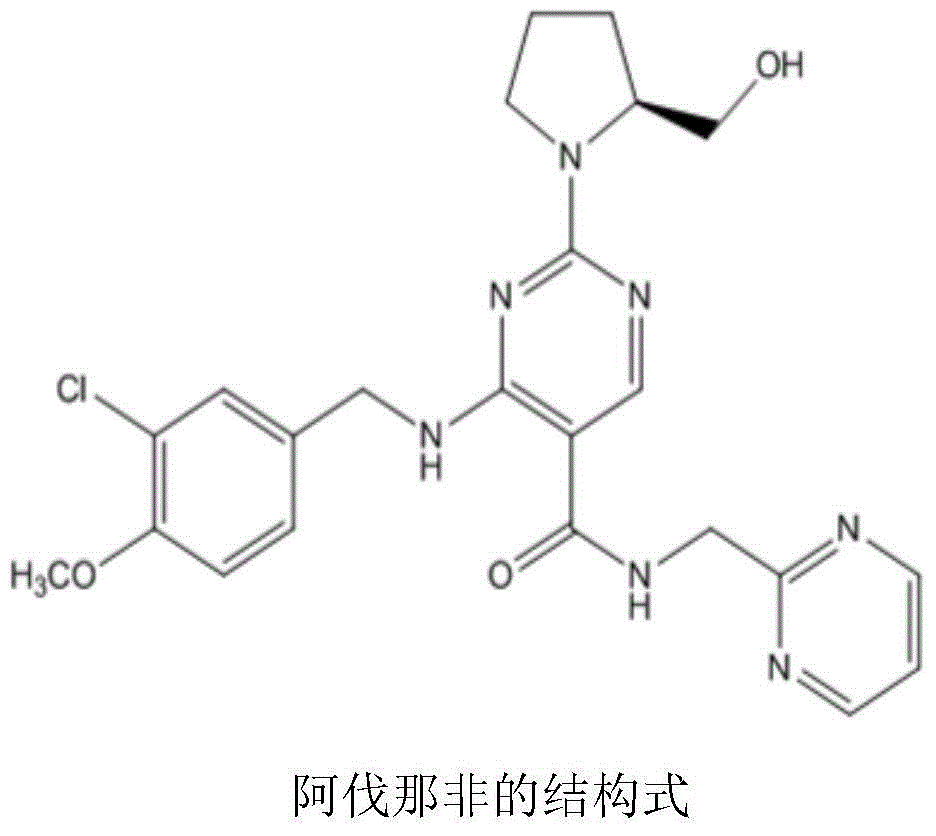
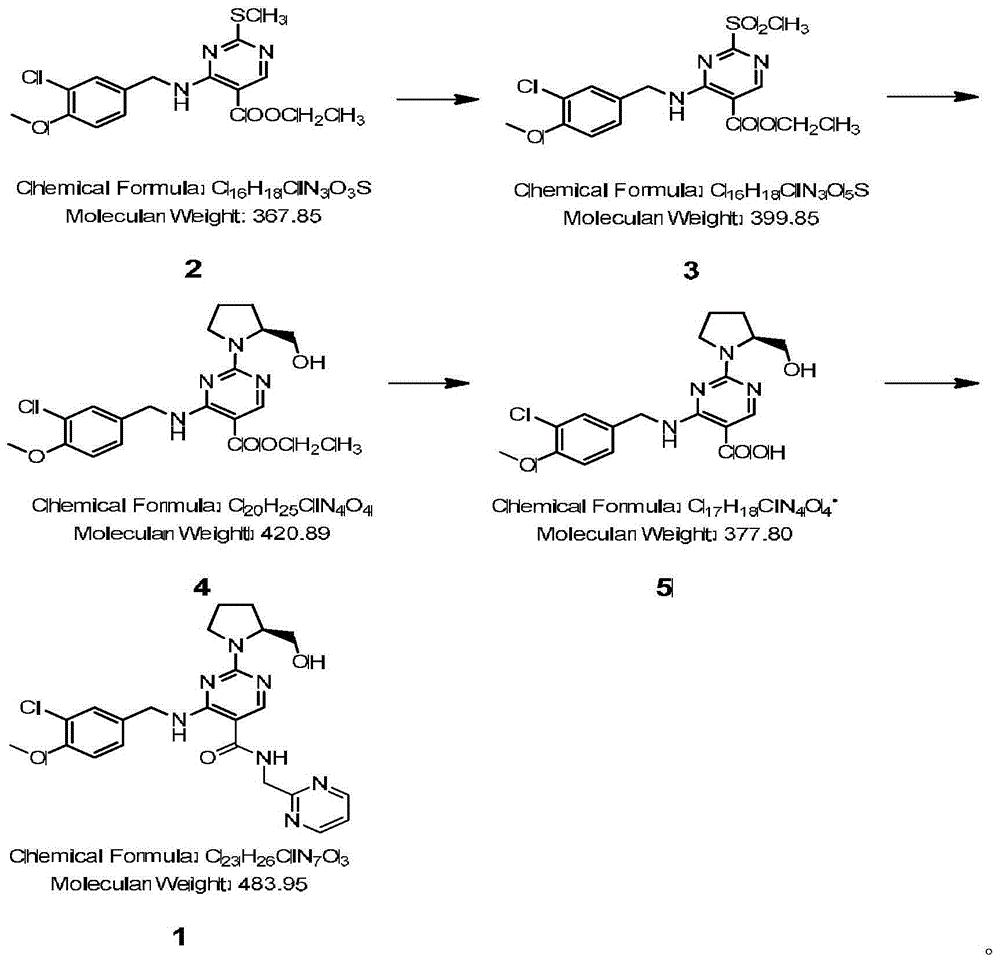
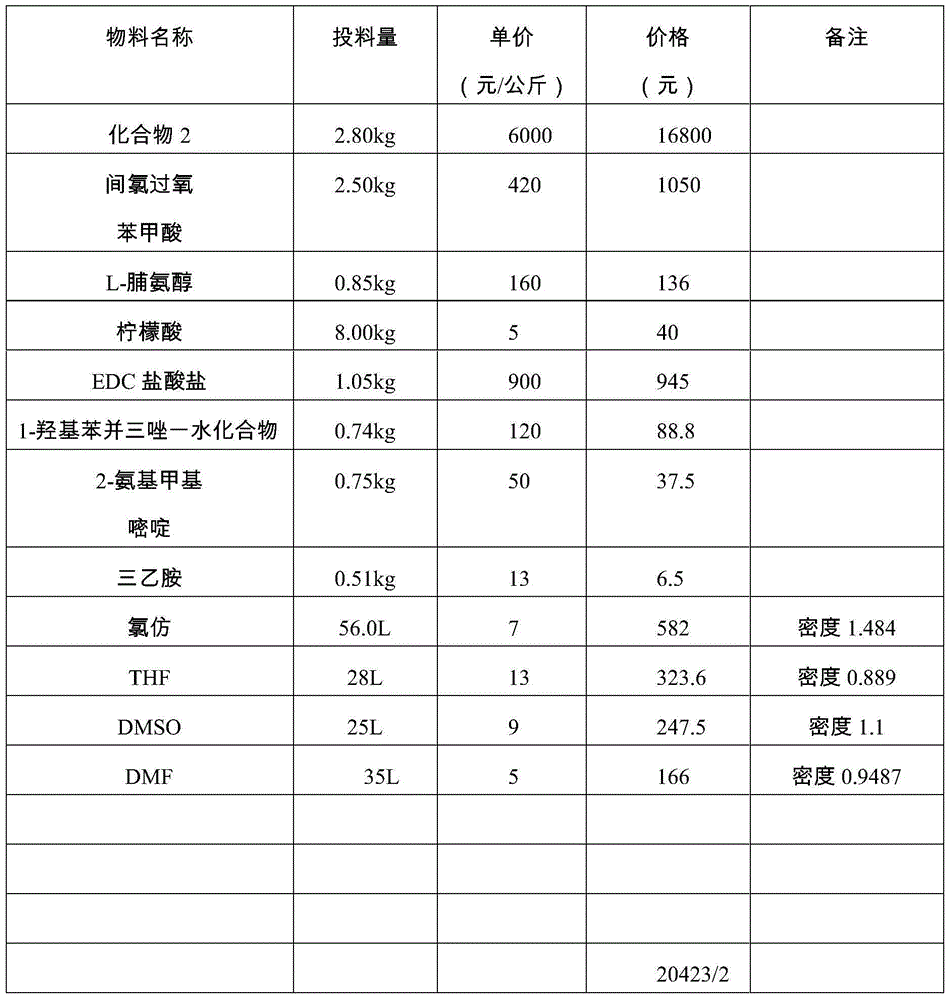
Avanafil preparation method
An avanafil and compound technology, which is applied in the field of drug synthesis, can solve the problems of low product purity, low reaction yield, complicated operation, etc., and achieves the advantages of reducing production cost, simple operation, and improving reaction yield and product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] first hydrolysis
[0037] Its reaction formula is as follows:
[0038]
[0039] Add 5g of compound 2 (sulfide) into a three-necked flask, add 50g of water, add 27% sodium hydroxide solution dropwise at room temperature (20°C), raise the temperature to 90°C, and heat until it dissolves. After 3.5 hours, the solution cleared and the system was light yellow. Cool down to room temperature (20°C), adjust the pH to 4.0 with 10% hydrochloric acid, share 6g of 10% hydrochloric acid (the initial pH value is around 12.5), filter with suction after the pH is stable, wash with 25ml of water for 3 Wash once with 20ml ethanol, dry in vacuum at 45°C until the water content is <0.1%. See Table 2 for the raw materials and the feeding amount of this step.
[0040] Table 2
[0041] name
[0042] second condensation
[0043] Its reaction formula is as follows:
[0044]
[0045]Add 15g of compound 6 (hydrolyzed acid) into a single-necked flask, add 150ml of toluene, 12g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com