Tetra-epoxy cage type sesquialter siloxane and preparation thereof
A technology of silsesquioxane and tetraepoxy group, applied in the direction of silicon organic compounds, etc., to achieve the effect of delaying glass transition, improving modification performance, improving mechanical properties and dielectric properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
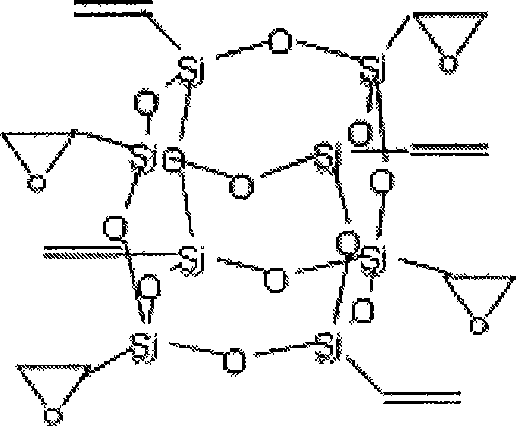
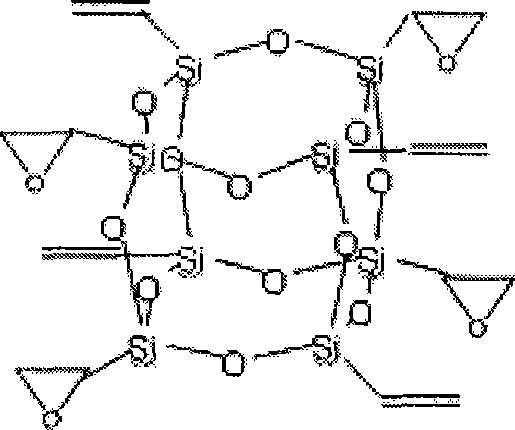
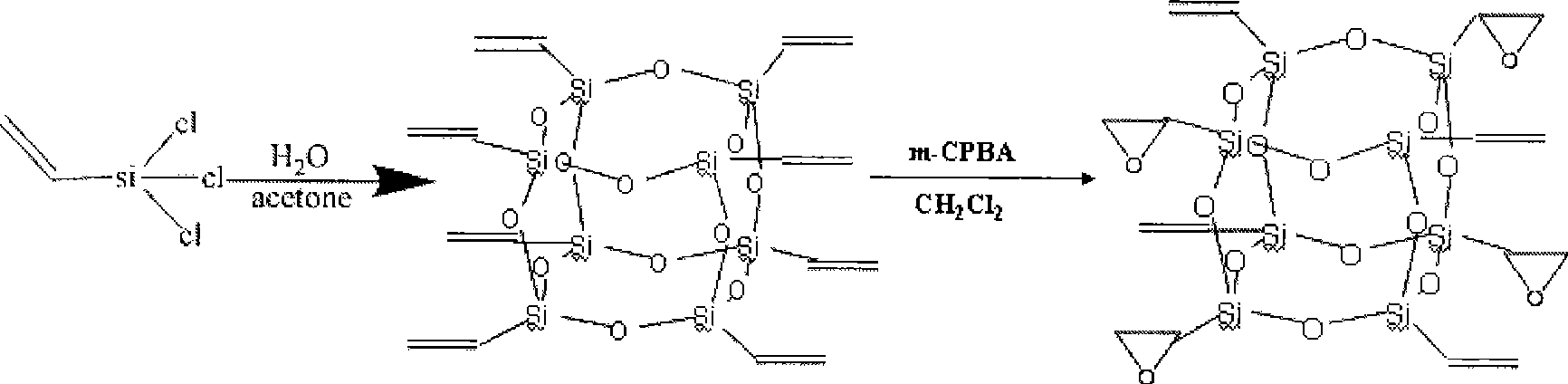
[0020] Synthesis of octavinyl cage silsesquioxane: Add 1200ml acetone and 60ml (0.47mol) vinyltrichlorosilane to a 2000ml three-necked flask in sequence, raise the temperature to 30°C, start stirring, then add 180ml deionized water dropwise, After about 20 minutes of dripping, react at 30°C for 160 hours, the solution turns dark reddish brown, white crystals precipitate out, stand for 10 hours, filter with suction, and vacuum dry at 45°C for one hour to obtain 9.18g (0.014mol) of white powder , is octavinyl cage silsesquioxane, and the mass yield is 25%. Its nuclear magnetic resonance and infrared absorption analysis characterization data are: 29 Si NMR (119MHz, CDCl 3 , 300K, acetone-d 6 , ppm): -80.23; FTIR (cm -1 , KBr): 1604.18 (ν C=C ), 1109.55(ν Si-O-Si ), 779.48(ν Si—C ), 1409.73 (in-plane bending α =CH ), 1276.76 (in-plane bending β =CH ), 971.21 (out-of-plane bending α =CH ), 1005.17 (out-of-plane bending β =CH ).
[0021] Synthesis of tetraepoxy cage silse...
Embodiment 2
[0023] Synthesis of tetraepoxy cage silsesquioxane in organic system: Weigh 8g of m-chloroperoxybenzoic acid and pour it into a 500ml round bottom flask, add 200ml of dichloromethane to it, and stir with a magnet to dissolve it completely . Then take by weighing 6g (0.0095mol) octavinyl cage type silsesquioxane (preparation method is the same as Example 1) and be dissolved in 100ml of dichloromethane, after it dissolves completely, use constant pressure funnel with 5ml / min Drop into the round-bottomed flask in which m-chloroperoxybenzoic acid was dissolved at a speed of 30°C for 48 hours under reflux. The resulting solution was then rotary evaporated at 50°C to give a white solid, which was dried under vacuum at 45°C for one hour. After taking out, the solid powder was dissolved in 300ml of methanol, and stirred at room temperature for 30 hours. Suction filtration and vacuum drying at 45° C. for one hour gave 6.3 g of white powder, which was tetraepoxy cage silsesquioxane, w...
Embodiment 3
[0025] Preparation of tetraepoxy cage silsesquioxane using inorganic system: the preparation method of vinyl cage silsesquioxane is the same as that in Example 1. Dissolve 5g of octavinyl cage silsesquioxane in 80mL of chloroform, and add it into a 250mL three-necked flask. Slowly pour 40mL of glacial acetic acid and 2mL of concentrated sulfuric acid into the mixture, stir it electromagnetically and heat up to 70°C. Add 80 mL of hydrogen peroxide dropwise with a dropping funnel at a rate of one drop per second, and condense and reflux for 12 hours. The reaction solution was washed successively with sodium carbonate solution and deionized water, the water phase was removed, dried over anhydrous magnesium sulfate, and the solvent was evaporated to dryness in vacuo to obtain 4.95 g of white powder, which was tetraepoxy cage silsesquioxane, with a mass yield of The rate is 90%. Its nuclear magnetic resonance and infrared absorption analysis characterization data are: 29 Si NMR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com