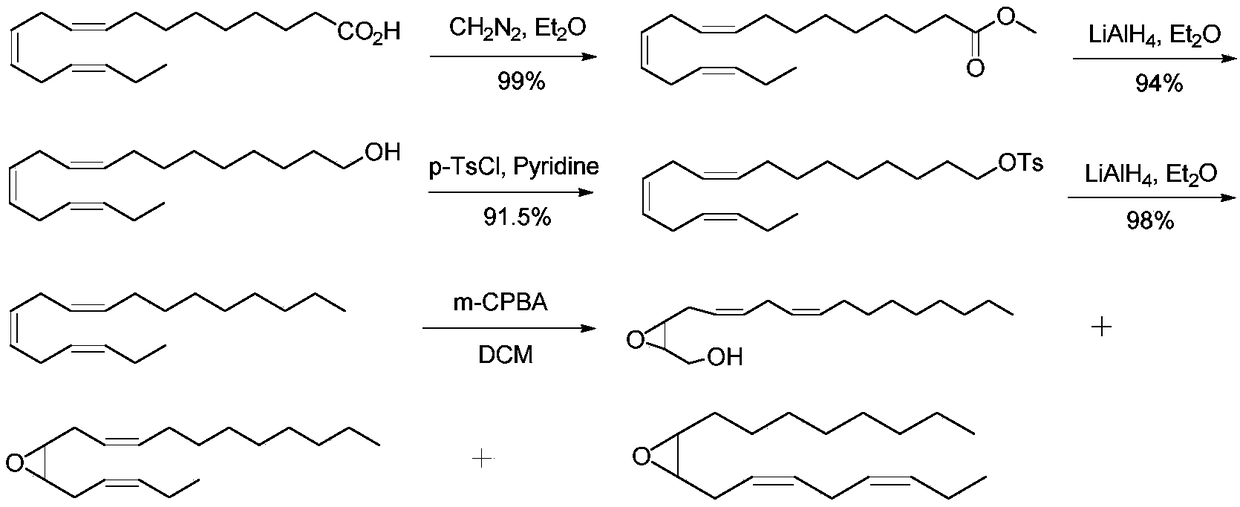
Synthesis method of (3Z,6Z)-9,10-epoxy-octadecadiene
A technology of octadecadiene and a synthesis method, applied in the field of pharmaceutical synthesis, can solve the problems of unsuitability for large-scale preparation, high price of natural linolenic acid, etc., and achieves the effects of low cost, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
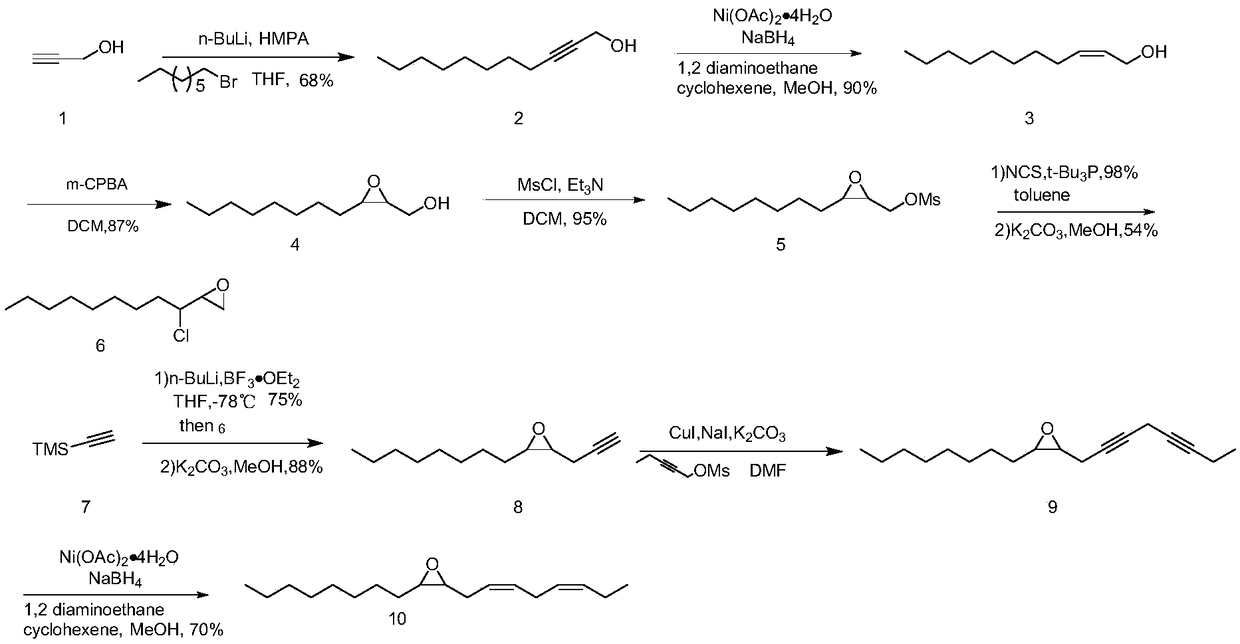
[0037] Step 1: Preparation of Undec-2-yn-1-ol
[0038] Embodiment 1: join propynyl alcohol (2g, 35.7mmol) and hexamethylphosphoric triamide (21mL, 107mmol) in anhydrous THF (100mL), under N 2 Under protection, cool down to -78°C, slowly add n-butyllithium (28.6mL, 71.4mmol, 2.5M in hexane), stir for 10min, then add bromooctane (7.5g, 39.3mmol), stir overnight at room temperature . After the reaction was complete, 10 mL of dilute hydrochloric acid was added to quench at 0°C. Extracted with 10% hydrochloric acid and methyl tert-butyl ether (MTBE), washed with saturated brine, collected the organic phase, dried with anhydrous sodium sulfate, and purified by column chromatography to obtain 4.1 g (yield 68%) of brown oily liquid One carbon-2-yn-1-ol. 1 H NMR (300MHz, CDCl 3 ):δ0.85(t,J=6.9Hz,3H),1.24-1.36(m,10H),1.43-1.50(m,2H),2.15-2.21(m,3H),4.21-4.23(m,2H ); 13 C NMR (75MHz, CDCl 3 ): δ14.2, 18.8, 22.7, 28.7, 28.9, 29.2, 29.3, 31.9, 51.3, 78.4, 86.6.
Embodiment 2
[0039] Embodiment 2: join propynyl alcohol (2g, 35.7mmol) and hexamethylphosphoric triamide (21mL, 107mmol) in anhydrous tetrahydrofuran (100mL), under N 2 Under protection, cool down to -10°C, slowly add n-butyllithium (28.6mL, 71.4mmol, 2.5M in hexane), stir for 10min, then