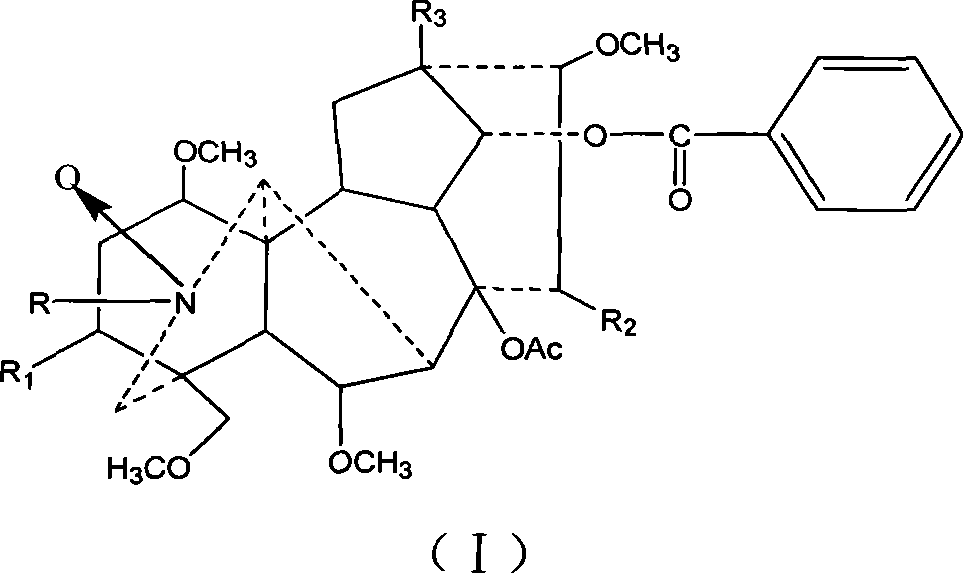
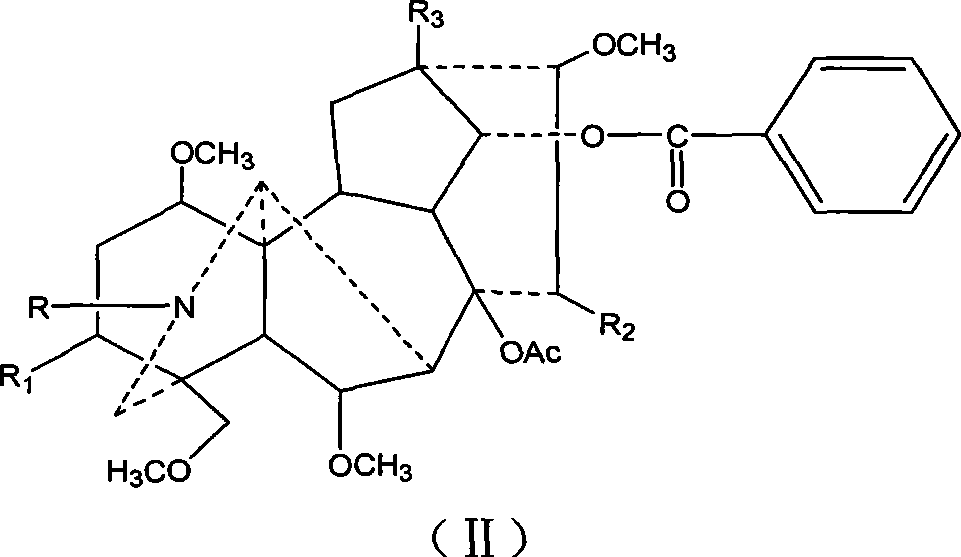
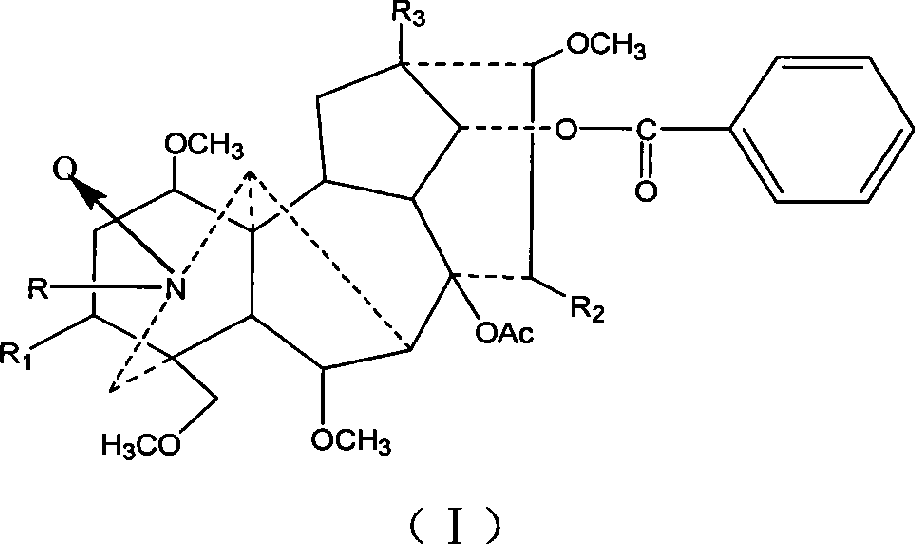
Mesaconitine acylated product nitrous oxide, preparation and use
A technology of aconitine acylate and nitrogen oxides, which is applied in the synthesis of pesticides and pharmaceuticals, and can solve the problems of poor nitrogen oxidation reaction, research on the activity of non-derivatives, and non-nitrogen oxidation reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Dissolve 209 mg of aconitine in 2 mL of pyridine, then add 1.5 mL of acetic anhydride dropwise, and stir at room temperature for 20 hours. The pH was adjusted to 9 with saturated sodium carbonate solution, and after extraction with chloroform, the chloroform layer was washed with water three times and dried, and distilled under reduced pressure to obtain 240.9 mg of the acetylated product of aconitine. Slowly add 246.3 mg of dissolved m-chloroperoxybenzoic acid to the chloroform solution of aconitine acetylation product under stirring at room temperature, and adjust the pH to 9 after reacting for 10 hours. After chloroform extraction, the chloroform layer is washed 3 times with water and dried. Distillation under reduced pressure gave 245.3 mg of the mixture. The mixture was separated on a silica gel column and eluted sequentially with 200 mL chloroform / methanol=150:1 and 300 mL chloroform / methanol=50:1 to obtain 56 mg of nitroxide-3-O-acetyl mesaconitine.
[0014] The...
Embodiment 2
[0018] Dissolve 200 mg of aconitine in 2 mL of pyridine, then add 1.5 mL of propionic anhydride dropwise, and stir at room temperature for 20 hours. Adjust the pH to 9 with saturated sodium carbonate solution, extract with chloroform, wash with water three times, dry, and distill under reduced pressure to obtain 219 mg of aconitine propionylation product. Then, 226.9 mg of dissolved m-chloroperoxybenzoic acid was slowly added to the solution of aconitine propionylation product under stirring at room temperature. After reacting for 10 hours, the pH was adjusted to 9. After extraction with chloroform, the chloroform layer was washed with water for 3 times and dried. , Distilled under reduced pressure to obtain 146.6 mg of the mixture. The mixture was separated on a silica gel column and eluted sequentially with 200ml chloroform / methanol=100:1 and 200mL chloroform / methanol=50:1 to obtain 71mg of nitroxide-3-O-propionyl mesaconitine.
[0019] The product structure was confirmed b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com