production method of high-optical-purity esomeprazole salt. The method takes m-chloro peroxy-sodium benzoate as an oxidizing agent and takes an organic solvent which is dissolved into water as a reaction solvent, so that the problem that the acidity of the m-chloro peroxy-sodium benzoate cannot be applied to preparing the esomeprazole is solved. The method is simple to operate and has a low cost; and the optical purify of the prepared product is high and the impurity content is low.
Owner:NANJING XUNAN PHARMA TECH
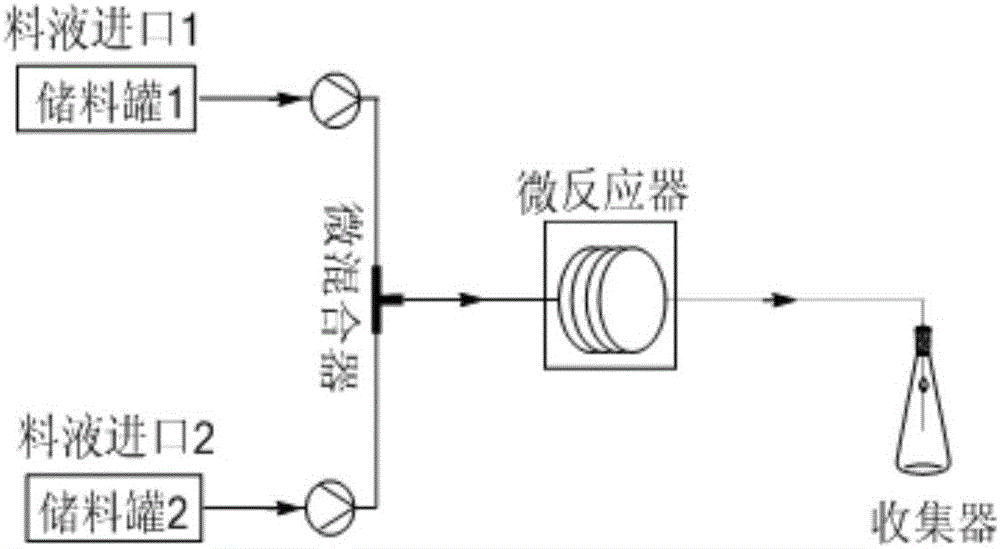
Method for preparing epsilon-caprolactone by using micro-reaction device
The invention discloses a method for preparing epsilon-caprolactone by using a micro-reaction device. The epsilon-caprolactone is prepared from meta-chloroperbenzoic acid and ethyl acetate as raw materials by using a micro-channel reaction technique. Compared with the prior art, the method adopts the micro-reaction device for synthesizing the epsilon-caprolactone, so that problems of conventional production can be overcome, the raw material conversion rate can be increased, the reaction time can be shortened, the content of byproducts can be reduced, the process procedures are simplified, circulation of an oxidant can be achieved, the production cost can be lowered, the production process security of the epsilon-caprolactone can be greatly improved, the product quality can be improved, and the industrial production can be facilitated.
Owner:NANJING UNIV OF TECH
Aqueous metal ion pentazole salts and preparation method thereof
The invention discloses aqueous metal ion pentazole salts and a preparation method thereof. The metal salts have a chemical structural formula of M(N5)x(H2O)y. When M represents a monovalent metal Li, Na, K or Ag, x is 1 and y is 4. When M represents a divalent metal Fe, Co, Ni, Mg, Ca, Ba, Cu, Zn or Pb, x is 2 and y is 8 or 10. The preparation method utilizes metachloroperbenzoic acid and ferrous glycinate as a cleavage reagent and an aid acting with aryl pentazole. Through an oxidative cleavage method, the C-N bond of the aryl pentazol molecule is selectively cut and a metal ion-containing salt is used as a capture reagent in preparation of the aqueous metal ion pentazole salts. The preparation method successfully prepares the metal pentazole salts. The metal pentazole salts can be widely used in the field of energetic materials.