add bromooctane (7.5g, 39.3mmol), stir overnight at room temperature . After the reaction was complete, 10 mL of dilute hydrochloric acid was added to quench at 0°C. Extracted with 10% hydrochloric acid and methyl tert-butyl ether (MTBE), washed with saturated brine, collected the organic phase, dried with anhydrous sodium sulfate, and purified by column chromatography to obtain 3 g (yield 50%) of brown oily liquid eleven Carbo-2-yn-1-ol. Compared with embodiment 1, change reaction temperature, other conditions are constant. product of 1 H NMR and 13 CNMR is completely consistent with Example 1.
[0040] Step 2: Preparation of cis-undec-2en-1-ol
Embodiment 3
[0041] Embodiment 3: Ni(OAc)·4H 2 O (6.4g, 25.9mmol) was dissolved in 60mL methanol, under N 2 Under protection, the temperature was cooled to 0°C, sodium borohydride (0.98g, 25.9mmol) was added, and reacted at room temperature for 20 minutes, then 1,2-ethylenediamine (3.2g, 51.8mmol) was added, and undecyl- 2-Alkyn-1-ol (8.7g, 51.8mmol) was added to the reaction solution, and reacted for 5 hours under hydrogen gas. After the reaction was complete, the insoluble matter was removed by suction filtration with diatomaceous earth, the filtrate was extracted with ethyl acetate and water, and the organic phase was collected and dried with anhydrous sodium sulfate. Spin-dry to obtain 7.9 g (90% yield) of cis-undec-2en-1-ol as a light yellow liquid. 1 H NMR (300MHz, CDCl 3 ): δ0.87(t,J=6.9Hz,3H),1.26-1.37(m,13H),2.02-2.10(m,4H),2.02-2.09(m,2H),4.12-4.19(m,2H ),5.49-5.63(m,2H); 13 C NMR (75MHz, CDCl 3 ): δ14.2, 22.8, 27.5, 29.3, 29.4, 29.6, 29.7, 31.9, 58.6, 128.5, 133.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com