Owner:NANJING UNIV OF SCI & TECH
Biomass-based epoxy resin and preparation method thereof
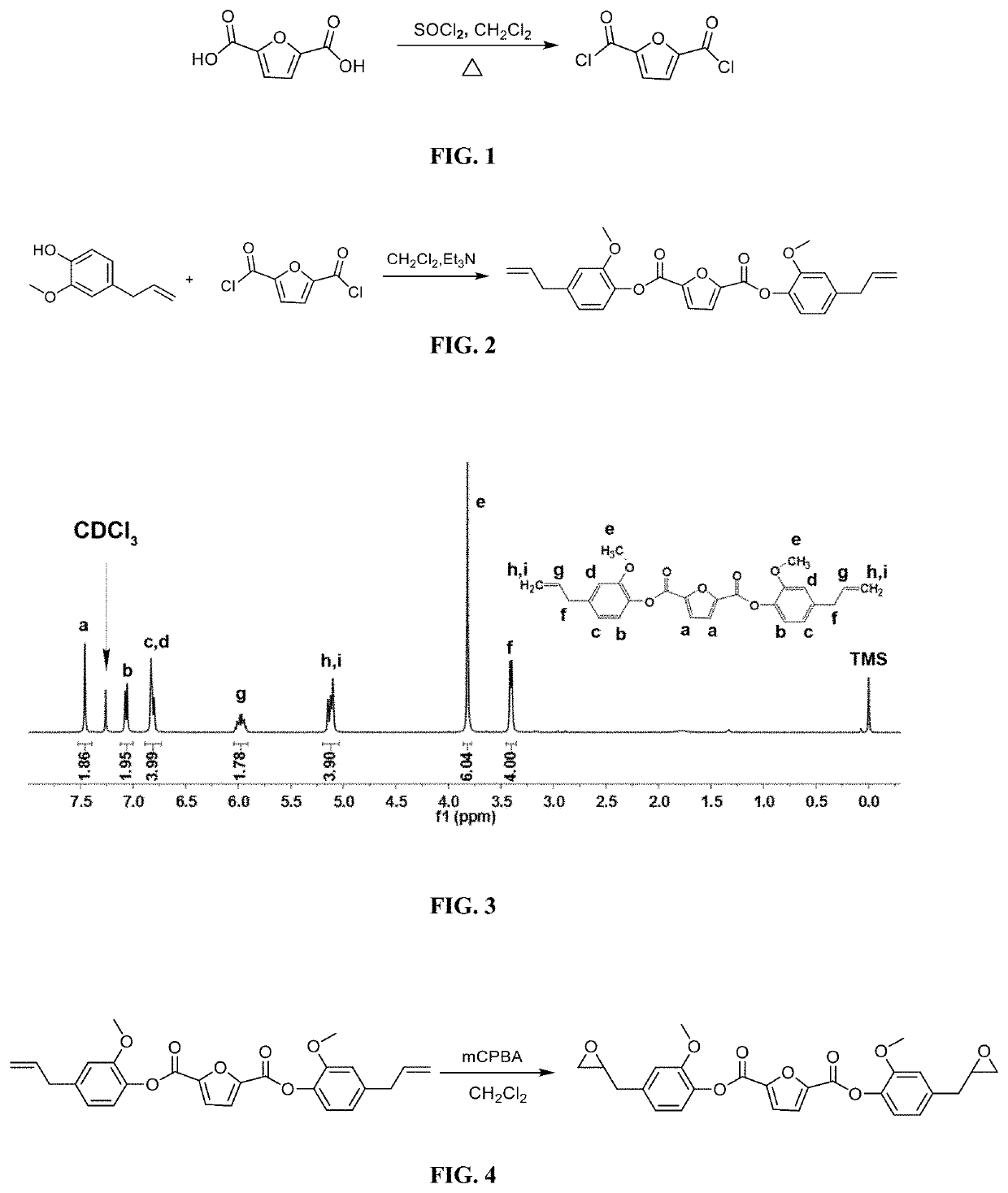
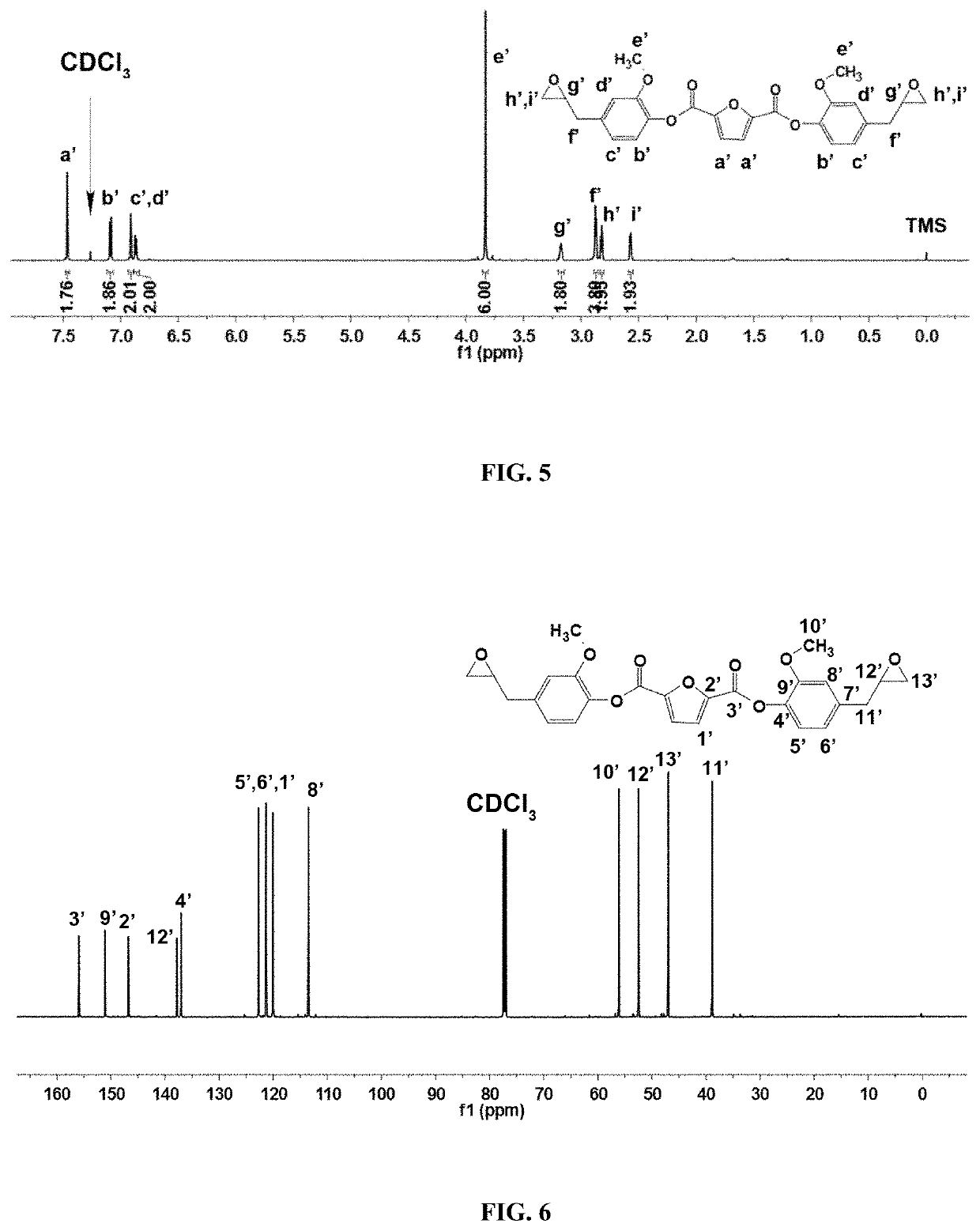
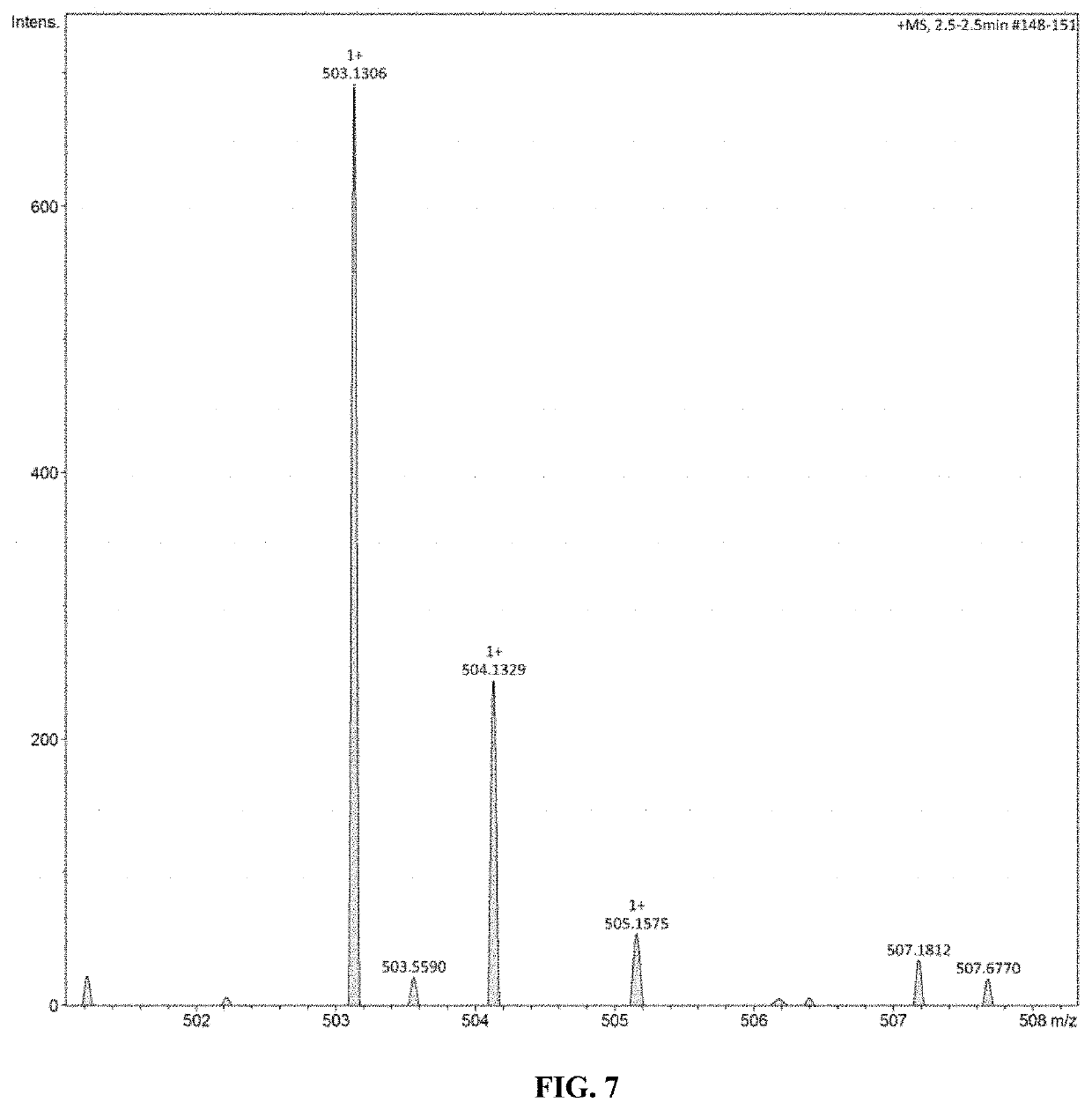
ActiveUS20200062888A1Improve mechanical propertiesGood thermal propertiesOrganic chemistryFuranBenzoic acid
The present invention discloses a biomass-based epoxy resin and preparation method thereof; under conditions of N,N-dimethylformamide as a catalyst, 2,5-furandicarboxylic acid and thionyl chloride are acylated to obtain 2,5-furan diformyl chloride; then it is dissolved with dichloromethane; under tertiary amine conditions an esterification reaction takes place, and bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylic acid ester is thus obtained; by means of meta-chloroperoxybenzoic acid, its unsaturated double bond is epoxidized to obtain a biomass-based epoxy resin. The process of the present invention is simple; the raw materials come from biomass 2,5-furandicarboxylic acid and eugenol; in comparison with bisphenol-A epoxy resin based on petroleum and coal resources, the raw materials are green and renewable, and are advantageous to reducing the consumption of renewable resources with regard to polymeric material. The obtained cured epoxy resin has excellent thermal properties and modulus, and has broad prospects for application.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com